ABSTRACT
Gestational diabetes mellitus (GDM) is a common disease in pregnant women, imposing risks on both mother and fetus. Dysregulated nesfatin-1 has been observed in women with GDM, but the specific role of nesfatin-1 underlying the pathological process of GDM is unclear. The main objective of this study is to investigate the role and the molecular mechanism of nesfatin-1 in GDM. HTR-8/SVneo cells were treated with high glucose (HG)/high lipid (HL) to mimic the injured trophoblast of GDM in vitro. Cell viability, cytotoxicity and apoptosis were measured using CCK-8, LDH and TUNEL assays, respectively. The levels of inflammatory cytokines and antioxidant factors were detected using their commercial kits. ATP level and cytochrome c were determined with corresponding detecting kits. Quantitative real-time PCR and Western blot were performed to detect the expression of corresponding genes. The results showed that nesfatin-1 was downregulated upon HG/HL stimulation. Nesfatin-1 treatment greatly alleviated HG/HL-induced cell viability loss, cytotoxicity, inflammatory response, oxidative stress, and apoptosis in HTR-8/SVneo cells. In addition, nesfatin-1 promoted ATP generation, reduced the leakage of cytochrome c from mitochondria to cytoplasm, and upregulated mitochondrial transcription factor A (TFAM) and nuclear respiratory factor 1 (NRF1), alleviating mitochondrial dysfunction. Furthermore, nesfatin-1 inhibited p38 MAPK signaling. p79350, an agonist of p38 MAPK signaling, remarkably hindered the protective role of nesfatin-1 in HG/HL-induced HTR-8/SVneo cells. In conclusion, nesfatin-1 exerted a protective effect on GDM model in vitro, by regulating p38 MAPK signaling pathway, providing novel insights of treating GDM.
Introduction
Gestational diabetes mellitus (GDM), historically defined as the occurrence of high blood glucose levels with onset or firstly detected during pregnancy, is a common pregnancy complication which is linked with adverse maternal and offspring events [Citation1]. Women with GDM trend to develop cardiovascular disease and type 2 diabetes mellitus (T2DM), and offspring are also have a high risk for the occurrence of T2DM or obesity [Citation2]. Recent years, the morbility of GDM is continuing increasing, companied with 2%-5% of pregnancies complicated with GDM worldwide [Citation3]. Therefore, early diagnosis and timely treatment strategies are crucial for patients with GDM to reduce the risk of these adverse pregnancy outcomes.
It has been generally recognized that the etiology and pathogenesis of GDM is complicated, and multiple factors, such as genetics, environment, insulin resistance and dysfunctions of islet β-cells and placental lactogen production, may jointly contribute to its development [Citation4]. Upon pregnancy, the placenta acts as the bridge between maternal and fetal circulation, exerting effects on hormone secretion, inflammation modulation and metabolic regulation [Citation5]. The mechanisms of placental pathology during GDM remain unclear.
The dysregulated expression of multiple genes associated with glucose and lipid metabolism is involved in the progression of GDM. It is reported that adipokines may influence metabolic modifications and participate into a variety of metabolic processes during pregnancy [Citation6], providing a crucial role in maternal-fetal demands. Up to now, the importance of adipokines in the placental pathology during GDM is still rarely understood. Nesfatin-1, an adipocyte factor, is reported to play a crucial role in the progression of metabolic diseases, such as obesity and diabetes, by regulating appetite, insulin secretion and glucose homeostasis [Citation7–9]. A lower circulating level of serum nesfatin-1 was observed in GDM women than that in non-GDM women, pointing out the possible role of nesfatin-1 as a potential biomarker for the prediction and early diagnosis of GDM [Citation10]. In addition, the level of nesfatin-1 in GDM was found to be negatively correlated with weight, glucose level, obesity and insulin resistance, indicating a potential role of nesfatin-1 on GDM pathogenesis [Citation11]. Therefore, it is greatly needed to understand the specific mechanism of nesfatin-1 related GDM pathogenesis.
In the present study, we aim to investigate the specific role of nesfatin-1 in GDM, and to explore the underlying mechanism of nesfatin-1 on the placental pathology during GDM.
Methods
Cell culture and treatment
Immortalized human chorionic trophoblast line HTR-8/SVneo was purchased from ATCC, and cultured in RPMI-1640 medium supplemented with 5% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) in a humidified incubator with 5% CO2 in air atmosphere at 37°C.
Before the co-treatment with high glucose/high lipid (HG/HL) or Nesfatin-1, cells were treated with low glucose (5.5 mM glucose) for 24 h. Subsequently, cells were washed and the medium was changed to HG (25 mM glucose) or/and HL (0.4 mM palmitic acid) [Citation12]. For Nesfatin-1 treatment group, cells were pre-incubated with Nesfatin-1 (1, 2, 5, 10 and 20 nM; BioVision, Milpitas, CA, USA) reconstituted in double-distilled water for 2 h prior to medium change to HG/HL [Citation12,Citation13].
Western blot
Proteins were extracted from cells using radio‐immunoprecipitation assay (RIPA) kit (Solarbio, Beijing, China). After determination of the protein concentration using a BCA kit (Solarbio), the proteins were resolved in 12% SDS-PAGE and electrophoretically transferred on to polyvinylidene fluoride (PVDF) membranes by wet method. After blocking with 5% skimmed milk for 1 h at room temperature, membranes were incubated with diluted primary antibodies against Nesfatin-1 (orb544751, 1: 200, Biorbyt), Bcl-2 (ab32124, 1: 1,000, Abcam), Bax (ab32503, 1: 1,000, Abcam), cleaved caspase-9 (#7237, 1: 1,000, Cell Signaling Techonology), cleaved caspase-3 (#9661, 1: 1,000, Cell Signaling Techonology), p-p38(ab195049, 1: 1,000, Abcam), p38(ab31828, 1: 1,000, Abcam), and GAPDH (ab9485, 1: 1,000, Abcam) overnight at 4°C. On the following day, the membrane was washed with Tris-buffered saline with Tween 20 (TBST) three times and then incubated with horseradish peroxidase-labeled immunoglobulin G for 2 h at room temperature. The bands were visualized using an enhanced chemiluminescence detection kit (Amersham LifeScience, Buckinghamshire, UK), and analyzed by Quantity One v4.6.2 software (Bio-Rad, Inc.).
Cell viability assay
Cell viability was determined using Cell Counting Kit-8 (CCK-8; DOJINDO Laboratories, Kumamoto, Japan) in the guide of the manufacturer’s instructions. In brief, cells were cultured in 96-well plates at a density of 5000 cells/well. After indicated treatment, CCK-8 solution was diluted with the culture medium and added into these wells. In the following procedure, the plate was kept in the incubator for 1 hour. Eventually, the absorbance was detected at 450 nm with a microplate reader (BioTek, Winooski, USA).
LDH assay
The cytotoxicity was evaluated by detecting the LDH leakage in to the culture medium according to the manufacturer’s instructions of Cytoscan-LDH cytotoxicity assay kit (G-Biosciences Inc., St Louis, MO, USA). Briefly, cells were seeded in 96-well plates (5000 cells/well). After indicated treatment, 100 μl of cellular supernatant was transferred to a clean 96-well plate, and 100 μl of reaction mixture was added to each well for another incubation for 30 min in the dark. The absorption at 490 nm was detected using a microplate reader (BioTek, Winooski, USA).
TUNEL assay
The cell apoptosis was assessed by terminal dUTP transferase nick-end labeling (TUNEL) assay kit (Roche, Switzerland). In brief, after indicated treatment, cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min. Subsequently, Triton X-100 (Beyotime, China) was used to enhance the permeability of cell membranes, followed by the incubation with TUNEL reagent for 2 h. The apoptotic cells were observed under an inverted fluorescence microscope (Olympus IX71, Tokyo, Japan).
Cytokines analysis
After indicated treatment, the cell culture medium supernatants were collected. Tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β in culture supernatants were detected in guide of the manufacturer’s instructions of enzyme-linked immunosorbent assay (ELISA) kits (TNA31-K01for TNF-α, IL639-K01 for IL-6, and L1B31-K01 for IL-1β, all from BD Biosciences, San Jose, USA).
Oxidative stress assessment
After indicated treatment, the cell culture medium supernatants were collected. Levels of catalase (CAT), malondialdehyde (MDA) and superoxide dismutase (SOD) in culture supernatants were detected using their corresponding commercial kits (A007-1-1 for CAT, A003-4-1 for MDA, and A001-3-2 for SOD, all from Jiancheng Bioengineering, Nanjing, China) according to the manufacturer’s instructions.
Quantitative real-time PCR
Isolation of the total RNA in PTC cells was conducted by means of utilizing TRIzol reagent (Invitrogen, USA). 1 μg of total RNA was reverse-transcribed to generate cDNA with M-MLV reverse transcription (Takara, Japan), which is then applied to perform qRT-PCR equipped with SYBR green fluorescent dyes (Vazyme Biotech Co., Nanjing, China). The primers were obtained from Sangon Biotech (Shanghai, China) and the primer sequences were as follows: NFR1, forward, 5ʹ-GGAGCAGCTTGCCTCCTCAGA-3ʹ, and reverse, 5ʹ-CCATCACACACATGGGGAGAGCT-5ʹ; TFAM, forward, 5ʹ-AGCTCATGGACTTCTGCCAGCA-3ʹ, and reverse, 5ʹ-CCTGCCTCCATAATATAAGGAAACAAGAGT-3ʹ; β-actin, forward, 5ʹ- TGGCACCCAGCACAATGAA-3ʹ and reverse, 5ʹ- CTAAGTCATAGTCCGCCTAGAAGCA-3ʹ. The mRNA level was calculated using 2-ΔΔCt method. β-actin was used as internal reference.
Adenosine triphosphate (ATP) and Cytochrome C assessment
Levels of ATP in culture supernatants were detected using ATP Assay Kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. The level of cytochrome c release was measured after subcellular fraction preparation. Then, the Cytochrome C activity in mitochondria or cytoplasm was detected using Cytochrome c Assay Kit (BioVision, USA) according to the manufacturer’s instructions.
Statistical analysis
Values were expressed as the mean ± SD from at least three individual experiments. Compassion among groups were carried out using one-way ANOVA, followed by Tueky’s post hoc test. P < 0.05 is the parameter that confirms the significance of the statistic difference.
Results
Nesfatin-1 improved cell viability in HG/HL-induced HTR-8/SVneo cells
To explore the role of nesfatin-1 in GDM, we firstly examined the expression level of nesfatin-1 upon HG and/or HL stimulation in HTR-8/SVneo cells. As shown in ), the upregulated protein expression of nesfatin-1 was observed upon HG or HL stimulation, especially upon HG/HL stimulation, indicating that nesfatin-1 was highly expressed in the GDM model in vitro. After that, to assess the biological role of nesfatin-1 in GDM, HTR-8/SVneo cells were treated with different concentrations of nesfatin-1 (1, 2, 5, 10 and 20 μM) 2 h prior to HG/HL stimulation. As exhibited, HG or HL caused a great reduction of cell viability, which was more obvious on simultaneous stimulation of HG and HL. Nesfatin-1 at 1 μM and 2 μM had no influence on cell HG/HL-induced cell viability loss, but Nesfatin-1 at 5, 10 and 20 μM raised cell viability, peaking at 10 μM. In addition, HG and HL caused excessive leakage of LDH, which was greatly blocked by nesfatin treatment, peaking at 10 μM. Thus, nesfatin-1 could inhibit HG/HL-induced cell viability loss and cytotoxicity in HTR-8/SVneo cells, and 10 μM of nesfatin-1 was used for the following experiment.
Figure 1. Nesfatin-1 improved cell viability in HG/HL-induced HTR-8/SVneo cells (a-b) HTR-8/SVneo cells were treated with high glucose (HG; 25 mM glucose) or/and high lipid (HL; 0.4 mM palmitic acid). The protein expression of nesfatin-1 was detected using Western blot. (c) HTR-8/SVneo cells were treated with HG or/and HL, or pre-incubated with Nesfatin-1 (1, 2, 5, 10 and 20 nM) for 2 h prior to HG/HL stimulation. Then, cell viability of each group was measured using CCK-8 assay. (d) The cytotoxicity was evaluated by detecting the LDH leakage in to the culture medium. Data were expressed as the mean ± SD. N = 3. *p < 0.05, **p < 0.01, ***p < 0.001
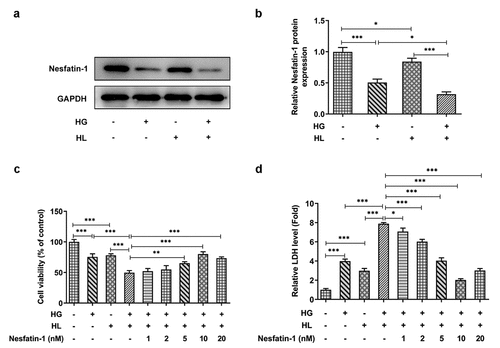
Nesfatin-1 lowered inflammatory response and oxidative stress in HG/HL-induced HTR-8/SVneo cells
Then, a series of inflammatory cytokines and antioxidant related factors were detected to assess the effects of nesfatin-1 on inflammatory response and oxidative stress in HG/HL-induced HTR-8/SVneo cells. The results revealed that the production of TNF-α, IL-6 and IL-1β was greatly elevated following HG or HL stimulation, especially following HG/HL stimulation; however, nesfatin-1 could remarkably reduce the elevation of these inflammatory cytokines (), indicating that nesfatin-1 inhibited inflammatory response in HG/HL-induced HTR-8/SVneo cells. In addition, an oxidative stress environment upon HG/HL stimulation was also observed, accompanied with the increased level of MDA and the decreased levels of SOD and CAT, whereas nesfatin-1 could alleviate this condition (), indicating that nesfatin-1 also inhibited oxidative stress in HG/HL-induced HTR-8/SVneo cells.
Figure 2. Nesfatin-1 lowered inflammatory response and oxidative stress in HG/HL-induced HTR-8/SVneo cells (a-c) HTR-8/SVneo cells were treated with HG or/and HL, or pre-incubated with Nesfatin-1 for 2 h prior to HG/HL stimulation. Then, the production of TNF-α, IL-6 and IL-1β in culture medium was assess using their corresponding ELISA kits. (d-f) The levels of MDA, SOD and CAT were elevated by their corresponding commercial kits. Data were expressed as the mean ± SD. N = 3. ***p < 0.001
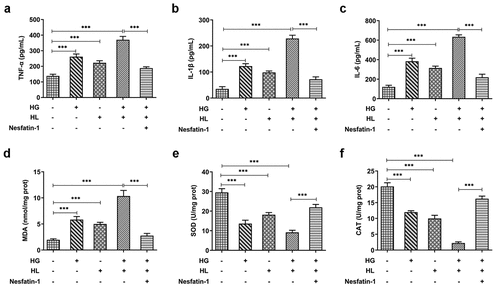
Nesfatin-1 reduced mitochondrial apoptosis in HG/HL-induced HTR-8/SVneo cells
To determine whether nesfatin-1 exerted a protective effect against HG/HL-induced apoptosis, TUNEL and Western blot assays were conducted. The elevated TUNEL-positive cells induced by HG and HL, as well as the downregulated Bcl-2 and upregulated Bax, cleaved caspase 3 and cleaved caspase 9, suggesting that HG/HL caused a significant apoptosis in HTR-8/SVneo cells, while these changes could be partly abolished by nesfatin-1 treatment (), demonstrating that nesfatin-1 exerted a protective effect against HG/HL-induced apoptosis. As mitochondrial pathway of apoptosis is the most commonly deregulated form of cell death [Citation14], we specifically focus upon mitochondrial function during this process. As shown in ), HG/HL resulted in the release of cytochrome c from mitochondria to cytoplasm, which was found to be reduced by nesfatin-1 treatment. Congruently, ATP level was also reduced upon HG/HL stimulation, which was alleviated by nesfatin-1 (). Nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM) were remarkably decreased following HG/HL stimulation, while nesfatin-1 hindered this decrease (). Taken together, these results suggested that nesfatin-1 could alleviate cell apoptosis and mitochondrial dysfunction in HG/HL-induced HTR-8/SVneo cells.
Figure 3. Nesfatin-1 reduced mitochondrial apoptosis in HG/HL-induced HTR-8/SVneo cells (a) HTR-8/SVneo cells were treated with HG or/and HL, or pre-incubated with Nesfatin-1 for 2 h prior to HG/HL stimulation. Cell apoptosis was assessed by TUNEL assay. (b) The protein expression of Bcl-2, Bax, Cleaved caspase 3 and cleaved caspase 9 was detected using Western blot. (c-d) The content of cytochrome C in mitochondria and cytoplasm is evaluated using Cytochrome c Assay Kit. (e) The ATP level in culture supernatants were detected using ATP Assay Kit. (f-g) The mRNA level of Nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM) was determined by qRT-PCR. Data were expressed as the mean ± SD. N = 3. *p < 0.05, **p < 0.01, ***p < 0.001
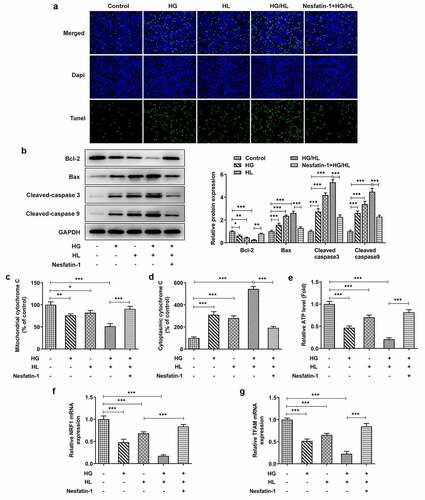
Nesfatin-1 inhibited p38 MAPK signaling in HG/HL-induced HTR-8/SVneo cells
P38 MAPK signaling pathway, is not only correlated with nesfatin-1 functioning, but also involved in pathological process of GDM [Citation15,Citation16], thus the involvement of p38 MAPK in HG/HL-induced HTR-8/SVneo cells upon nesfatin-1 was deserved to be investigated. As exhibited in , the protein expression of phosphorylated (p)-p38 was remarkably increased by HG or HL stimulation, especially in HG/HL group, while this increase was reversed by nesfatin-1.
Figure 4. Nesfatin-1 inhibited p38 MAPK signaling in HG/HL-induced HTR-8/SVneo cells HTR-8/SVneo cells were treated with HG or/and HL, or pre-incubated with Nesfatin-1 for 2 h prior to HG/HL stimulation. The protein expression of p-p38 and p38 was evaluated using Western blot. Data were expressed as the mean ± SD. N = 3. ***p < 0.001
Activation of p38 MAPK signaling abolished the protective effect of nesfatin-1 in HG/HL-induced HTR-8/SVneo cells
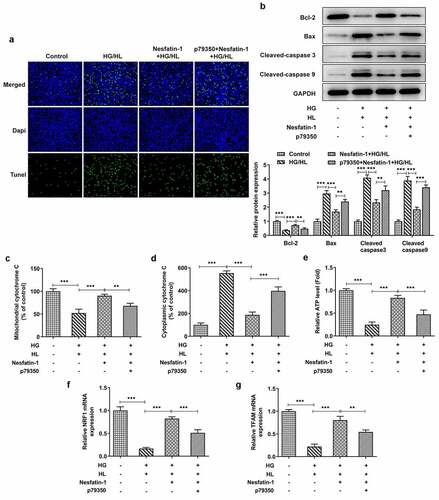
Finally, to confirm the involvement of p38 MAPK signaling pathway in the results observed here, we repeated the above experiments by adding p79350, an agonist of p38 MAPK signaling. CCK-8 and LDH assays exhibited that the improved cell viability and reduced cytotoxicity were eliminated by p79350 treatment (). The protective effects of nesfatin-1 against inflammatory response and oxidative stress were also weakened by p79350 treatment, as p79350 treatment increased the production of TNF-α, IL-6 and IL-1β, elevated the level of MDA, and reduced the levels of SOD and CAT (). Furthermore, the elevated apoptotic cells in p79350+ nesfatin-1+ HG/HL group was observed, compared to nesfatin-1+ HG/HL group (). Meanwhile, p79350 treatment erased the effects of nesfatin-1 on cell apoptosis-related proteins (). Moreover, compared to nesfatin-1+ HG/HL group, additional p79350 treatment remarkably promoted the cytochrome c leakage from the mitochondria to cytoplasm, decreased ATP level, and downregulated expression of Nrf1 and TFAM (), suggesting that p79350 erased the protective effect of nesfatin-1 against mitochondrial dysfunction in HG/HL-induced HTR-8/SVneo cells.
Figure 5. P79350 treatment erased the effects of nesfatin-1 on cell viability, inflammatory response and oxidative stress in HG/HL-induced HTR-8/SVneo cells (a) HTR-8/SVneo cells were treated with HG/HL, or pre-incubated with Nesfatin-1 with or without p79350 for 2 h prior to HG/HL stimulation. Cell viability was measured using CCK-8 assay. (b) The cytotoxicity was evaluated by detecting the LDH leakage in to the culture medium. (c-e) The production of TNF-α, IL-6 and IL-1β in culture medium was assess using their corresponding ELISA kits. (f-h) The levels of MDA, SOD and CAT were elevated by their corresponding commercial kits. Data were expressed as the mean ± SD. N = 3. *p < 0.05, **p < 0.01, ***p < 0.001
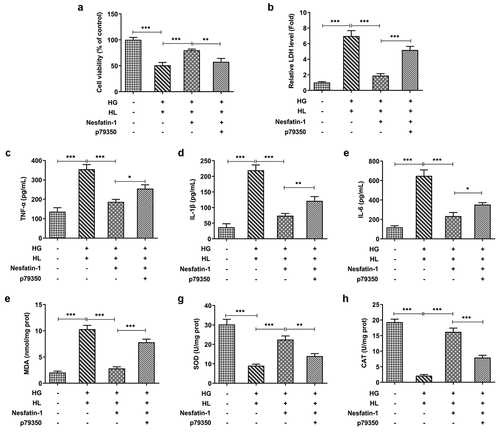
Figure 6. P79350 treatment erased the effects of nesfatin-1 on cell apoptosis and mitochondrial dysfunction in HG/HL-induced HTR-8/SVneo cells (a) HTR-8/SVneo cells were treated with HG/HL, or pre-incubated with Nesfatin-1 with or without p79350 for 2 h prior to HG/HL stimulation. Cell apoptosis was assessed by TUNEL assay. (b) The protein expression of Bcl-2, Bax, Cleaved caspase 3 and cleaved caspase 9 was detected using Western blot. (c-d) The content of cytochrome C in mitochondria and cytoplasm is evaluated using Cytochrome c Assay Kit. (e) The ATP level in culture supernatants were detected using ATP Assay Kit. (f-g) The mRNA level of Nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM) was determined by qRT-PCR. Data were expressed as the mean ± SD. N = 3. **p < 0.01, ***p < 0.001
Discussion
In the present study, we found that the expression of nesfatin-1 was notably downregulated upon HG/HL stimulation. Nesfatin-1 treatment was demonstrated to alleviate HG/HL-caused inflammatory response, oxidative stress, and apoptosis, as well as mitochondrial dysfunction. In addition, the inhibitory effect of nesfatin-1 on this pathological status of GDM was weakened by p38 MAPK signaling activation, indicating that p38 MAPK signaling was indispensable for nesfatin-1 protecting against GDM. These findings suggested that nesfatin-1 could be a possible candidate for treating GDM.
Although the correlation between nesfatin-1 and GDM pathogenesis has been revealed from previous reports [Citation10,Citation11], as well as the protective role of nesfatin-1 in diabetes and diabetic complications [Citation17,Citation18], there is so far no evidence showing the effect of nesfatin-1 on injured trophoblast cells during GDM. In addition to maintaining metabolite homeostasis in diabetes-related diseases, the anti-inflammatory, anti-oxidant, and anti-apoptotic activities of nesfatin-1 also contribute to its outstandingly protective role in multiple pathological processes. Concretely, Nesfatin-1 suppressed inflammation-related signaling pathways, thus reducing the inflammatory response and oxidative stress of injured alveolar epithelial cells, thereby alleviating acute lung injury [Citation13]; Nesfatin-1 protected against high glucose-induced PC12 cell injury by inhibiting oxidative stress and apoptosis [Citation19]. In the present study, we found that nesfatin-1 also exerted protective effects against HG/HL-induced cell viability loss, cytotoxicity, inflammatory response, oxidative stress and cell apoptosis in HTR-8/SVneo cells, suggesting that nesfatin-1 might protect against trophoblast cell injury during GDM by inhibiting inflammation, oxidative stress and cell apoptosis.
Furthermore, GDM can cause mitochondrial dysfunction in the placental trophoblast, exhibited as lower mitochondrial activity in the human foeto-placental unit. Mitochondria is known to primarily function as providing cellular chemical energy in the form of ATP, the efficient generation of which in placenta is necessary to ensure fetal growth and development [Citation20]. Thus, the sufficient ATP not only represents the abnormality of mitochondria, but also indicates a pathological state during GDM. As expected, HG/HL resulted in a notable reduction of ATP level of HTR-8/SVneo cells, while nesfatin-1 rescued the reduced ATP, indicating that nesfatin-1 might alleviate mitochondrial dysfunction by improving ATP level in GDM. In addition, mitochondrial damage causes apoptotic cascades, as it leaks pro-apoptotic protein cytochrome c into cytoplasm, leading to caspase 3-dependent cell apoptosis [Citation21]. In the present study, nesfatin-1 strictly hindered the leakage of cytochrome from mitochondria to cytoplasm in HG/HL-induced HTR-8/SVneo cells, which might be the explanation for its anti-apoptotic activity as aforementioned. Moreover, we further detected the effect of nesfatin-1 on Nrf1 and TFAM expression level. Nrf1 is an effector of nucleo-mitochondrial interactions, and is recognized to be eminently pertinent to the biogenesis of mitochondria [Citation22]. TFAM is a principal mitochondrial gene-regulator, crucial to mitochondrial homeostasis. The results showed that the expression level of Nrf1 and TFAM was nesfatin-1 distinctly reduced following HG/HL stimulation, which was reversed by nesfatin-1, indicating that nesfatin-1 alleviated HG/HL-caused mitochondrial dysfunction in trophoblast cells, in line with previous work showed that nesfatin-1 exerted anti-apoptotic property by ameliorating mitochondrial dysfunction to prevent Parkinson’s disease [Citation21].
P38 MAPK, a critical subtype of MAPKs family, is classically known as a responsive element to stress stimuli. Several studies have shown that the p38 MAPK signaling is activated either in response to high glucose or high lipid stimulation [Citation23,Citation24]. In addition, p38 MAPK is reported to be involved in the pathophysiological mechanism of reproductive diseases, such as preeclampsia, recurrent pregnancy loss and GDM [Citation25–27]. Here, the expression of p-p38 was notably elevated following HG or HL stimulation, especially following the simultaneous stimulation of HG and HL, demonstrating that the p38 MAPK signaling was activated in HG/HL-induced HTR-8/SVneo cells. In particular, recent evidence revealed that p38 MAPK activation contributes to mitochondrial dysfunction by disrupting mitochondrial homeostasis, including causing DNA damage, reducing ATP production, and increasing ROS production [Citation28]. In addition, p38 MAPK activation also inhibited autophagy to cause mitochondrial impairment by negatively regulating Parkin activity, which disrupted mitophagy [Citation29]. Coincidentally, nesfatin-1 exerted its protective role in acute lung injury by inhibiting p38 MAPK signaling pathway, thus reducing inflammatory response and oxidative stress [Citation13]. In the present study, nesfatin-1 also exhibited an inhibitory effect on p38 MAPK signaling. Additionally, the protective effects of nesfatin-1 against HG/HL-induced inflammatory response, oxidative stress, apoptosis, and mitochondrial dysfunction of HTR-8/SVneo cells were partly diminished by p79350, an agonist of p38 MAPK signaling, confirming the involvement of p38 MAPK signaling pathway underlying the mechanism of the protective role of nesfatin-1 in GDM.
Conclusion
In conclusion, nesfatin-1 prevents against HG/HL-induced inflammatory response, oxidative stress, and apoptosis of HTR-8/SVneo cells by alleviating mitochondrial dysfunction, which is mediated by p38 MAPK signaling. This study provides a neoteric insight and potential therapeutic strategy for the treatment of GDM.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Availability of Data and Materials
All data generated or analyzed during this study are included in this published article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- International Association of D, Pregnancy Study Groups Consensus P, Metzger BE, Gabbe SG, Persson B, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):e98–e98.
- Group HSCR. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int J Gynaecol Obstet. 2002;78(1):69–77.
- Rosik J, Szostak B, Machaj F, et al. The role of genetics and epigenetics in the pathogenesis of gestational diabetes mellitus. Ann Hum Genet. 2020;84(2):114–124.
- Plows JF, Stanley JL, Baker PN, et al. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018;19(11):11.
- Legg-St Pierre CB, Mackova M, Miskiewicz EI, et al. Insulinotropic nucleobindin-2/nesfatin-1 is dynamically expressed in the haemochorial mouse and human placenta. Reprod Fertil Dev. 2018;30(3):519–532.
- Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. 2016;231(3):R77–R99.
- Garcia-Galiano D, Navarro VM, Roa J, et al. The anorexigenic neuropeptide, nesfatin-1, is indispensable for normal puberty onset in the female rat. J Neurosci. 2010;30(23):7783–7792.
- Cao X, Liu XM, Zhou LH. Recent progress in research on the distribution and function of NUCB2/nesfatin-1 in peripheral tissues. Endocr J. 2013;60(9):1021–1027.
- Garces MF, Poveda NE, Sanchez E, et al. Regulation of NucB2/Nesfatin-1 throughout rat pregnancy. Physiol Behav. 2014;133:216–222.
- Mierzynski R, Poniedzialek-Czajkowska E, Dluski D, et al. Nesfatin-1 and vaspin as potential novel biomarkers for the prediction and early diagnosis of gestational diabetes mellitus. Int J Mol Sci. 2019;20(1):1.
- Kucukler FK, Gorkem U, Simsek Y, et al. Low level of Nesfatin-1 is associated with gestational diabetes mellitus. Gynecol Endocrinol. 2016;32(9):759–761.
- Wang Y, Xue J, Li Y, et al. Telmisartan protects against high glucose/high lipid-induced apoptosis and insulin secretion by reducing the oxidative and ER stress. Cell Biochem Funct. 2019;37(3):161–168.
- Wang ZZ, Chen SC, Zou XB, et al. Nesfatin-1 alleviates acute lung injury through reducing inflammation and oxidative stress via the regulation of HMGB1. Eur Rev Med Pharmacol Sci. 2020;24(9):5071–5081.
- Lopez J, Tait SW. Mitochondrial apoptosis: killing cancer using the enemy within. Br J Cancer. 2015;112(6):957–962.
- Jiang L, Xu K, Li J, et al. Nesfatin-1 suppresses interleukin-1beta-induced inflammation, apoptosis, and cartilage matrix destruction in chondrocytes and ameliorates osteoarthritis in rats. Aging (Albany NY). 2020;12(2):1760–1777.
- Aye IL, Jansson T, Powell TL. TNF- αstimulates System A amino acid transport in primary human trophoblast cells mediated by p38 MAPK signaling. Physiol Rep. 2015;3(10):e12594.
- Ranjan A, Choubey M, Yada T, et al. Nesfatin-1 ameliorates type-2 diabetes-associated reproductive dysfunction in male mice. J Endocrinol Invest. 2020;43(4):515–528.
- Chen K, Zhuo T, Wang J, et al. Saxagliptin upregulates nesfatin-1 secretion and ameliorates insulin resistance and metabolic profiles in type 2 diabetes mellitus. Metab Syndr Relat Disord. 2018;16(7):336–341.
- Nazarnezhad S, Rahmati M, Shayannia A, et al. Nesfatin-1 protects PC12 cells against high glucose-induced cytotoxicity via inhibiting oxidative stress, autophagy and apoptosis. Neurotoxicology. 2019;74:196–202.
- Fisher JJ, Vanderpeet CL, Bartho LA, et al. Mitochondrial dysfunction in placental trophoblast cells experiencing gestational diabetes mellitus. J Physiol. 2021;599(4):1291–1305.
- Tan Z, Xu H, Shen X, et al. Nesfatin-1 antagonized rotenone-induced neurotoxicity in MES23.5 dopaminergic cells. Peptides. 2015;69::109–114.
- Hickson-Bick DL, Jones C, Buja LM. Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. J Mol Cell Cardiol. 2008;44(2):411–418.
- Shan L, Yang D, Zhu D, et al. High glucose promotes annulus fibrosus cell apoptosis through activating the JNK and p38 MAPK pathways. Biosci Rep. 2019;39(7):7.
- Li X, Cui K, Fang W, et al. High level of dietary olive oil decreased growth, increased liver lipid deposition and induced inflammation by activating the p38 MAPK and JNK pathways in large yellow croaker (Larimichthys crocea). Fish Shellfish Immunol. 2019;94:157–165.
- Zhao H, Li Y, Dong N, et al. LncRNA LINC01088 inhibits the function of trophoblast cells, activates the MAPK signaling pathway and associates with recurrent pregnancy loss. Mol Hum Reprod. 2021;27(8). DOI:10.1093/molehr/gaab047.
- Cawyer CR, Horvat D, Leonard D, et al. Hyperglycemia impairs cytotrophoblast function via stress signaling. Am J Obstet Gynecol. 2014;211(5):541 e1–8.
- Harlev A, Aricha-Tamir B, Shaco-Levy R, et al. Macrophage infiltration and stress-signaling in omental and subcutaneous adipose tissue in diabetic pregnancies. J Matern Fetal Neonatal Med. 2014;27(12):1189–1194.
- Gui C, Ren Y, Chen J, et al. p38 MAPK-DRP1 signaling is involved in mitochondrial dysfunction and cell death in mutant A53T alpha-synuclein model of Parkinson’s disease. Toxicol Appl Pharmacol. 2020;388:114874.
- Chen J, Ren Y, Gui C, et al. Phosphorylation of Parkin at serine 131 by p38 MAPK promotes mitochondrial dysfunction and neuronal death in mutant A53T alpha-synuclein model of Parkinson’s disease. Cell Death Dis. 2018;9(6):700.
