ABSTRACT
MicroRNA (miR)-150-5p has been investigated in many studies, while the role of exosomal miR-150-5p from bone arrow mesenchymal stromal cells (BMSCs) on cerebral ischemia/reperfusion (I/R) injury is not fully explored. This research aims to probe the effects of exosomal miR-150-5p from BMSCs on cerebral I/R injury via regulating B-cell translocation gene 2 (TLR5). Bone marrow mesenchymal stem cell-derived exosomes (BMSCs-Exo) were isolated and identified. The middle cerebral artery occlusion (MCAO) rat model was established and treated by BMSCs-Exo. Then, functional assays were conducted to explore neurological function, pathological changes, neuron apoptosis and inflammatory factors in MCAO rats. miR-150-5p and TLR5 expression in rat brain tissues were detected. Then, gain and loss-function assays were conducted to determine the impact of exosomes, miR-150-5p and TLR5 on neurological function, pathological changes, neuron apoptosis and inflammatory factors of MCAO rats. The binding relation between miR-150-5p and TLR5 was validated. It was found that miR-150-5p expression was decreased while TLR5 level was augmented in MCAO rats. BMSCs-Exo could improve neurological function, pathological changes, decelerate neuron apoptosis and reduce inflammatory factors in MCAO rats. Enriched miR-150-5pcould enhance the protective effects of BMSCs-Exo on cerebral I/R injury. The elevated TLR5 reversed the impacts of elevated exosomal miR-150-5p on cerebral I/R injury. TLR5 was targeted by miR-150-5p. This research manifested that exosomal miR-150-5p from BMSCs exerts protective effects on cerebral I/R injury via repressing TLR5. This study provided novel therapeutic targets for the treatment of cerebral I/R injury.
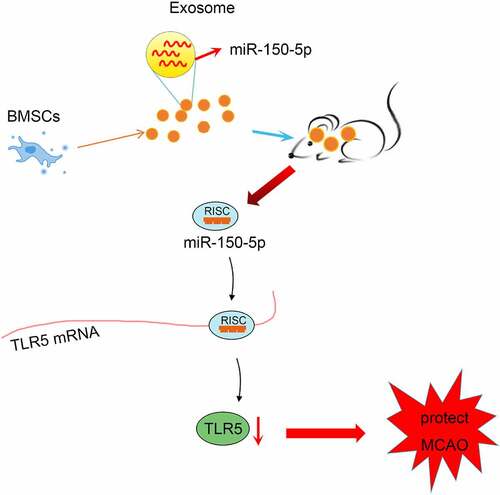
Graphical abstract
Introduction
Cerebral ischemia is defined as perturbations in blood flow to the brain that trigger complex metabolic and cellular pathology, inducing neuronal cell death and cerebral infarction [Citation1]. In addition, the reperfusion after cerebral ischemia can lead to brain injury, further causing cerebral edema, brain hemorrhage, and neuronal death. Such a process is termed as cerebral ischemia/reperfusion (I/R) injury [Citation2]. Intravenous thrombolysis and endovascular therapy are two major treatments for ischemic reperfusion injury [Citation3]. However, I/R injury still contributes to high mortality and mobility in a wide range of pathologies, such as myocardial infarction, ischemic stroke and acute kidney injury [Citation4]. Therefore, novel therapeutic avenues for cerebral I/R injury treatment require further exploration.,
Bone marrow mesenchymal stromal cells (BMSCs) are a group of nonhematopoietic skeletal progenitor cells with salient importance for the hematopoietic microenvironment [Citation5]. It has been validated that BMSCs and oxiracetam treatment can mitigate cerebral I/R injury [Citation6]. Furthermore, it has been illustrated that BMSCs-derived exosomes may attenuate cell pyroptosis by promoting autophagic flux in cerebral I/R injury [Citation7]. As small, single-membrane organelles that aggregate in proteins, lipids, nucleic acids, and glycoconjugates, exosomes also function as the regulator of the extracellular matrix and the mediator for signals and molecules [Citation8]. Yu et al. have validated that the suppression of exosomes secretion slashes the inhibitory effects of conditioned medium of BMSCs on inflammatory factors in I/R injury [Citation9]. Exosomes from BMSCs effectively protect renal I/R injury and endoplasmic reticulum stress at incipient reperfusion stages [Citation10]. Additionally, exosomal microRNAs (miRNAs) derived from BMSCs have been clarified to mitigate renal and myocardial I/R injury [Citation11,Citation12]. miRNAs are also involved in I/R injury through the regulation of critical signaling pathways that are related to necrosis, apoptosis, inflammation, fibrosis and neoangiogenesis [Citation13]. For instance, miR-150 has been revealed to reduce the vascular density of infarct border zone in middle cerebral artery occlusion (MCAO) rats and constrain the growth of brain microvascular endothelial cells [Citation14]. The enrichment of miR-150-5p can repress the cardiomyocyte apoptosis in I/R rats [Citation15]. Furthermore, it was predicated through the bioinformatic website that there was a binding relationship between miR-150-5p and toll-like receptor 5 (TLR5). Shreds of evidences have suggested that toll-like receptors (TLRs) exert crucial impacts on the adult cerebral ischemic injury. For instance, TLR-2 is highly expressed in the brain and TLR-2 deficiency contributes to attenuating hypoxia-ischemia injury in the mice brain [Citation16]. Moreover, Qiao et al. have implied that TLR5 deletion helps to relieve neurological deficit, reduce infarct volume and block edema after ischemic stroke [Citation17]. Nevertheless, the efficacy of exosomal miR-150-5p in cerebral I/R injury development through mediating TLR5 still necessitated further extensive exploration.
Concerning these previous studies mentioned above, we hypothesized that the exosomal miR-150-5p from BMSCs might be involved in the progression of cerebral ischemia/reperfusion injury via modulating TLR5. Therefore, this study committed to evidence the detailed effects of exosomal miR-150-5p from BMSCs on cerebral I/R injury, affording novel therapeutic targets and a distinguished research direction for the treatment of cerebral I/R injury.
Material and methods
Ethics statement
Animal experiments were conformed to the Guide to the Management and Use of Laboratory Animals issued by the National Institutes of Health. The protocol of animal experiments was approved by the Institutional Animal Care and Use Committee of Daqing Oilfield General Hospital.
Laboratory animal
Specific pathogen-free grade sprague dawley male rats (260–280 g) were purchased from Guangzhou Jennio Biotech Co., Ltd (Guangzhou, China) for the construction of the MCAO rat model. Female rats were characterized by high estrogen levels with neuroprotective function and periodic estrogen secretion. During the experiment, the estrogen level could not be calculated accurately, causing difficulties to the application of female animal models with cerebrovascular disease. Because of the instability of the female animal model, male rats were selected for the preparation of the MCAO rat model.
Isolation and culture of BMSCs as well as the extraction and identification of exosomes
The fetal bovine serum was ultra-centrifuged (120,000 × g, 4°C, 18 hours) to remove exosomes in serum. The exosomes-removing 10% fetal bovine serum and 1% penicillin-streptomycin were added into Dulbecco’s modified Eagle’s medium. The bone marrow cavity of femur and tibia of rats was washed with a prepared culture medium in a sterile environment. The obtained bone marrow cells were seeded in a 25 cm2 culture dish for incubation. Then, the culture medium was replaced every 2 days. The morphology and growth of the cells were observed under a microscope. When the cell confluence reached 80–90%, the cells were detached with 0.25% trypsin-ethylene diamine tetraacetic acid solution and passaged in 1:2.
The BMSCs in passage 3 were detached, adjusted to 1.0 × 106 pc/mL and put into 1.5 mL eppendorf tubes. CD29-PE, CD45-PE, CD54-PE and CD90-PE monoclonal antibodies (BD phamingen company, CA, USA) were added, respectively. A homeotype of negative control (NC) was established, reacted for 30 minutes and determined by a flow cytometer (Bio-Rad Laboratories, Hercules, CA, USA).
BMSCs in passage 3 with 90% confluence were seeded into a 6-well plate with 1 × 105 cells/mL. The cells were induced to differentiation with the adipogenic differentiation mediumand osteogenic differentiation medium , respectively. Fourteen days later, the ability of adipogenic differentiation of BMSCs was detected with oil red O staining. The cells were washed three times with phosphate buffered saline, fixed with 4% paraformaldehyde at 20°C for 30 minutes, and then stained with oil red O solution at 20°C for 1 hour. The stained cells were washed with phosphate buffered saline and observed under a microscope. After 21 days, the osteogenic differentiation ability of BMSCs was examined through alizarin staining, Then, the cells were stained with 0.1% alizarin red S in distilled water (pH 4.2) at 20°C for 60 minutes. After staining, the cells were rinsed with distilled water for three times, and observed with a microscope to quantify the stained cells [Citation18,Citation19].
BMSCs were cultured with 10% exosomes-removing fetal bovine serum culture medium for 48 hours. The collected supernatant was centrifuged at 500 g for 15 minutes, and at 2000 g for 15 minutes. Then, the vacuole was removed by centrifugation at 10,000 × g at 4°C for 20 minutes. Thereafter, the supernatant was filtered with a 0.22 micron filter, and then ultra-centrifuged at 110,000 × g at 4°C for 70 minutes two times. Afterward, the supernatant was resuspended with 100 µL sterile phosphate buffered saline for downstream experiments[Citation20]. Then, the exosomes were stained with 3% aqueous phosphotungstic acid for 5 minutes. Finally, the samples were observed with a transmission electron microscope running at 100 kV, and the particle size of the exosomes was detected with the ZetaView (Particle Metrix, Meerbusch, Germany). The marker proteins CD63, CD9 and CD81 were detected by Western blot assay [Citation21,Citation22].
BMSCs transfection
Lipofectamine 3000 (Thermo Fisher Scientific Inc., Waltham, MA, USA) was used for cell transfection. Mimic-NC (5′-UUCUCCGAACGUGUCACGU-3′) and miR-150-5p-mimic (5′-UCUCCCAACCCUUGUACCAGUG-3′) were transfected into BMSCs to obtain exosomes [Citation23,Citation24].
MCAO rat model establishment
MCAO rat model was constructed with a modified nylon suture method. Rats were fasted before the ischemic injury. Then, rats were anesthetized and midline of the ventral neck was incised. The right common carotid artery, external carotid artery and internal carotid artery were exposed. The right common carotid artery and the external carotid artery were ligated through the ventral midline neck incision. Ischemia was caused through blocking the middle cerebral artery of rats with the insertion of nylon monofilament sutures into the ICA. The filaments were held for 2 hours and the monofilament was removed to restore the blood perfusion of the internal carotid artery-middle cerebral artery for around 24 hours. The rats in the sham group underwent the same surgery without ligating the arteries. The rats temperature was maintained at 37°C by a thermostatically controlled heating pad until rats recovered from surgery.
The experimental groups were as follows: sham-operated group (sham group), MCAO group, Exo group (MCAO rats injected with BMSCs-Exo), Exo- mimic-NC group (MCAO rats injected with BMSCs-Exo that had transfected with mimic-NC), Exo-miR-150-5p-mimic group (MCAO rats injected with BMSCs-Exo that transfected with miR-150-5p-mimic), Exo+short hairpin RNA (sh)-NC group (MCAO rats injected with BMSCs-Exo andlentivirus cloned with sh-NC sequence), Exo + sh-TLR5 group (MCAO rats injected with BMSCs-Exo and lentivirus cloned with sh-TLR5 sequence [5′-GGACTGCGATGAAGAGGAA-3′]), Exo-miR-150-5p-mimic + overexpression (oe)-NC group (MCAO rats injected with BMSCs-Exo that had transfected with miR-150-5p-mimic andlentivirus cloned with oe-NC sequence), Exo-miR-150-5p-mimic + oe-TLR5 group (MCAO rats injected with BMSCs-Exo that had transfected with miR-150-5p-mimic and lentivirus cloned with oe-TLR5 sequence) [Citation25]. After 2-hour establishment of the MCAO rat model that conformed to the method in a preceding study [Citation26], the rats were performed with stereotactic injection of exosomes (100 μg/kg) and lentivirus (5 × 107 TU/mL) into lateral ventricle [Citation27]. After 72 hours, rats were euthanized and the brain tissue was collected.
Neurological function score
The neurological function of rats was evaluated according to the description in previous literature [Citation28]. The neurological deficit in MCAO rats was classified into five levels as follows: 0 score, no obvious neurological deficit symptoms; 1 score, when the rats were pulled up, the left forelimb could not be fully extended; 2 scores, when the tail was pulled up, the left forelimb was flexed; when rats were put on the ground to left, the crawling resistance was lower than the right; 3 scores, when the crawling condition was the same as 2 scores, the rats displayed involuntary left turn; 4 scores, coma, including death within 24 hours.
Hematoxylin-eosin (HE) staining
The brains (5 in each group) were collected and the paraffin-embedded tissues were sliced into 6 μm coronal sections at 4°C after dehydration and hydration. The tissue sections were dewaxed with xylene, rehydrated with gradient ethanol and stained with hematoxylin and eosin. Then, the sections were observed under a light microscope (Olympus, Tokyo, Japan) [Citation29,Citation30].
Nissl staining
Paraffin sections were dewaxed in xylene and hydrated with graded ethanol (95%, 85% and 75%). After rinsing with distilled water, the slices were stained with 0.5% crystal violet for 10 minutes and observed under the BX53 light microscope (Olympus).
Transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining
The cell apoptosis in brain tissue was analyzed using the TUNEL apoptosis detection kit (Beyotime Institute of Biotechnology, Shanghai, China). Paraffin sections were dewaxed and hydrated, and 20 mg/mL DNase-free protease K was added dropwise. Then, the mixture was restored for 20 minutes and incubated with 3% hydrogen peroxide solution for 20 minutes. Then, 50 mL TUNEL working solution was added and restored for 60 minutes. The sample was added with 0.3 mL labeled reaction termination solution and incubated for 10 minutes. Then, 50 mL streptavidin-horseradish peroxidase working solution was added, and then incubated for 15 minutes. Thereafter, 0.5 mL diaminobenzidine color solution was added for 30-minute incubation. After counter-staining of hematoxylin, ethanol gradient dehydration, xylene permeabilization and neutral resin sealing, the samples were observed under a light microscope. Then, 10 visual fields in each section were randomly selected. The cells with brown-yellow nuclei were positive, and the cells with blue nuclei were normal. The apoptosis rate of neurons was calculated as the ratio of the number of brown-yellow cells to that of blue cells [Citation31].
Enzyme-linked immunosorbent assay (ELISA)
Brain tissues were collected and sequentially homogenized in ice-cold PBS, and the supernatants were centrifuged at 8000g for 10 min at 4°C.The tumor necrosis factor alpha (TNF-α), interleukin-1beta (IL-1β) and IL-6 levels were determined by ELISA kits (Nanjing KeyGen Biotech, Jiangsu, Chain). The experiment was repeated for 3 times [Citation32].
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
RNApure kit (BioTeKe corporation, Jiangsu, China). was used for extracting total RNA from brain tissue. The mass and concentration of RNA were measured by ultraviolet-visible spectrophotometry (ND-1000, Nanodrop, USA). cDNA was synthesized using the TaKaRa PrimeScript RT reagent kit with gDNA Eraser. qRT-PCR was conducted using TaKaRa SYGB Premix EX Taq (Tli RNaseH Plus, CA). For miRNA analysis, the One Step PrimeScript miRNA cDNA Synthesis Kit (TaKaRa) and the SYBR PrimeScriptTM miRNA RT-PCR Kit (TaKaRa) were used. Primers used are listed in Supplementary Table 1. U6 was the endogenous reference of miR-150-5p, and glyceraldehyde-3-phosphate dehydrogenase was the internal reference of the remained genes. The gene expression was calculated by 2−ΔΔCt method [Citation33,Citation34].
Western blot assay
Brain tissue was collected and lysed with radio-immunoprecipitation assay cell lysis buffer . The eppendorf tube was then centrifuged at 10,000rpm for 20 minutes. Protein concentrations were examined through the bicinchoninic acid method. The protein and sample buffer were treated with sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membrane. The polyvinylidene fluoride membrane was sealed in the blocking buffer consisting of tris-buffered saline with Tween 20 and 5% skim milk. Then, the membrane was placed on a shaking table for 1 hour and incubated overnight with primary antibodies at 4°C. GAPDH (1:5000, Abcam Inc., Cambridge, MA, USA) and TLR5 (1:1000, Abcam Inc.) were prepared for immunoblotting. Thereafter, the polyvinylidene fluoride membrane was incubated overnight at 4°C in secondary antibody and washed again with tris-buffered saline with Tween 20 for 10 minutes. The bands were photographed through a Bio-Rad image analysis system (BIO-RAD Company, CA, USA), and then analyzed by the Quantity One v4.6.2 (Bio- Rad Laboratories). The relative protein content was expressed as the gray value of the corresponding protein bands/gray value of GAPDH protein bands[Citation35].
Dual luciferase reporter gene assay
The targeting relation between miR-150-5p and TLR5 was predicted through the bioinformatic website TargetScan. The 3'-untranslated region (UTR) of APC containing miR-150-5p binding sites was cloned into the pGL3-Basic luciferase vector (Promega, Madison, United States) to generate TLR5 wild type (WT) and mutated to generate TLR5 mutant (MUT). 293T cells were co-transfected with TLR5-WT, TLR5-MUT and miR-150-5p mimic or mimic NC using Lipofectamine 3000ʹ. After 48 hours, cell lysates were collected. The activity of luciferase was measured using the Luciferase Reporter Assay Kit (Promega, Madison, United States) in accordance with the manufacturer’s instructions. Each experiment was repeated for 3 times [Citation23].
Statistical analysis
The data were expressed as mean ± standard deviation, and each experiment was repeated at least 3 times. The statistical analysis was conducted by GraphPad Prism 8.0 (GraphPad Software, Inc., CA, USA). The t-test was used for the comparison between two groups; one-way analysis of variance (ANOVA) was adopted for comparisons among multiple groups and followed by Tukey’s post hoc test for pairwise comparison. P < 0.05 was an indicator of statistical significance [Citation33].
Results
The identification of BMSCs and exosomes
It has been reported that exosomal miR-150-5p from BMSCs can attenuate cardiomyocyte apoptosis and improve cardiac function in myocardial infarction rats via targeting B-cell lymphoma-2 associated X (BAX) [Citation36]. Hence, we speculated that miR-150-5p from BMSCs might also be involved in the progression of cerebral I/R injury. In this study, we focused on the regulatory mechanism of exosomal miR-150-5p from BMSCs on the progression of cerebral I/R injury through mediating TLR5, and aimed to afford a novel research direction for cerebral I/R injury treatment.
Initially, after the isolation of BMSCs, we identified the BMSCs and it was found that BMSCs were mainly consisted of spindle-shaped cells with single and uniform shapes. The BMSCs were arranged in a vortex-like and radial manner, and conformed to the typical growth pattern of BMSCs ()). The outcomes of flow cytometry showed that CD29, D54 and CD90 were positively expressed and CD45 was negatively expressed ()). The results of oil red O staining and alizarin red staining implied that lipid droplets were stained red, and there existed nodules, indicating that the isolated BMSCs were capable of osteogenic and adipogenic differentiation ()).
Figure 1. The identification of BMSCs and exosomes. A, observation of morphology of BMSCs (scale bar = 100 μm); B, identification of surface markers of BMSCs by flow cytometry; C, the adipogenic ability and osteogenic ability were examined by oil red O and alizarin red staining; D, the exosome particle size was measured by NTA; E, the exosome morphology was observed by transmission electron microscope (scale bar = 100 nm); F, the exosome surface markers were detected by Western blot assay; G, miR-150-5p expression in BMSCs and exosomes after transfected with miR-150-5p-mimic was examined by RT-qPCR; *P < 0.05 vs. the mimic-NC group; the data were expressed as mean ± standard deviation; the t-test was used for the comparison between two groups.
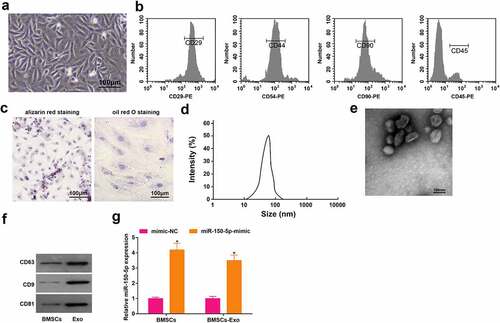
Exosomes were extracted from the culture medium of BMSCs. The outcomes of nanoparticle tracking analysis (NTA) particle size detection revealed ()) that the diameter of the BMSCs-derived exosomes was mainly 30–150 nm. Exosome morphology was observed under the transmission electron microscope ()), which exhibited many saucer-shaped vesicles . The surface markers of BMSCs were identified by Western blot assay ()), which uncovered that the expression of CD63, CD9 and CD81 was positive, suggesting that exosomes were successfully isolated.
The result of RT-qPCR implied that miR-150-5p level was significantly augmented in BMSCs-Exo transfected with miR-150-5p-mimic compared with BMSCs-Exo transfected with mimic NC ()).
Exosomes can effectively mitigate cerebral I/R injury
It has been reported that exosomes exert protective effects on cerebral I/R injury [Citation37], but the potential mechanism of BMSCs-derived exosomes in the protection of cerebral I/R injury was unclear. To further explain the mechanism, we constructed the MCAO rat model by the modified nylon suture method. The neurological function of MCAO rats was scored by single blind trail, which showed that ()) the score of MCAO rats was saliently high, and the score was decreased after exosomes injection in MCAO rats. Subsequently, the pathological and histological lesions of the rat brain were examined through HE staining, Nissl staining and TUNEL staining. The outcome of HE staining ()) demonstrated that the pathological changes were obvious in MCAO rats, which displayed necrotic and degenerated neurons with the disordered arrangement, concentrated neuron cytoplasm, condensed and unclear nucleus. However, in MCAO rats injected with exosomes, the neuron loss and damage were attenuated, only a few neurons showed degeneration and necrosis. The results of Nissl staining implied that ()) the structure of neurons in MCAO rats was destroyed during I/R, the number of Nissl bodies decreased. In MCAO rats injected with exosomes, the quantity of Nissl body was elevated. As reflected in TUNEL staining ()), there were more apoptotic neurons in MCAO rats, while after the injection of exosomes, the number of apoptotic neurons was depleted in MOCA rats. In addition, RT-qPCR was adopted to examine the apoptosis-related gene expression in brain tissue of rats. The results implied that ()) B cell Bax mRNA was enriched yet lymphoma-2 (Bcl-2) mRNA expression was depleted in MCAO rats; while Bax mRNA expression was reduced and Bcl-2 mRNA expression was elevated in rats after being injected with exosomes. The levels of inflammatory factors were assessed by ELISA ()), which disclosed that the levels of TNF-α, IL-1β and IL-6 were significantly enriched in the brain of the MCAO rats while reduced in rats injected with exosomes.
Figure 2. Exosomes can effectively mitigate cerebral I/R injury. A, the neurological function score of MCAO rats after injection with exosomes; B, pathological tissue damage in rat brain after injection with exosomes was detected by HE staining (scale bar = 50 μm); C, the pathological damage in rat brain (scale bar = 50 μm) after injection with exosomes was examined by Nissl staining; D, cell apoptosis in rat brain after injection with exosomes was assessed by TUNEL staining (scale bar = 50 μm); E, Bax and Bcl-2 mRNA levels in rat brain after injection with exosomes were examined by RT-qPCR; F, the levels of inflammatory factors (TNF-α, IL-1β, IL-6) in rat brain was after injection with exosomes were detected by ELISA; A-F, n = 6; * P < 0.05 vs. the Sham group; # P < 0.05 vs. the MCAO group; the data were expressed as mean ± standard deviation; one-way ANOVA was used for comparisons among multiple groups and Tukey’s post hoc test was used for pairwise comparisons.
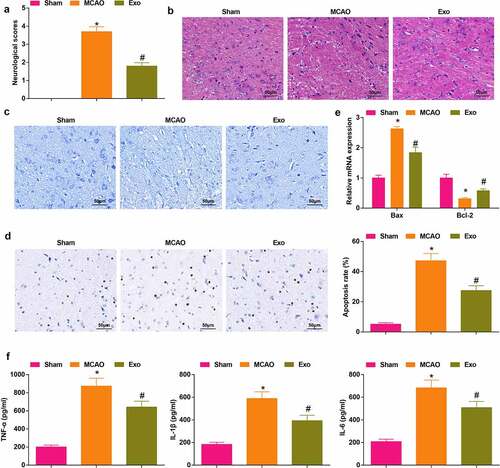
These findings validated that the MCAO rat model was successfully constructed, and the exosomes secreted by BMSCs could effectively protect the brain injury.
Elevated miR-150-5p promotes BMSCs-exosomes-mediated protective effects on cerebral I/R injury
It has been elucidated that miR-150 regulates the myocardial ischemia injury [Citation38], and the low-expressed miR-150 is accountable for inducing accelerated cell activities in smoking-related disease non-small cell lung cancer [Citation39,Citation40], so we speculated that miR-150 might also be involved in exosomes-mediated protection of cerebral I/R injury. We examined the expression of miR-150-5p first ()), and it was found that miR-150-5p was low-expressed in MCAO rats and its expression was elevated after injection with exosomes by RT-qPCR. Next, exosomes from BMSCs that transfected with miR-150-5p-mimic were injected into the MCAO rats, the outcomes implied that miR-150-5p was augmented in rats injected with Exo-miR-150-5p-mimic. In response to the enriched miR-150-5p, the neurological function score was lowered ()); the cell arrangement tended to be regular with decreased necrotic neurons ()); the quantity of Nissl body was increased ()); the apoptotic neurons was decreased ()); the Bax mRNA level was reduced and Bcl-2 mRNA level was amplified ()) and the levels of TNF-α, IL-1β and IL-6 were reduced ()).
Figure 3. Elevated miR-150-5p promotes BMSCs-exosomes-mediated protective effects on cerebral I/R injury. A, miR-150-5p level in rat brain tissue was detected by RT-qPCR; B, the neurological function score in MCAO rats after injection with Exo- miR-150-5p-mimic; C, the pathological damage in rat brain (scale bar = 50 μm) after injection with Exo-miR-150-5p-mimic was examined by HE staining; D, the pathological damage in rat brain (scale bar = 50 μm) after injection with Exo-miR-150-5p-mimic was detected by Nissl staining; E, cell apoptosis in rat brain tissue (scale bar = 50 μm) after injection with Exo-miR-150-5p-mimic was assessed by TUNEL staining; F, the levels of Bax and Bcl-2 mRNA in rat brain tissue after injection with Exo-miR-150-5p-mimic were examined by RT-qPCR; G, the levels of inflammatory factors (TNF-α, IL-1β, IL-6) in rat brain after injection with Exo-miR-150-5p-mimic were detected by ELISA; A-G, n = 6; * P < 0.05 vs. the Sham group; # P < 0.05 vs. the MCAO group; & P < 0.05 vs. the Exo-mimic-NC group. the data were expressed as mean ± standard deviation, the t-test was used for the comparison between two groups; one-way ANOVA was used for comparisons among multiple groups and Tukey’s post hoc test was used for pairwise comparisons.
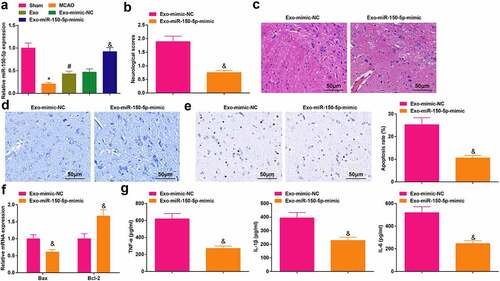
The results showed that miR-150-5p was silenced in rats with cerebral I/R injury. The exosomes injection elevated the miR-150-5p expression, and the induction of miR-150-5p could further facilitate the therapeutic efficiency of BMSCs-exosomes for cerebral I/R injury.
TLR5 is a target gene of miR-150-5p
Next, we studied the downstream target genes of miR-150-5p. A previous finding has clarified that TLR5 is robustly expressed in rats with intestinal I/R, and TLR5 knockout relieves the degree of injury and inflammatory response after intestinal I/R [Citation41]. The TargetScan predicted that there were binding sites between miR-150-5p and TLR5 ()). Subsequently, the targeting relationship between miR-150-5p and TLR5 was validated through the dual luciferase reporter gene assay ()), which revealed that the luciferase activity of 293 T cells was impaired after co-transfection with TLR5-WT and miR-150-5p-mimic.
Figure 4. TLR5 is a target gene of miR-150-5p. A, the targeting relationship between miR-150-5p and TLR5 was predicted by TargetScan; B, the targeting relationship between miR-150-5p and TLR5 was validated by the dual luciferase reporter gene assay; C, TLR5 expression in rat brain tissue after injection with Exo + miR-150-5p-mimic was detected by RT-qPCR and Western blot assay. A-B, N = 3; C, n = 6; * P < 0.05 vs. the mimic NC group; # P < 0.05 vs. the Exo- mimic-NC group; the data were expressed as mean ± standard deviation, the t-test was used for the comparison between two groups.
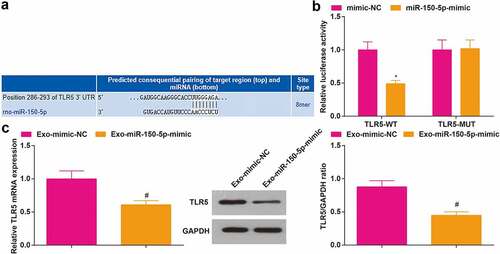
To further verify the targeting relationship between miR-150-5p and TLR5, we detected TLR5 expression in rats by RT-qPCR and Western blot assay. The results disclosed that TLR5 was low-expressed in rats injected with exosomes from BMSCs that transfected with miR-150-5p-mimic ()).
These discoveries evidenced that TLR5 was a downstream target gene of miR-150-5p, and the elevation of miR-150-5p restrained TLR5 expression.
Inhibited TLR5 enhances the BMSCs-exosomes-mediated protective effects on cerebral I/R injury
The TLR5 level in rat brain tissue was assessed by RT-qPCR and Western blot assay. It was found that ()) TLR5 level was elevated in MCAO rats, while TLR5 was decreased after the injection of exosomes, meanwhile, in rats injected with Exo + sh-TLR5, TLR5 was drastically reduced. As a result of TLR5 silencing, the rats displayed a low neurological function score, fewer necrotic neurons, more Nissl bodies, fewer apoptotic neurons, reduced Bax mRNA level, enhanced Bcl-2 mRNA level and ablated TNF-α, IL-1β and IL-6 level ().
Figure 5. Inhibited TLR5 enhances the BMSCs-exosomes-mediated protective effects on cerebral I/R injury. A, TLR5 level in rat brain tissue was detected by RT-qPCR and Western blot assay; B, the neurological function score in MCAO rats after injection with Exo + sh-TLR5; C, the pathological damage in rat brain (scale bar = 50 μm) after injection with Exo + sh-TLR5 was examined by HE staining; D, the pathological damage in rat brain (scale bar = 50 μm) after injection with Exo + sh-TLR5 was detected by Nissl staining; E, cell apoptosis in rat brain tissue (scale bar = 50 μm) after injection with Exo + sh-TLR5 was assessed by TUNEL staining; F, the levels of Bax and Bcl-2 mRNA in rat brain tissue after injection with Exo + sh-TLR5 were examined by RT-qPCR; G, the levels of inflammatory factors (TNF-α, IL-1β, IL-6) in rat brain was after injection with Exo + sh-TLR5 were detected by ELISA; A-G, n = 6; * P < 0.05 vs. the sham group; # P < 0.05 vs. the MCAO group; $ P < 0.05 vs. the Exo + sh-NC group; the data were expressed as mean ± standard deviation, the t-test was used for the comparison between two groups, one-way ANOVA was used for comparisons among multiple groups and Tukey’s post hoc test was used for pairwise comparisons.
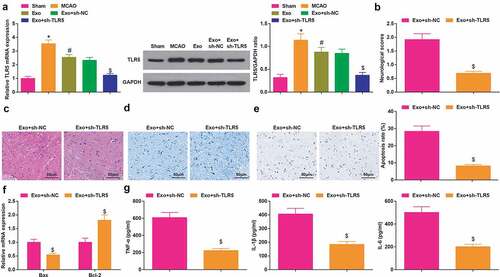
These findings reflected that TLR5 exhibited a high level in MCAO rats, and silenced TLR5 could strengthen the therapeutic effect of exosomes.
Elevated TLR5 inhibits the therapeutic effect of exosomal miR-150-5p from BMSCs on cerebral I/R injury
To probe whether exosomal miR-150-5p from BMSCs protects against cerebral I/R injury by regulating the expression of TLR5, exosomes from BMSCs that transfected with miR-150-5p-mimic as well as the oe-TLR5 lentivirus were co-transfected into MCAO rats. As suggested by RT-qPCR and Western blot assay, TLR5 was increased in rats injected with Exo-miR-150-5p-mimic + oe-TLR5 ()). The Exo-miR-150-5p-treated MCAO rats with enriched TLR5 exhibited high neurological function score, numerous necrotic and degenerated neurons, with disordered arrangement, concentrated neuron cytoplasm, condensed and unclear nuclei, destroyed neurons, Moreover, the Nissl bodies were decreased, apoptotic neurons was increased, and elevated Bax mRNA level, reduced Bcl-2 mRNA level and enriched levels of TNF-α, IL-1β and IL-6 were also observed ().
Figure 6. Elevated TLR5 inhibits the therapeutic effect of exosomal miR-150-5p from BMSCs on cerebral I/R injury. A, TLR5 level in rat brain tissue after injection with Exo- miR-150-5p-mimic + oe-TLR5 was detected by RT-qPCR and Western blot assay; B, the neurological function score in MCAO rats after injection with Exo-miR-150-5p-mimic + oe-TLR5; C, the pathological damage in rat brain (scale bar = 50 μm) after injection with Exo-miR-150-5p-mimic + oe-TLR5 was examined by HE staining; D, the pathological damage in rat brain (scale bar = 50 μm) after injection with Exo-miR-150-5p-mimic + oe-TLR5 was detected by Nissl staining; E, cell apoptosis in rat brain tissue (scale bar = 50 μm) after injection with Exo -miR-150-5p-mimic + oe-TLR5 was assessed by TUNEL staining; F, the levels of Bax and Bcl-2 mRNA in rat brain tissue after injection with Exo-miR-150-5p-mimic + oe-TLR5 were examined by RT-qPCR; G, the levels of inflammatory factors (TNF-α, IL-1β, IL-6) in rat brain was after injection with Exo -miR-150-5p-mimic + oe-TLR5 were detected by ELISA; A-G, n = 6; * P < 0.05 vs. the Exo-miR-150-5p + mimic + oe-NC group; the data were expressed as mean ± standard deviation, the t-test was used for the comparison between two groups.
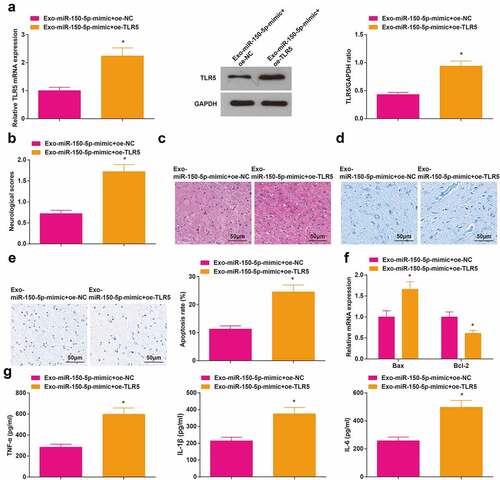
It was demonstrated that the TLR5 overexpression abrogated the therapeutic effects of exosomal miR-150-5p from BMSCs on cerebral I/R injury.
Discussion
Cerebral ischemia is one of the most malignant complications in the perioperative period with severe clinical sequelae [Citation42]. In this study, we focused on the regulatory mechanism of exosomal miR-150-5p from BMSCs on the progression of cerebral I/R injury through mediating TLR5. Collectively, it was demonstrated that exosomal miR-150-5p from BMSCs exerted protective effects on cerebral I/R injury by suppressing TLR5 ().
Initially, this research validated that miR-150-5p displayed a low level in rats with cerebral I/R injury. Similarly, a previous study has demonstrated that thelow-expressed miR-150-5p existed in patients with ischemic stroke, and the silencing miR-150-5p is accountable for inducing unfavorable outcome and high mortality of patients with ischemic stroke [Citation43]. Additionally, miR-150-5p is also depleted in patients with advanced heart failure, and the reduction of miR-150-5p leads to severe clinical exacerbation, causing death from progressive pump failure or even requiring hospitalization [Citation44]. Thereafter, our study manifested that BMSCs-derived exosomes could effectively relieve the cerebral I/R injury in MCAO rats. Similarly, Wang et al. have verified that the exosomes from BMSCs can also attenuate renal I/R injury [Citation10], and activate cell viability in oxygen-glucose deprivation/reoxygenation as well as constrain the pyroptosis, thereby mitigating the cerebral I/R injury [Citation7]. Thereafter, our research further illustrated that the augmented miR-150-5p enhanced the protective effects of BMSCs-derived exosomes on cerebral I/R injury. Consistently, in rats with myocardial I/R injury, miR-150-5p also exhibits a low level, and the extracellular vesicles derived from miR-150-5p effectively suppress the cardiomyocyte apoptosis and myocardial remodeling, contributing to favor the treatment of myocardial I/R injury [Citation15]. The overexpression of miR-150-5p effectively enhances the Bax level and promotes the cell viability of mouse embryonic cardiomyocytes H9c2 cells [Citation45]. Moreover, this research further discovered that miR-150-5p induction enhanced the therapeutic effects of BMSCs-derived exosomes on relieving pathological changes of MCAO rat neurons. As similarly reported by Wu et al., exosomal miR-150-5p from BMSCs can protect cardiac function, mitigate pathological changes of the myocardium and reduce apoptosis rate of cardiomyocytes in myocardial infarction mice [Citation46]. Additionally, Liu et al. have implied that miR-211 protects cerebral I/R injury by inhibiting cell apoptosis [Citation47]. Compared to such finding, this study highlighted the function miR-150-5p from exosomes. It was further validated the augmented miR-150-5p enhanced the protective impact of BMSCs-derived exosomes on cerebral I/R injury, thus the therapeutic targets for cerebral I/R treatment were fully extended by introducing exosomes compared with Liu’s conclusion.
Furthermore, the binding relationship between miR-150-5p and TLR5 was predicted through the bioinformatics website. This study validated that TLR5 exhibited a high level in MCAO rats, and the silenced TLR5 could strengthen the therapeutic effects of BMSCs-derived exosomes on cerebral I/R injury via repressing inflammatory factor levels, blocking neuron apoptosis, mitigating pathological change and improving neurological function in MCAO rats. Previous studies for directly probing the role of TLR5 in cerebral I/R injury were rare, yet some related studies about ischemia still offer valuable references for this research. For instance, in line with our findings, Gu et al. have concluded that TLR5 is robustly expressed in the ischemic brain, and they further illustrated that TLR5 deletion helps to strengthen the anti-inflammation effects of Radix Paeoniae treatment via decreasing IL-6 levels in MCAO rats [Citation48]. The an-inflammatory effects of TLR5 silencing have also been validated in intestinal I/R injury, moreover, TLR5 deficiency even contributes to relieving intestinal I/R-induced damage of remote organs [Citation41]. In addition, Qiao et al. have elucidated that TLR5 down-regulation is accompanied by alleviated neurological deficit, reduced infarct volume and dampened edema after ischemic stroke [Citation17]. As for TLR5 effects on neuron apoptosis, Ifuku et al. have uncovered that TLR5 activation is accounted for inducing accelerated neuron apoptosis in microglia [Citation49].
Conclusion
Collectively, this study reveals that miR-150-5p expression is decreased while TLR5 level is elevated in MCAO rats, and the exosomal miR-150-5p from BMSCs exerts a protective effect on cerebral I/R injury via reducing TLR5 expression. The current discovery contributes to exploring novel therapeutic targets for cerebral I/R injury by highlighting the regulatory mechanism of exosomal miR-150-5p from BMSCs and TLR5. However, adequate study samples and groupings were required in future study to minimize potential data errors in the experimental results; and the detailed role of miR-150-5p knockout or TLR5 elevation on cerebral I/R injury can be another research topic in future works.
Highlights
Exosomal miR-150-5p from BMSCs mitigates cerebral I/R injury via repressing TLR5.
MiR-150-5p targets TLR5.
MiR-150-5p is depleted and TLR5 is enriched in MCAO rats.
Exosomes from BMSCs improve neurological function in MCAO rats.
Enriched exosomal miR-150-5p attenuates neurological function in MCAO rats.
Supplemental Material
Download Zip (1.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Shin TH, Lee DY, Basith S, et al. Metabolome changes in Cerebral Ischemia. Cells. 2020;9(7). DOI:10.3390/cells9071630.
- Yang Z, Weian C, Susu H, et al. Protective effects of mangiferin on cerebral ischemia-reperfusion injury and its mechanisms. Eur J Pharmacol. 2016;771: pp. 145–151.
- Catanese L, Tarsia J, Fisher M. Acute Ischemic Stroke Therapy Overview. Circ Res. 2017;120(3): pp. 541–558.
- Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17(11): pp. 1391–1401.
- Li H, Ghazanfari R, Zacharaki D, et al. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci. 2016;1370(1): pp. 109–118.
- Wang J, Sun R, Li Z, et al. Combined bone marrow stromal cells and oxiracetam treatments ameliorates acute cerebral ischemia/reperfusion injury through TRPC6. Acta Biochim Biophys Sin (Shanghai). 2019;51(8): pp. 767–777.
- Zeng Q, Zhou Y, Liang D, et al. Exosomes secreted from bone marrow mesenchymal stem cells attenuate oxygen-glucose deprivation/reoxygenation-induced pyroptosis in pc12 cells by promoting ampk-dependent autophagic Flux. Front Cell Neurosci. 2020;14: p. 182.
- Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88(1): pp. 487–514.
- Yu H, Xu Z, Qu G, et al. Hypoxic Preconditioning Enhances the Efficacy of mesenchymal stem cells-derived conditioned medium in switching microglia toward anti-inflammatory polarization in ischemia/reperfusion. Cell Mol Neurobiol. 2021;41(3): pp. 505–524.
- Wang C, Zhu G, He W, et al. BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J. 2019;33(4): pp. 5440–5456.
- Zhu G, Pei L, Lin F, et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J Cell Physiol. 2019;234(12): pp. 23736–23749.
- Chen Q, Liu Y, Ding X, et al. Bone marrow mesenchymal stem cell-secreted exosomes carrying microRNA-125b protect against myocardial ischemia reperfusion injury via targeting SIRT7. Mol Cell Biochem. 2020;465(1–2): pp. 103–114.
- Fan ZX, Yang J. The role of microRNAs in regulating myocardial ischemia reperfusion injury. Saudi Med J. 2015;36(7): pp. 787–793.
- He QW, Li Q, Jin H-J, et al. MiR-150 regulates poststroke cerebral angiogenesis via vascular endothelial growth factor in rats. CNS Neurosci Ther. 2016;22(6): pp. 507–517.
- Ou H, Teng H, Qin Y, et al. Extracellular vesicles derived from microRNA-150-5p-overexpressing mesenchymal stem cells protect rat hearts against ischemia/reperfusion. Aging (Albany NY). 2020;12(13): pp. 12669–12683.
- Stridh L, Smith PL, Naylor AS, et al. Regulation of toll-like receptor 1 and −2 in neonatal mice brains after hypoxia-ischemia. J Neuroinflammation. 2011;8(1): p. 45.
- Qiao H, Zhang X, Zhu C, et al. Luteolin downregulates TLR4, TLR5, NF-kappaB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res. 2012;1448: pp. 71–81.
- Fang S, Li Y, Chen P. Osteogenic effect of bone marrow mesenchymal stem cell-derived exosomes on steroid-induced osteonecrosis of the femoral head. Drug Des Devel Ther. 2019;13: pp. 45–55.
- Li T, Zhang C, Ding Y, et al. Umbilical cord-derived mesenchymal stem cells promote proliferation and migration in MCF-7 and MDA-MB-231 breast cancer cells through activation of the ERK pathway. Oncol Rep. 2015;34(3): pp. 1469–1477.
- Yan W, Wu X, Zhou W, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20(5): pp. 597–609.
- Waldner F, Hansen MC, Potapov PV, et al. National-scale cropland mapping based on spectral-temporal features and outdated land cover information. PLoS One. 2017;12(8): p. e0181911.
- Teng X, Chen L, Chen W, et al. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and Anti-Inflammation. Cell Physiol Biochem. 2015;37(6): pp. 2415–2424.
- Yu Y, Zhang, X, Han, Z, et al. Expression and regulation of miR-449a and AREG in cerebral ischemic injury. Metab Brain Dis. 2019;34(3): pp. 821–832.
- Wu J, Yan, W, Wu, X, et al. Protective effects of Corbrin Capsule against permanent cerebral ischemia in mice. Biomed Pharmacother. 2020;121: p. 109646.
- Zhang D, et al. Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging (Albany NY). 2021;13(3): pp. 4079–4095.
- Zhang G, Ge M, Han Z, et al. Wnt/beta-catenin signaling pathway contributes to isoflurane postconditioning against cerebral ischemia-reperfusion injury and is possibly related to the transforming growth factorbeta1/Smad3 signaling pathway. Biomed Pharmacother. 2019;110: pp. 420–430.
- Huang X, Ding, J, Li, Y, et al. Exosomes derived from PEDF modified adipose-derived mesenchymal stem cells ameliorate cerebral ischemia-reperfusion injury by regulation of autophagy and apoptosis. Exp Cell Res. 2018;371(1): pp. 269–277.
- Li X, Zheng L, Xia Q, et al. A novel cell-penetrating peptide protects against neuron apoptosis after cerebral ischemia by inhibiting the nuclear translocation of annexin A1. Cell Death Differ. 2019;26(2): pp. 260–275.
- Fard N, Saffari A, Emami G, et al. Acute respiratory distress syndrome induction by pulmonary ischemia-reperfusion injury in large animal models. J Surg Res. 2014;189(2): pp. 274–284.
- Nemery B, Vanlommel S, Verbeken EK, et al. Lung injury induced by paraquat, hyperoxia and cobalt chloride: effects of ambroxol. Pulm Pharmacol. 1992;5(1): pp. 53–60.
- Zhang M, Ge DJ, Su Z, et al. miR-137 alleviates focal cerebral ischemic injury in rats by regulating JAK1/STAT1 signaling pathway. Hum Exp Toxicol. 2020;39(6): pp. 816–827.
- Li M, Luan L, Liu Q, et al. MiRNA-199a-5p Protects Against Cerebral Ischemic Injury by Down-Regulating DDR1 in Rats. World Neurosurg. 2019;131: pp. e486–e494.
- Zhu L, Zhou, X, Li, S, et al. miR1835p attenuates cerebral ischemia injury by negatively regulating PTEN. Mol Med Rep. 2020;22(5): pp. 3944–3954.
- Khan-Malek R, Wang Y. Statistical analysis of quantitative RT-PCR results. Methods Mol Biol. 2017;1641: pp. 281–296.
- Wang SL, Duan L, Xia B, et al. Dexmedetomidine preconditioning plays a neuroprotective role and suppresses TLR4/NF-kappaB pathways model of cerebral ischemia reperfusion. Biomed Pharmacother. 2017;93: pp. 1337–1342.
- Wu Z, Cheng S, Wang S, et al. BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int Immunopharmacol. 2021;93: p. 107389.
- Jiang Y, He R, Shi Y, et al. Plasma exosomes protect against cerebral ischemia/reperfusion injury via exosomal HSP70 mediated suppression of ROS. Life Sci. 2020;256: p. 117987.
- Tang Y, Wang Y, Park K-M, et al. MicroRNA-150 protects the mouse heart from ischaemic injury by regulating cell death. Cardiovasc Res. 2015;106(3): pp. 387–397.
- Lu W, Zhang H, Niu Y, et al. Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol Cancer. 2017;16(1): p. 118.
- Guohua H, Hongyang L, Zhiming J, et al. Study of small-cell lung cancer cell-based sensor and its applications in chemotherapy effects rapid evaluation for anticancer drugs. Biosens Bioelectron. 2017;97: pp. 184–195.
- Ito H, Sadatomo A, Inoue Y, et al. Role of TLR5 in inflammation and tissue damage after intestinal ischemia-reperfusion injury. Biochem Biophys Res Commun. 2019;519(1): pp. 15–22.
- Zhou ZB, Meng L, Gelb AW, et al. Cerebral ischemia during surgery: an overview. J Biomed Res. 2016;30(2): pp. 83–87.
- Scherrer N, Fays F, Mueller B, et al. MicroRNA 150-5p improves risk classification for mortality within 90 days after acute ischemic stroke. J Stroke. 2017;19(3): pp. 323–332.
- Scrutinio D, Conserva F, Passantino A, et al. Circulating microRNA-150-5p as a novel biomarker for advanced heart failure: a genome-wide prospective study. J Heart Lung Transplant. 2017;36(6): pp. 616–624.
- Zhou J, Li, D, Yang, BP, et al. LncRNA XIST inhibits hypoxia-induced cardiomyocyte apoptosis via mediating miR-150-5p/Bax in acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2020;24(3): pp. 1357–1366.
- Wu Z, Cheng S, Wang S, et al. BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int Immunopharmacol. 2021;93: p. 107389.
- Liu W, Miao Y, Zhang L, et al. MiR-211 protects cerebral ischemia/reperfusion injury by inhibiting cell apoptosis. Bioengineered. 2020;11(1): pp. 189–200.36. Gubern, C., et al., miRNA expression is modulated over time after focal ischaemia: up-regulation of miR-347 promotes neuronal apoptosis. FEBS J, 2013. 280(23): p. 6233-46.
- Gu J, Su S, Guo J, et al. Anti-inflammatory and anti-apoptotic effects of the combination of Ligusticum chuanxiong and Radix Paeoniae against focal cerebral ischaemia via TLR4/MyD88/MAPK/NF-κB signalling pathway in MCAO rats. J Pharm Pharmacol. 2018;70(2): pp. 268–277.
- Ifuku M, Hinkelmann L, Kuhrt LD, et al. Activation of Toll-like receptor 5 in microglia modulates their function and triggers neuronal injury. Acta Neuropathol Commun. 2020;8(1): p. 159.
