ABSTRACT
We aimed to clarify the therapeutic effects of imperatorin (IMP) on knee osteoarthritis (KOA). Thirty 3-month-old SD male rats were randomly divided into Normal, monosodium iodoacetate (MIA) and MIA+IMP groups. Their synovial tissues were subjected to histopathological analysis. Primary synovial fibroblasts obtained from additional normal rats were treated by lipopolysaccharide (LPS) and then IMP. The mRNA and protein expressions of factors related to synovitis and synovial fibrosis were detected by qRT-PCR and Western blotting, respectively. The concentrations of inflammatory factors IL-1β and IL-18 were measured by ELISA. IMP reduced HIF-1α, NLR family pyrin domain-containing 3 inflammasome expression and IL-1β, IL-18 production in synovial fibroblasts induced by LPS. IMP also downregulated synovial fibrosis markers. In vitro study revealed that MIA-induced synovitis and synovial fibrosis were relieved by IMP. IMP relieves the inflammation associated with synovitis and synovial fibrosis. It reduces the production of pro-inflammatory mediators and cytokines and inhibits TGF-β1, TIMP-1 and VEGF expressions that promote synovial fibrosis.
Background
Knee osteoarthritis (KOA) is a common and disabling condition that represents a substantial and increasing health burden with notable implications for the affected individuals, health-care systems, and wider socioeconomic costs [Citation1,Citation2]. Synovial fibrosis is an important pathological process characterized by abnormal deposition of extracellular matrix (ECM), as well as cell migration and proliferation in the occurrence and development of KOA. Synovial fibrosis is very common in the case of KOA, as the main cause for pain and joint stiffness. Upregulation of fibrogenic factors, such as TGF-β1 [Citation3], are signs of the development of synovial fibrosis. TGF-β1 is the most well-known fibrosis factor, and plays a key role in many profibrotic processes, including promoting tissue matrix metalloproteinase (TIMP) expressions [Citation4]. TIMPs are elevated in some diseases related to fibrosis (e.g. liver fibrosis), as well as in the synovium of mice with OA and end-stage OA patients. In addition, vascular endothelial growth factor (VEGF) is an effective stimulator of angiogenesis and may also promote synovial fibrosis through extravasation [Citation5]. These three are confirmed to be fibrogenic factors [Citation6].
KOA is a complex disease, the pathogenesis of which involves not only mechanical but inflammatory and metabolic factors, leading to the destruction and failure of knee joints [Citation7]. Each common osteoarthritis risk factor can lead to different mechanisms of KOA, such as increase of inflammatory factors [Citation8] and oxidative stress. In addition, the extended metabolic characteristics of knee synovium contribute to imbalance of oxygen homeostasis and enhance hypoxia in the microenvironment [Citation9]. Hypoxia-induced factor (HIF)-1α is recognized as a major regulator of hypoxia signaling, which mediates the adaptive response of cells to hypoxia by activating the transcription of genes encoding proteins. HIF-1α expression can be triggered in an inflammatory microenvironment even under normoxic conditions [Citation10]. Clinical studies have shown that the HIF-1α concentrations in serum, synovial fluid and articular cartilage of patients with KOA are associated with progressive joint damage. It can be used as a biomarker for KOA progression and prognosis. HIF-1α is associated with the upregulation of genes encoding pro-inflammatory cytokines and growth factors, thereby activating fibroblasts and mediating fibrosis. Besides, the NLR family pyrin domain-containing 3 (NLRP3) inflammasome can be activated by HIF-1α [Citation11]. The NLRP3 inflammasome has been implicated in the pathogenesis of a number of arthritic disorders, producing proinflammatory cytokines and degradative enzymes, such as interleukin-1 beta (IL-1β) which drives cartilage degeneration and synovial inflammation [Citation12]. Inhibiting NLRP3 inflammasome can alleviate many types of fibrosis, especially synovial fibrosis [Citation13,Citation14].
Imperatorin (IMP) is a secondary metabolite of plants. It is one of furanocoumarin derivatives and widely used in many traditional Chinese herbal drugs (e.g. Angelica dahurica) which are employed to treat KOA. It has antitumor [Citation15], antibacterial [Citation16] and anti-inflammatory effects [Citation17], but little is known about its role in the suppression of synovial inflammatory and fibrosis. Also, IMP is one of the active ingredients in ‘Yiceng’, a tropical drug used to treat KOA [Citation18].
Therefore, in this study, we intended to assess the effects of IMP on synovial fibrosis induced by monosodium iodoacetate (MIA) in vivo, and an inflammatory model in primary synovial fibroblasts induced by lipopolysaccharide (LPS) in vitro. The therapeutic effect of IMP on synovitis and synovial fibrosis reveals it as a potential candidate for drug development.
Materials and methods
Reagents and antibodies
IMP (purity >99%) was purchased from Yuanye (Shanghai, China). MIA and dimethyl sulfoxide were obtained from Sigma (St Louis, USA). Antibodies against NLRP3, ASC, Caspase-1, VEGF, TIMP1, TGF-β1 and HIF-1α were purchased from Abcam (Cambridge, UK). HRP-conjugated affinipure goat anti-rabbit IgG(H + L) (Proteintech Group, Inc., SA00001-2, 1:20,000), HRP-conjugated affinipure goat anti-mouse IgG(H + L) (Proteintech Group, Inc., SA00001-1, 1:20,000). ECL luminescent liquid (Shanghai Tianneng, 180–5001), protein marker (Shanghai Tianneng, 180–6003), BCA protein assay kit (Thermo Fisher, 23,227). Bovine serum albumin, fetal bovine serum (FBS), Dulbecco’s Modified Eagle’s Medium (DMEM), TRIzol and 0.25% trypsin-EDTA were purchased from Gibco (Life Technologies Corp., California, USA). TransStart Green qPCR SuperMix was obtained from Takara (Dalian, China). The primers and rat GAPDH Endogenous Reference were supplied by Sangon Biotech (Shanghai, China). Enzyme-linked immunosorbent assays (ELISA) kits for IL-1β and IL-18 were supplied by Invitrogen (Life Technologies Corp., California, USA). All other chemicals were of reagent grade.
Animals
Thirty 3-month-old SD male rats weighing from 250 to 290 g, 10 for each group (provided by Beijing Vital River Laboratory Animal Technology Co., Ltd.), were used for experimental KOA studies. The animals were maintained in a specific pathogen-free laminar-flow housing apparatus with controlled temperature, humidity, and 12 h light/dark regimen. All animal protocols were approved by the Animal Care and Use Committee of the Nanjing University of Chinese Medicine. All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Induction of KOA and drug administration
The rat osteoarthritis model was established according to a previous literature [Citation6]. Thirty rats were randomly divided into three groups (Normal group, MIA group, MIA+IMP group). For the Normal group, injection of 50 µl 0.9% saline into articular joint was performed. For MIA group, 2 mg MIA dissolved in 50 µl 0.9% saline [Citation11]. For MIA +IMP group, 5 mg/kg/day was selected as oral administration concentration from 2 weeks after injection as previously described [Citation19]. IMP was dissolved in 0.5% carboxymethylcellulose sodium (CMC-Na), and 0.5% CMC-Na was given by intragastric administration alone for sham group and MIA group every day for 6 weeks until the rats were sacrificed. All rats were sacrificed 8 weeks after injection, and the synovial tissues were treated for histological analysis and further experiments.
Histopathological analysis
For hematoxylin and eosin (H&E) staining, synovial tissues were frozen and fixed in 4% paraformaldehyde, soaked in 0.5 M EDTA and embedded in paraffin. The paraffin blocks were sectioned at a thickness of 5 μm [Citation20].
Isolation and primary culture of synovial fibroblasts
Primary rat synovial fibroblasts were obtained from additional normal rats. In brief, synovial tissues were washed 2–3 times with phosphate-buffered saline, minced into pieces of 2–3 mm2 and digested in 0.1% collagenase type II (Sigma Aldrich, St. Louis, MO, USA) for 30 min. Following cell dissociation, the samples were filtered through a cell strainer. After dissociation, fibroblasts were pelleted by centrifugation at 1500 rpm for 4 min and cultured in DMEM supplemented with 10% FBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin). The cells were cultured at 37°C in a humidified 95% air and 5% CO2 atmosphere. Passages 3–6 of the synovial fibroblasts were used for experiments.
Fibroblasts were treated with LPS (5 μg/ml) in DMEM for 6 h to stimulate inflammatory response and activate the NLRP3 inflammasome. Fibroblasts exposed to DMEM with the same volume of saline served as control. Before administration of LPS, IMP (50 μM) was used for 24 h or 48 h [Citation21].
Synovial extraction and preservation in rats
After 14 days of modeling or 14 days of treatment, the rats were sacrificed by CO2 asphyxia, and the knee joint hair was removed. The ligament was incised on both sides of the patellofemoral ligament. The upper edge of the humerus was transversely cut to the distal end of the quadriceps muscle until the femur. The free tibia and its surrounding tissue were opened until the distal end. The pale yellow translucent synovial membrane was discerned. The synovial tissue was carefully cut with a surgical blade. Paraformaldehyde was used for pathological sectioning, and the rest was placed in a cryotube at −70°C [Citation22].
Real-time PCR
RNA was isolated from synovial tissues and fibroblasts with Trizol (Invitrogen, CA, USA), respectively. Reverse transcription was performed by using a first-strand cDNA synthesis kit (Takara, Otsu, Japan) according to manufacturer’s instructions. qPCR was performed using Premix Ex Taq SYBR-Green PCR (Takara) according to manufacturer’s instructions on an ABI PRISM 7300 system (Applied Biosystems, Foster City, CA, USA).
Primers were designed and synthesized by Shanghai Biotechnology Service Co. Ltd. in accordance with the gene sequence in GenBank gene sequence design, together with Oligo v6.6. Sequences for primers are shown in . The mRNA levels of individual genes were normalized to that of GAPDH and calculated by the 2−ΔΔCt method [Citation23].
Table 1. Primer sequences
Western blotting
The synovial tissue was dissected, weighed and mixed with RIPA lysate. The samples were centrifuged at 15,000 r/min for 15 minutes at 4°C. The cultured fibroblasts were washed and lysed. Protein concentrations were then quantified using the BCA protein assay kit. Protein samples were electrophoresed by SDS-PAGE to separate protein bands. The products were transferred from the gel to a PVDF membrane which was then blocked with 5% skimmed milk for 2 h. The membrane was incubated with polyclonal rabbit antibodies specific for NLRP3, caspase-1, ASC, TGF-β1, VEGF and TIMP-1 overnight at 4°C. On the next day, the membrane was incubated with secondary antibody for 2 h. The bands were visualized using the ECL method, and ImageJ software was used to quantify the total gray value (average gray value × gray value area) of protein band to calculate the relative value of target protein [Citation24].
ELISA
The peripheral serum of rats and culture supernatant of cells were collected and centrifuged at 10,000 rpm for 20 min at 4°C, after which the concentrations of IL-1β and IL-18 were measured by ELISA kits. All steps were performed according to the manufacturer’s instructions [Citation25].
Statistical analysis
Graphpad prism 8.0 software (San Diego, CA, USA) was used to statistically analyze the data. The measurement data were based on (χ ± s), and the numerical data were subjected to the χ2 test. The three groups of data conformed to normal distribution, and one-way ANOVA was used, including LSD and SNK tests. The Kruskal–Wallis H test was used for non-normal distribution. The difference was statistically significant when P < 0.05.
Results
Synovial fibrosis and hypoxia were found in MIA-induced KOA model rats
The NLRP3 inflammasome has been implicated in the pathogenesis of several arthritic disorders, producing degradative enzymes and proinflammatory cytokines to drive cartilage degeneration and synovial inflammation. Inhibiting NLRP3 inflammasome can mitigate many types of fibrosis, especially synovial fibrosis. Given that IMP has long been used to treat KOA, its effects on NLRP3 inflammasome cascade-induced synovial fibrosis and synovitis in KOA rats were assessed.
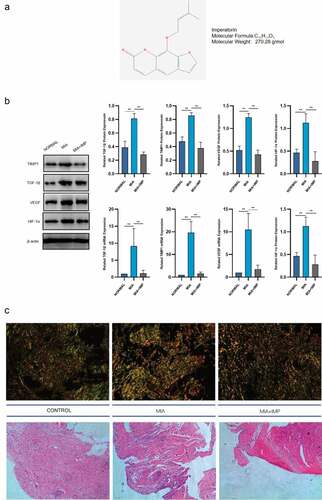
The chemical structure of IMP is shown in . The upregulation of HIF-1α in pathological conditions suggested that the synovial membrane was under hypoxia conditions with synovial fibrosis induced by MIA in rats. HIF-1α protein and mRNA levels were markedly elevated in KOA synovial membranes (). The mRNA and protein expression levels of TGF-β1, TIMP1 and VEGF in fibrotic synovium were all upregulated in pathological conditions induced by MIA in rats (). The representative synovium tissues after staining are exhibited in .
Figure 1. Fibrogenic and hypoxia markers were upregulated in MIA-induced KOA rats and downregulated by IMP. (a) Chemical structure of imperatorin. (b) mRNA and protein levels of HIF-1α, TGF-β1, TIMP1 and VEGF in Normal, MIA-induced KOA and KOA rats treated with IMP determined by qRT-PCR and Western blotting. (c) Representative synovium tissues undergoing Sirius red staining or H&E staining, 200×, scale bar = 100 μm. Values are represented as mean ± SEM (*p < 0.05, **p < 0.01)
Activation of NLRP3 inflammasome in synovial membranes resulted in synovitis in rats
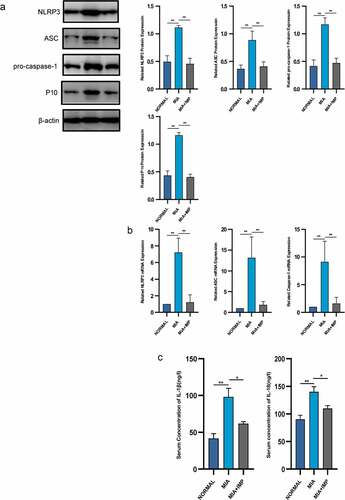
The key inflammatory factors of NLRP3 inflammasome-dependent cytokines, IL-1β and IL-18, were significantly upregulated in the MIA group compared with those of the NORMAL group (). Caspase-1 recruited by NLRP3 inflammasome cleaved pro-IL-1β and pro-IL-18 that promoted the maturation of inflammatory cytokines. We then evaluated the mRNA and protein expressions of NLRP3 inflammasome in the MIA group. Both mRNA and protein levels were upregulated compared to those of the NORMAL group ( and b).
Figure 2. NLRP3 inflammasome was activated in MIA-induced KOA rats and partly inhibited by IMP. (a) Expression of NLRP3, ASC, pro-caspase-1 and P10 determined by Western blotting. The upregulation of NLRP3 inflammasome protein expression was inhibited by IMP. (b) mRNA levels were quantified by qRT-PCR in fibroblasts treated with or without LPS (5 μg/mL) or treated with LPS (5 μg/mL) and IMP. The upregulation of NLRP3 inflammasome mRNA expression was inhibited by IMP. (c) Serum concentrations of IL-1β and IL-18. Values are represented as mean ± SEM (*p < 0.05, **p < 0.01)
IMP inhibited experimental synovitis and synovial fibrosis in rats
IMP, one of the representative components in furanocoumarin, is extracted from Angelica dahurica. The MIA+IMP group had milder resident cell hyperplasia, formation of lining cell layers and inflammatory infiltration than those of the MIA group. Sirius red staining showed that the collagen fibers were red, the nucleus was green, and other components were yellow. The MIA group showed more collagen fibers than those of the Control group, suggesting that IMP ameliorated synovitis and synovial fibrosis (). After IMP administration, the expressions of NLRP3, ASC, caspase-1 and caspase-1 p10 were determined by qRT-PCR and Western blotting. All these components related to NLRP3 inflammasome were significantly downregulated in MIA+IMP group compared with those of MIA group at both mRNA and protein levels. The production of caspase-1 (p10) was also significantly reduced by IMP compared with that of MIA group, as well as IL-1β and IL-18 (‒c). Besides, fibrogenic factors TGF-β1, TIMP-1 and VEGF were downregulated after IMP administration at both mRNA and protein levels (). Collectively, the administration of IMP inhibited synovitis, synovial fibrosis and hypoxia pathogenesis in rats.
IMP inhibited LPS-induced upregulation of fibrogenic factors in fibroblasts
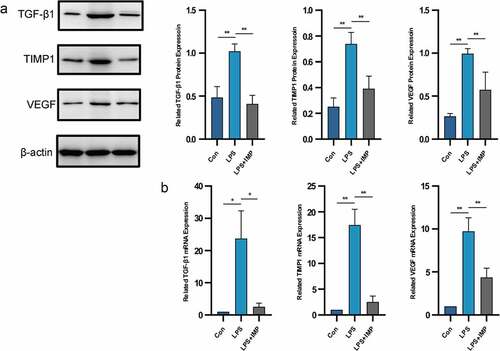
We next examined the in vitro role of IMP in LPS-stimulated primary cultured fibroblast-like synoviocytes. The upregulated expression of fibrogenic factors suggested their possible involvement in synovial fibrosis. Treatment of fibroblast-like synoviocytes with LPS (5 μg/mL) significantly upregulated TGF-β1, TIMP-1 and VEGF at both mRNA and protein levels. These fibrogenic factors contributed to synovial fibrosis in vivo. After IMP administration, all those factors were downregulated significantly ( and b).
Figure 3. Fibrogenic marker expressions induced by LPS in fibroblasts were upregulated and downregulated by IMP. (a) Expression of TGF-β1, TIMP1 and VEGF determined by Western blotting. The protein expressions of these fibrogenic markers were inhibited by IMP. (b) mRNA levels were quantified by qRT-PCR in fibroblasts treated with or without LPS (5 μg/mL) or treated with LPS (5 μg/mL) and IMP (50 μM). The mRNA expressions of these fibrogenic markers were inhibited by IMP. Values are represented as mean ± SEM (*p < 0.05, **p < 0.01)
IMP inhibited activation of NLRP3 inflammasome in LPS-stimulated fibroblasts
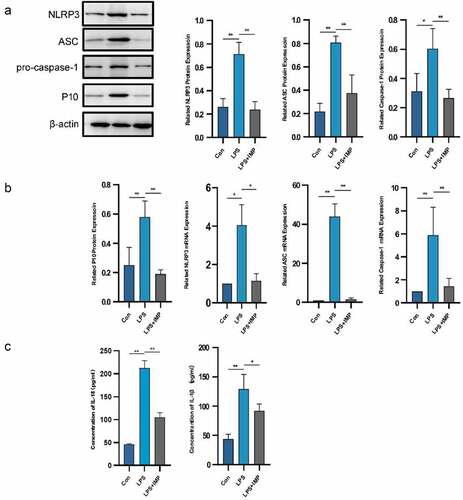
NLRP3, ASC and caspase-1 expressions were significantly upregulated in the LPS group compared with those of the NORMAL group at both mRNA and protein levels. IMP significantly inhibited the mRNA and protein expressions of NLRP3 inflammasome compared with those of the LPS group. The production of cleaved caspase-1 and caspase-1 p10 was also confirmed by Western blotting and qRT-PCR. It showed significant upregulation in the LPS group compared with that of the NORMAL group, which was suppressed by IMP ( and b). The downstream production of IL-1α and IL-18 was upregulated after LPS stimulation, and the inflammatory cytokines secreted by fibroblasts were downregulated by IMP ().
Figure 4. NLRP3 inflammasome was activated by LPS in fibroblasts and partly inhibited by IMP. (a) Expression of NLRP3, ASC, pro-caspase-1 and P10 determined by Western blotting. The protein expression levels were inhibited by IMP compared to those in the LPS group. (b) The mRNA levels were quantified by qRT-PCR in fibroblasts treated with or without LPS (5 μg/mL) or treated with LPS (5 μg/mL) and IMP (50 μM). The mRNA expression levels were inhibited by IMP compared to those in LPS group. Values are represented as mean ± SEM (*p < 0.05, **p < 0.01). (c) Inflammation cytokines IL-1β and IL-18 were measured by ELISA. Values are represented as mean ± SEM (*p < 0.05, **p < 0.01)
Discussion
Hypoxia, inflammation and fibrosis persistently exist in the pathological progress of KOA [Citation26]. Herein, we demonstrated the therapeutic effects of IMP on KOA. IMP significantly inhibited the expressions of HIF-1α mRNA and protein, activated the NLRP3 inflammasome, and downregulated the levels of fibrogenic factors in KOA. KOA is characterized by the progressive destruction of articular cartilage and surrounding tissues, especially synovial tissue [Citation27]. In this study, we verified for the first time that IMP intervened with the pathological processes of KOA. The therapeutic effects of IMP may be related to the suppression of inflammation and synovial fibrosis.
Increased HIF-1α is widely involved in the progression of diseases, as well as synovial fibrosis and inflammation. It is well established that low oxygen tension exists in the synovium when KOA occurs because of synovial angiogenesis and inflammatory cell infiltration [Citation28]. Hypoxia in the microenvironment is mainly marked by HIF-1α expression. Compared with normal rats, the expressions of HIF-1α and its target genes VEGF and TIMP-1 in synovial tissue significantly increased in KOA group [Citation29]. We inferred that the inhibition of HIF-1α expression suppressed synovial fibrosis. In addition, HIF-1α can regulate NLRP3 expression. In the case of hypoxia, the expressions of HIF-1α, NLRP3, caspase-1 and IL-1β increased [Citation30]. HIF-1α is known to regulate a plethora of human diseases. By regulating NLRP3 (transcript) expression under these conditions, it becomes a key node in linking hypoxia response to pro-inflammatory status. Increased expression of NLRP3 and enzymatic activation of caspase-1 are one of the conditions for the upregulation of IL-1β and IL-18. These increased inflammatory factors were driven by the NLRP3 inflammasome. We found that IMP inhibited not only the expression of HIF-1α in fibroblasts, but also the activation of NLRP3 inflammasome and the expressions of downstream IL-1β and IL-18. Inhibiting HIF-1α can lead to synovial fibrosis in KOA rats; so, we observed the changes of three fibrogenic factors (TGF-β1, VEGF and TIMP-1) at the same time.
Synovial fibrosis is a pathological process observed in several musculoskeletal diseases. HIF-1α regulates the expressions of genes and proteins related to angiogenic growth factors, such as VEGF and TGF-β1 [Citation31]. TGF-β1 is one of the major indicators of synovial fibrosis that activate myoblasts, promote ECM gene expression, and inhibit ECM degradation [Citation32]. However, the triggering mechanism of synovial fibrosis in the knee joint remains largely unknown. Ko et al. found that TIMP1 was upregulated in OA fibroblasts stimulated with TGF-β1 and in mice with TGF-β-induced fibrosis [Citation33]. TIMP-1 is an inhibitor of matrix metalloproteinases, which is also elevated in the synovium of end-stage OA patients [Citation5]. TIMP-1 is induced by TGF-β, usually as an accelerator of fibrosis development [Citation34]. A recent study showed that the articular capsule of fixed knee joint was in the hypoxia state, and VEGF was upregulated at the mRNA and protein levels after immobilization. The decoy ODN transfected with HIF-1 successfully suppressed the transcriptional activation of HIF-1. VEGF expression was subsequently suppressed [Citation35]. Upon RA, hypoxia is caused by increasing metabolic requirements for white blood cells to enter RA joints, which can cause HIF-1α to accumulate in the cytoplasm and induce VEGF expression. Positive feedback regulation of the HIF-1α and VEGF pathways can trigger angiogenesis during hypoxia. In addition, the levels of VEGF and HIF-1α in synovial tissue were positively correlated with microvascular density [Citation36]. In this study, IMP decreased TGF-β1 and TIMP1 mRNA and protein levels in vitro and in vivo. Therefore, IMP may inhibit synovial fibrosis by suppressing the expressions of fibrogenic factors.
IMP is one of the furanocoumarin derivatives and exists in many Chinese herbal drugs with anti-tumor, antibacterial, cardiovascular, anti-inflammatory activities. In our previous studies, we found that synovial fibrosis was highly correlated with the activation of HIF-1α and NLRP3 inflammasome. These results indicate that IMP can mitigate synovitis and synovial fibrosis by inhibiting HIF-1α/NLRP3 inflammasome signaling.
Conclusions
In summary, IMP can alleviate synovial hypoxia and synovitis, and thus MIA-induced synovial fibrosis in KOA rats. It can reduce the release of inflammatory mediators and upregulate fibrosis markers induced by MIA or LPS by inhibiting the activation of NLRP3 inflammasome. Taken together, IMP may be a potentially effective therapy for KOA, especially for synovial fibrosis. Further studies are required to determine the signal pathway involved in HIF-1α/NLRP3 inflammasome activation/fibrosis. Its mechanism provides new ideas for treating KOA-related synovitis and synovial fibrosis.
Abbreviations
CMC-Na: carboxymethylcellulose sodium; ECM: extracellular matrix; FBD: fetal bovine serum; H&E: hematoxylin and eosin; HIF: hypoxia-induced factor; IL: interleukin; IMP: imperatorin; KOA: knee osteoarthritis; LPS: lipopolysaccharide; MIA: monosodium iodoacetate; TIMP: tissue matrix metalloproteinase; VEGF: vascular endothelial growth factor.
Highlights
1. KOA synovium shows both inflammatory and fibrotic phenotypes.
2. Imperatorin relieves inflammatory and fibrotic phenotypes in LPS-induced FLSs.
3. Imperatorin alleviates synovitis and synovial fibrosis in MIA-induced KOA rats.
Authors’ contributions
Haosheng Zhang, Liang Ding and Peimin Wang participated in the design of the study. Haosheng Zhang and Xiaoqing Shi performed the experiments, analyzed the data and wrote the manuscript. Liang Ding, Xiaoqing Shi, Wei Mei, Zhengquan Huang, Li Zhang, Xiaochen Li and Li Zhang performed the experiments and analyzed the data. Peimin Wang considerably revised this manuscript. All authors read and approved the final manuscript.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All animal protocols were approved by the Animal Care and Use Committee of the Nanjing University of Chinese Medicine. All experiments were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Consent for publication
All authors have consented the manuscript been published.
Acknowledgements
We are grateful to the considerable contributions of all coauthors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Prieto-Alhambra D, Judge A, Javaid MK, et al. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. 2014;73:1659–1664.
- Hunter DJ, Schofeld D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–441.
- Lafyatis R, Thompson NL, Remmers EF, et al. Transforming growth factor-beta production by synovial tissues from rheumatoid patients and streptococcal cell wall arthritic rats. Studies on secretion by synovial fibroblast-like cells and immunohistologic localization. J Immunol. 1989;143:1142–1148.
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827.
- Remst DF, Blom AB, Vitters EL, et al. Gene expression analysis of murine and human osteoarthritis synovium reveals elevation of transforming growth factor β-responsive genes in osteoarthritis-related fibrosis. Arthritis Rheumatol. 2014;66:647–656.
- Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148–156.
- Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology. 2005;44:7–16.
- Felson DT. Identifying different osteoarthritis phenotypes through epidemiology. Osteoarthr Cartil. 2010;18:601–604.
- Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759.
- Cramer T, Yamanishi Y, Clausen BE, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657.
- Zhang L, Huang Z, Xing R, et al. Increased HIF-1α in knee osteoarthritis aggravate synovial fibrosis via fibroblast-like synoviocyte pyroptosis. Oxid Med Cell Longev. 2019;2019:6326517.
- McAllister MJ, Chemaly M, Eakin AJ, et al. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthr Cartil. 2018;26:612–619.
- Li Y, Zheng JY, Liu JQ, et al. Succinate/NLRP3 inflammasome induces synovial fibroblast activation: therapeutical effects of clematichinenoside AR on arthritis. Front Immunol. 2016;7:532.
- Zhang L, Xing R, Huang Z, et al. Inhibition of synovial macrophage pyroptosis alleviates synovitis and fibrosis in knee osteoarthritis. Mediators Inflamm. 2019;2019:2165918.
- Mi C, Ma J, Wang KS, et al. Imperatorin suppresses proliferation and angiogenesis of human colon cancer cell by targeting HIF-1α via the mTOR/p70S6K/4E-BP1 and MAPK pathways. J Ethnopharmacol. 2017;203:27–38.
- Rosselli S, Maggio A, Bellone G, et al. Antibacterial and anticoagulant activities of coumarins isolated from the flowers of Magydaris tomentosa. Planta Med. 2007;73:116–120.
- Chowdhury AA, Gawali NB, Shinde P, et al. Imperatorin ameliorates lipopolysaccharide induced memory deficit by mitigating proinflammatory cytokines, oxidative stress and modulating brain-derived neurotropic factor. Cytokine. 2018;110:78–86.
- Wu P, Huang Z, Shan J, et al. Interventional effects of the direct application of “Sanse powder” on knee osteoarthritis in rats as determined from lipidomics via UPLC-Q-Exactive Orbitrap MS. Chin Med. 2020;15:9.
- Oh HA, Kim HM, Jeong HJ. Distinct effects of imperatorin on allergic rhinitis: imperatorin inhibits caspase-1 activity in vivo and in vitro. J Pharmacol Exp Ther. 2011;339:72–81.
- Dai M, Sui B, Xue Y, et al. Cartilage repair in degenerative osteoarthritis mediated by squid type II collagen via immunomodulating activation of M2 macrophages, inhibiting apoptosis and hypertrophy of chondrocytes. Biomaterials. 2018;180:91–103.
- Guo C, Fu R, Wang S, et al. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 2018;194:231–243.
- Wang Q, Ye C, Sun S, et al. Curcumin attenuates collagen-induced rat arthritis via anti-inflammatory and apoptotic effects. Int Immunopharmacol. 2019;72:292–300.
- Estakhri F, Panjehshahin MR, Tanideh N, et al. The effect of kaempferol and apigenin on allogenic synovial membrane-derived stem cells therapy in knee osteoarthritic male rats. Knee. 2020;27:817–832.
- Yang G, Li S, Yuan L, et al. Effect of nobiletin on the MAPK/NF-κB signaling pathway in the synovial membrane of rats with arthritis induced by collagen. Food Funct. 2017;8:4668–4674.
- Mao T, He C, Wu H, et al. Silencing lncRNA HOTAIR declines synovial inflammation and synoviocyte proliferation and promotes synoviocyte apoptosis in osteoarthritis rats by inhibiting Wnt/β-catenin signaling pathway. Cell Cycle. 2019;18:3189–3205.
- Li YZ, Chen JH, Tsai CF, et al. Anti-inflammatory property of imperatorin on alveolar macrophages and inflammatory lung injury. J Nat Prod. 2019;82:1002–1008.
- Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257.
- Qing L, Lei P, Liu H, et al. Expression of hypoxia-inducible factor-1α in synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis. Exp Ther Med. 2017;13:63–68.
- Pufe T, Petersen W, Tillmann B, et al. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001;44:1082–1088.
- Gupta N, Sahu A, Prabhakar A, et al. Activation of NLRP3 inflammasome complex potentiates venous thrombosis in response to hypoxia. Proc Natl Acad Sci U S A. 2017;114:4763–4768.
- Li Y, Liu Y, Wang C, et al. Succinate induces synovial angiogenesis in rheumatoid arthritis through metabolic remodeling and HIF-1α/VEGF axis. Free Radic Biol Med. 2018;126:1–14.
- Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338.
- Ko JH, Kang YM, Yang JH, et al. Regulation of MMP and TIMP expression in synovial fibroblasts from knee osteoarthritis with flexion contracture using adenovirus-mediated relaxin gene therapy. Knee. 2019;26:317–329.
- Remst DF, Blaney Davidson EN, Van der Kraan PM. Unravelling osteoarthritis-related synovial fibrosis: a step closer to solving joint stiffness. Rheumatology. 2015;54:1954–1963.
- Sotobayashi D, Kawahata H, Anada N, et al. Therapeutic effect of intra‐articular injection of ribbon‐type decoy oligonucleotides for hypoxia inducible factor‐1 on joint contracture in an immobilized knee animal model. J Gene Med. 2016;18:180–192.
- Xu J, Feng Z, Chen S, et al. Taxol alleviates collagen-induced arthritis in mice by inhibiting the formation of microvessels. Clin Rheumatol. 2019;38:19–27.
