ABSTRACT
This review investigates the findings of the most up-to-date literature on bioremediation via composting technology. Studies on bioremediation via composting began during the 1990s and have exponentially increased over the years. A total of 655 articles have been published since then, with 40% published in the last six years. The robustness, low cost, and easy operation of composting technology make it an attractive bioremediation strategy for organic contaminants prevalent in soils and sediment. Successful pilot-and large-scale bioremediation of organic contaminants, e.g., total petroleum hydrocarbons, plasticizers, and persistent organic pollutants (POPs) by composting, has been documented in the literature. For example, composting could remediate >90% diesel with concentrations as high as 26,315 mg kg−a of initial composting material after 24 days. Composting has unique advantages over traditional single- and multi-strain bioaugmentation approaches, including a diverse microbial community, ease of operation, and the ability to handle higher concentrations. Bioremediation via composting depends on the diverse microbial community; thus, key parameters, including nutrients (C/N ratio = 25–30), moisture (55–65%), and oxygen content (O2 > 10%) should be optimized for successful bioremediation. This review will provide bioremediation and composting researchers with the most recent finding in the field and stimulate new research ideas.
1. Introduction
Composting is a self-heating biological process that has been used for centuries as an organic waste management solution. Apart from managing organic waste, the composting product can be used as a soil amendment and organic fertilizer. Composting research has made substantial advances over the years, especially on shortening the composting process and improving compost quality. The research has been aided by the knowledge of parameters affecting the composting process, including initial particle size, nutrients, oxygen content, moisture content, pH, and temperature. In addition, because composting can biodegrade organic products, researchers have been interested in using this technology to treat recalcitrant organic contaminants, including polycyclic aromatic hydrocarbons (PAHs) [Citation1–3], total petroleum hydrocarbons (TPHs) [Citation4], diesel [Citation5], phthalate-based plasticizers [Citation6,Citation7], organochlorine pesticides [Citation8], polychlorinated dibenzo-p-dioxins and furans (PCDD/Fs) [Citation9,Citation10], and polychlorinated biphenyls (PCBs) [Citation11].
The diverse microbial communities present in composting materials are responsible for the biodegradation of recalcitrant organic contaminants. This degradation process could take the form of either complete mineralization/metabolism, co-metabolism, or nonspecific extracellular oxidation. Several studies have identified species that can mineralize these contaminants. For instance, Acinetobacter lwoffii, Bacillus subtilis and Raoultella ornithinolytica can degrade crude oil [Citation12]. Moreover, high temperatures during the composting (thermophilic phase) also enhance degradation by making the contaminants less viscous and more bioavailable. Biosurfactants, e.g., rhamnolipids produced by certain microbial species during composting, also enhance biodegradation by solubilizing the organic contaminants.
Therefore, this review is aimed at presenting, reviewing, and discussing the recent bioremediation via composting literature. Past review papers, published in the past ten years, have only focused on specific organic contaminants, including TPH [Citation13], PAHs [Citation14–17], and pesticides [Citation16,Citation18]. This review will include all the organic contaminants that have been reported to degrade during composting. Because composting is a biological process and is influenced by some key physicochemical parameters, this review also includes an overview of the composting process and these parameters to enable the readers to understand that the key to effective bioremediation via composting lies in the optimization of the parameters. In addition, the composting studies are compared to commonly used single- and multi-strain bioremediation approaches to gauge the competitiveness of this technology. We conclude the review by offering some future perspectives in this research field that we believe would stimulate research ideas that are equally beneficial and interesting.
2. Overview of organic waste composting process
2.1. Basics of the composting process
The composting process has been neatly categorized into four phases: mesophilic, thermophilic, cooling, and maturation. These phases have different temperature, oxygen demand, microbial community structure, stability, carbon content, nitrogen content, and pH profiles. After the initial composting mixture has been prepared, the mesophilic phase commences. The microbes utilize readily degradable organic matter as a nutrient source. As a result, the temperature rises above the ambient temperature after hours, or even a few days, depending on the composting scale, initial material, and composting conditions. If the compost mixture has soluble organic compounds such as sugars, organic acids may be produced during the fermentation of these compounds, resulting in a pH drop into the acidic range. However, the pH will not stay in this range for long due to the further decomposition of organic acids, volatilization, and the production of NH3. This phase lasts until the temperature reaches 55°C, ushering in the thermophilic phase. This stage has the highest temperature during the composting process.
Since temperature is an indicator of microbial activity, the initial stage of the thermophilic phase is considered the period with the highest activity. Mesophilic microbes are temperature sensitive and deactivated during the thermophilic phase, while thermophilic microbes populate the microbial community. Less biodegradable and complex organic substances like cellulose and hemicellulose start biodegrading during this phase. Ammonia produced from the degradation of nitrogen-containing organic matter causes an increase in pH [Citation19]. The high temperature in this period also destroys most human and animal pathogens such as Escherichia coli and Salmonella sp. Microbial activity slows down as nutrient sources deplete, causing a decrease in temperature and the beginning of the curing phase, which consists of the cooling and the maturation phase. The temperature during the cooling phase is similar to the mesophilic phase, and mesophilic organisms thrive during this stage. The available nutrient source comprises complex organic materials that are lignocellulosic. Macrofungi, which consume these complex materials, are usually observed in the compost, while the pH remains alkaline but drops slightly, approaching the neutral range. The cooling phase generally takes several weeks and can easily be mistaken for the maturation phase, the last stage of the composting process when the compost is stable and mature. The end product is a humus-like substance with an earthy smell. At this period, the compost temperature is similar to the ambient temperature, and the pH is neutral or slightly alkaline. Several indicators, including the germination index and soluble C/N ratio, are used to determine the maturity and stability of the compost [Citation20].
2.2. Key parameters for effective composting
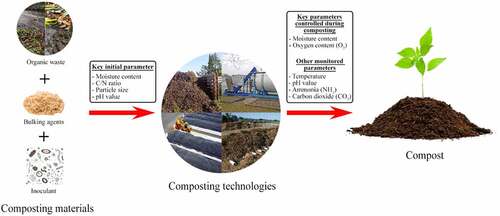
Composting, like other biological processes, is affected by nutrient availability and environmental conditions. This subsection will discuss the key parameters that influence the process and also include the optimal conditions for effective composting where necessary. summarizes these parameters and separates them into those initially adjusted and those monitored and/or controlled throughout the composting process. Moisture content, C/N ratio, particle size, and in some cases pH, are initially controlled to provide microorganisms with a suitable environment for thriving. Throughout the composting process, parameters, including oxygen and moisture content, that influence microbial activity during the four stages of the process, are monitored and controlled.
2.2.1. Initial compost materials and nutrient balance
The microorganisms in the compost require macronutrients such as carbon, nitrogen, phosphorus, and potassium, and micronutrients, including essential metals and minerals. The source of these nutrients is the substrate or feedstock available for these microorganisms. Another aspect to consider is how readily the microorganisms can break down the substrates. For example, recalcitrant substances like cellulose and lignin would take longer to break down compared to fructose. Consequently, although nutrients might be present in a substrate, they must be in a form that the microbes can utilize. Additionally, the decomposition depends on the enzymatic composition of individual microorganisms, strongly suggesting that some microbes can break down specific substrates while others might only break down the intermediate products.
In composting, carbon and nitrogen contents of composting materials are described as the main nutritional characteristic of the substrate. Carbon is used mainly as an energy source, while nitrogen is necessary for cell growth and function. The C/N ratio is used in composting to assess whether the microbes have sufficient nutrients. shows the nitrogen content and C/N ratio of commonly used composting materials. Generally, animal manure and sewage sludge are usually rich in nitrogen from urine and have lower C/N ratios, while lignocellulosic materials such as wheat and rice straw have more carbon and, therefore, a high C/N ratio. The consensus among most researchers is that an initial C/N ratio of 25–30 is ideal for the composting process. However, since the range assumes complete carbon mineralization, lower C/N ratios of up to 14 have been shown to work well [Citation21].
Table 1. Commonly used compost materials and their nitrogen and C/N ratio reported in literature
Lower ratios in excess of the requirements of the microbial population would lead to nitrogen loss as volatilized ammonia [Citation22], leading to malodor pollution. On the other hand, higher ratios lead to longer composting processes due to limited nitrogen resources. Therefore, the initial C/N ratio is usually adjusted before composting. Materials such as sucrose [Citation23–25], glucose [Citation26], spent mushroom [Citation24], and cellulose [Citation26] have been used to increase the C/N ratio of the compost mixture and reduce ammonia loss. For instance, Meng, et al. [Citation26] showed that 4% addition of sucrose to sewage sludge increased the C/N ratio from 8.06 to 9.56 and decreased nitrogen loss by 46.3%. Dry leaves and straw, which are common bulking agents, have a very high C/N ratio and, when added to the compost mixture, increase the initial C/N ratio. However, the types of carbon, for instance, lignin in these bulking agents, are complex and difficult to degrade. Another strategy that has been used to inhibit nutrient loss, and most specifically nitrogen loss in the form of ammonia, comprises biochar and other adsorbents [Citation27,Citation28]. These substances have high surface areas and adsorb ammonia preventing volatilization. High C/N ratios have been adjusted using ammonium fertilizers to decrease the C/N ratio, especially for some commercial-scale composting facilities [Citation29]. However, this adds to the operational cost.
2.2.2. Initial particle size
The particle size of the initial composting materials is important in two aspects. First, the size of the particle determines the surface area on which microbes can consume. Second, the particle size dictates how homogenous the initial materials mix. Smaller particles have larger surface areas which would allow for effective degradation. They also improve the homogenous mixing of the initial materials. However, small particles might also inhibit air and water penetration within the mixture leading to anaerobic zones. Conversely, larger particle sizes can lead to excessive ventilation, diminished water holding capacity, and slower degradation [Citation30]. There is no consensus about the best possible particle size for composting. Studies have used different particle sizes in their investigations, for instance, ≤ 1 cm in food waste composting [Citation9,Citation20,Citation31] and 1.5–3.0 cm in composting of cattle, chicken, kitchen, and municipal solid waste [Citation21]. Some researchers have studied the effect of particle size of the bulking agents on the composting process. For example, He, et al. [Citation32] found that granular biochar reduced methane emissions during pig manure and wheat straw composting by 22.2%, while powdered biochar increased emissions by 56.8%. This observation implies that anaerobic conditions occurred more frequently in the treatments with powdered biochar since methane is produced by methanogens which are anaerobic microorganisms. Bulking agents are supposed to give compost structural integrity, and the powdered biochar was too fine, resulting in poor air and water penetration. For the engineering and financial aspects, the grinding/cutting cost versus the additional benefit should be weighed when choosing the preferred particle size.
2.2.3. Moisture content
Moisture content is an important parameter in the composting process because microorganisms need adequate moisture to survive. Water is necessary for the transport of nutrients, making them accessible to microbes. Moisture influences air penetration, nutrients, oxygen uptake, and temperature. Higher moisture (usually >70%) content during the composting process forms waterlogs that lead to anaerobic conditions. Lower moisture content (usually <40%) could cause early dehydration during composting, hindering the biological process. However, the optimal moisture content depends on the feedstock’s physical characteristics, including the particle size and water-holding capacity, but a range of 55–65% has been utilized by most composting studies treating various types of organic materials [Citation13,Citation31–33]. The moisture content will also vary throughout the composting process depending on the temperature and aeration. For this reason, the moisture content is continuously adjusted, especially during the thermophilic phase.
2.2.4. Oxygen content
The aerobic microorganisms in compost require oxygen for respiration, so oxygen supply is crucial during composting. It is important that the microorganisms are provided with adequate oxygen to maintain their metabolic activities throughout composting. The oxygen content of > 10% in the compost gas throughout composting is recommended [Citation13]. Oxygen is supplied either through turning the compost manually or mechanically or with the aid of an aeration pump using positive or negative pressure depending on the size of the compost and resources. Among all other parameters mentioned in this review, aeration is the most influenced by the technology. In addition to supplying oxygen, aeration influences temperature and moisture during composting. Inadequate aeration leads to anaerobic conditions, while too high might dry out the compost and inhibit the composting process [Citation34]. Furthermore, since oxygen demand is proportional to microbial activities, aeration should be the highest during the thermophilic phase and the lowest during the curing phases.
Besides the composting scale, the desired aeration rate will depend on the characteristics of the composting materials, including particle size and moisture content [Citation35,Citation36]. Specifically, the particle size of the bulking agents, which provide the structural integrity of the compost mixture, will influence the oxygen supply in the compost. Therefore, a bulking agent that provides adequate voids that allow oxygen penetration throughout the compost is recommended. As Cao, et al. [Citation35] showed, powdered bulking agents increased methane emissions. Water and air compete for these interstitial voids, and therefore high moisture implies that the voids are occupied with water instead of air. In their extensive literature review, Tran, et al. [Citation13] concluded that an optimal aeration rate of 1–2 L kg dry wt.−1 min−1 could meet the aeration requirements for a successful pilot-scale composting process. However, it is not possible to maintain complete aerobic conditions during composting, especially for large-scale composting. Therefore, the goal is to maximize aeration within the constraints of financial feasibility.
2.2.5. Temperature
Composting is a self-heating biological process, and the heat is a product of aerobic microbial degradation of organic matter. The produced heat influences moisture and microbial community structure [Citation37]. High temperatures have been shown to dry out the compost and inhibit the composting process. In addition, the microbial diversity decreases in high temperatures, and only thermophiles, e.g., Thermus genus, survive and thrive under such conditions. For instance, Yu, et al. [Citation38] observed that the Shannon index, a measure of microbial diversity, dropped from 7.86 at day 0 to 4.03 at day 3 when the temperature reached 93.4°C, during hyperthermophilic composting. However, this is not necessarily a bad thing. Hyperthermophilic composting is garnering growing interest among researchers because of the shorter composting period and less nitrogen being lost compared to conventional composting [Citation39–41].
Ambient temperature has also been reported to influence the composting period. This effect is more pronounced for composting carried outdoors, e.g., windrow and onsite composting that are exposed to the elements [Citation42–44]. Zhou, et al. [Citation43] studied windrow composting in summer and winter and concluded that the temperature took one day longer to increase during winter. The maximum temperature reached during the thermophilic phase was also lower. Heat loss from the surface of the compost to the environment is also more pronounced in colder temperatures. In large-scale composting operation, uneven temperature distribution may occur due to non-homogeneous mixing or aeration and may end up affecting the compost quality.
2.2.6. pH
The initial pH of the composting materials is influenced by the type of organic wastes. For instance, food waste is slightly acidic, while animal manure is alkaline. Furthermore, the bacterial community in the compost prefers neutral or near-neutral pHs, while the fungal community prefers slightly acidic conditions [Citation45]. Therefore, the optimal pH range varies noticeably for composting, 5.5–8.0 [Citation27,Citation35,Citation46,Citation47]. This explains why pH is not usually adjusted during composting when compared to other biological treatment technologies. However, lower pH values have been shown to influence composting negatively [Citation35,Citation48]. For example, Cao, et al. [Citation35] demonstrated that the initial pH of 5 delayed degradation by seven to ten days and also increased the electrical conductivity of the final mature compost above the acceptable standards (≤4 mS cm−1). Such low pHs are characteristic of food waste composting, and some researchers have increased the initial pH of the compost mixture [Citation49].
The pH also varies throughout the composting process. When the process commences, the production of organic acids lowers the pH of the compost. The production of ammonia from the decomposition of nitrogen-containing organic matter increases the pH in the thermophilic stage [Citation33]. NH4+ and HCO3−, other decomposition products, act as buffers that maintain the high pH throughout the composting process [Citation35].
2.3. The history and current state of the composting process
Composting has a long history that evolved alongside human settlements and the practice of agriculture. Diaz and De Bertoldi [Citation50] present an exhaustive history of composting from the Neolithic period to the 20th century. Research on the composting process and influencing factors can be traced back to the late 1940s and 1950s. Mechanization of composting technology also began during this period, with composting digesters such as the Hardy digester and Dano drums becoming commercially available. The 1960s through to the 1980s witnessed more research on the technical aspects and financial viability of composting facilities, use of compost, the effect of compost on plant growth, and the hygienization aspects of composting [Citation51,Citation52]. Well-known composting technologies such as the aerated static pile were invented during this period [Citation53]. Zheng, et al. [Citation54] refer to this period as the budding stage of composting technology based on the number of patents filed worldwide. The period between 1990 and 2007 was designated as the developing stage and more recent years as the expanding stage. Their comprehensive bibliometric analysis suggests that composting technology research interest has grown steadily over the years, especially with the rise of the sustainable development movement and efforts to minimize wastage and needless pollution.
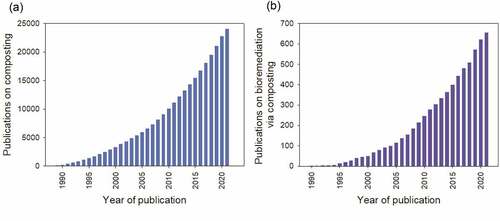
represents the cumulative publications on composting covering the years 1989 to 2021. The data was accessed from the Web of Science database and only included Scientific Citation Index Expanded (SCIE) research, review, and early access articles. According to , a total of 24,000 articles were published in the past three decades. Interest in composting research can also be seen to increase over the period steadily. The research has primarily focused on optimizing and shortening the composting process [Citation55,Citation56], odor control [Citation57,Citation58], microbial community structure [Citation47,Citation59,Citation60], composting application including heat recovery [Citation61–63] and bioremediation [Citation6,Citation9,Citation31]. shows the publication of articles on bioremediation in those three decades. Based on the exponential growth, bioremediation research via composting appears to be on the rise. A total of 655 articles have been published between 1990 to 2021, with 40% published in the last six years.
3. Bioremediation of recalcitrant organic contaminants
Bioremediation technologies employ microorganisms to degrade organic contaminants. This degradation process can occur via three main pathways: (i) mineralization or metabolism, whereby the microorganisms utilize the contaminant as a nutrient source; (ii) co-metabolism, whereby contaminants that do not serve as a nutrient source are broken down in parallel with metabolic reactions; and (iii) nonspecific oxidation, which involves the extracellular degradation of contaminants [Citation64]. Traditionally, bioremediation has been carried out in various ways, including bioaugmentation and biostimulation. Bioaugmentation involves incorporating specific microbial species capable of mineralizing a contaminant and using it as a nutrient source into a contaminated environment. Biostimulation involves providing rate-limiting nutrients such as phosphorus and nitrogen and supplements such as biosurfactants to microorganisms in a contaminated environment. Bioaugmentation has been conducted using single or multiple strains of bacteria or fungi (mycoremediation).
Bioremediation via composting presents unique advantages over single- or multi-strain bioremediation. Firstly, the composting process comprises several microorganisms that participate in mutualistic, synergistic, and/or competitive relationships. In this diverse microbial structure, a handful of species could completely mineralize or metabolize a contaminant, while other species are only able to co-metabolize or nonspecifically oxidize the contaminants. This makes the composting process robust and highly effective in degrading organic contaminants. In addition, the composting process undergoes certain physicochemical transformations that govern the fate of the organic contaminant in the compost. For example, the high temperatures during the thermophilic stage could cause the volatilization of volatile and semi-volatile compounds. On the other hand, the high temperatures might also increase the bioavailability of certain compounds by making them less viscous. Furthermore, some species can produce biosurfactants that increase the bioavailability of the compounds [Citation65].
This section presents the bioremediation of some common recalcitrant organic pollutants found in the pedosphere and sediment, including polycyclic aromatic hydrocarbons (PAHs), di(2-ethylhexyl) phthalate (DEHP), polychlorinated dibenzo-p-dioxins/furans (PCDD/Fs), total petroleum hydrocarbons (TPH), and pesticides. These compounds are highly hydrophobic and prevalent in the environment as a result of anthropogenic activities. Bioremediation studies, including composting, single- and multi-strain bioremediation, and biostimulation approaches, published in the past ten years, are presented in .
Table 2. Bioremediation and composting of petroleum and petroleum-related contaminants by biological treatment approaches
Table 3. Bioremediation of phthalate-based plasticizers by biological treatment approaches
Table 4. Bioremediation of pesticides by biological treatment approaches
Table 5. Bioremediation and composting of halogenated biphenyls, dioxins, and furans by biological treatment approaches
3.1. Bioremediation of petroleum and petroleum-related organic contaminations
Petroleum contaminants are among the most prevalent organic contaminants in the environment because of anthropogenic activities, including petroleum extraction, processing, transportation, storage, and usage. presents results from recent studies on the bioremediation of petroleum and petroleum-related contaminants at different scales. Petroleum-related contaminants that have been remediated via bioremediation include diesel, PAH, and TPH. It can be observed that the efficiency in removing petroleum contaminants utilizing biological approaches varies according to the initial concentration, biological approach, and scale. Except for composting, other bioremediation approaches were generally small-scale. This is an advantage of composting in that the scale of remediating TPH can be increased without significantly compromising the removal efficiency. For example, Lin, et al. [Citation5] showed that over 90% of diesel of the initial concentration of 26,315 mg kg−1 was degraded via composting for 24 days. This suggests that petroleum products are relatively easier to biodegrade.
Numerous microbial species capable of mineralizing petroleum and petroleum-related compounds have been reported in various studies [Citation12]. These species have been isolated from petroleum-contaminated environments, cultured, and used in bioremediation studies. For example, Abena, et al. [Citation12] identified crude oil-degrading bacterial strains belonging to Raoultella ornithinolytica, Bacillus subtilis, Serratia marcescens, and Acinetobacter lwoffii species and augmented the strains in contaminated soils, increasing the TPH-degradation to 48.1%. These species release certain enzymes, including alkane hydroxylases and methane monooxygenases, which assist in the breakdown of petroleum. For example, methane monooxygenase can oxidize the C-H bonds of alkanes.
The aerobic bacterial degradation mechanism of petroleum products, especially n-alkanes, is well documented [Citation13,Citation66]. The n-alkanes are broken down into a carbon source for the bacteria through the main pathways: terminal, subterminal, β-, and ω-oxidations. Details of the mechanisms have been extensively presented in review papers on petroleum degradation [Citation66,Citation67]. Briefly, these pathways are catalyzed by monooxygenases to convert them into alcohols. The dehydrogenases catalyze the conversion of the alcohols into aldehydes and ketones, then further into fatty acids. The fatty acids are further oxidized into tricarboxylic acid cycle (TCA) intermediates. On the other hand, the degradation mechanism of aromatic petroleum compounds is more complicated and has been reported to be initiated via oxidative attack with the help of monooxygenases or dioxygenases to produce catechol-like structures before ring cleavage reactions by dioxygenases. The resultant straight chain product goes through the above-mentioned n-alkane oxidation reactions.
Fungal species from genera such as Aspergillus, Alternaria, Penicillium, and Graphium have been reported to degrade petroleum and petroleum-related compounds [Citation68]. The degradation of complex petroleum-related compounds like PAHs by ligninolytic and non-ligninolytic fungi have been reported in detail by some studies [Citation69,Citation70]. Ligninolytic fungi extracellularly degrade PAHs using lignin-degrading enzymes, including peroxidases and laccase. Both groups of compounds can degrade these compounds intracellularly in reactions mediated by hydrolases and cytochrome P450 monooxygenases. The extracellular degradation produces polar and water-soluble products that can be accessible for fungal and the other microbial metabolisms in that environment. This process can occur during composting, and because of the microbial diversity, there would be a high probability of several species that would be able to metabolize these extracellular degradation products.
Several petroleum-degrading species have been reported in literature, including mesophilic microbes such as Acinetobacter calcoaceticus, Bacillus simplex, Paenibacillus pabuli, Bacillus pumilus, and Pseudomonas aeruginosa; and thermophilic microbes like Bacillus megaterium, Aspergillus sp, Pseudoxanthomonas sp., Mucor sp, Rhizopus sp., and Shigella flexneri [Citation13,Citation17,Citation71,Citation72]. This suggests that biodegradation can occur at all stages of the composting process.
3.2. Bioremediation of phthalate-based plasticizers
Phthalate-based plasticizers are common plastic and rubber additives that increase flexibility and durability. Consequently, they have become quite prevalent in the environment. Environmentalists and public health experts are greatly concerned about the links of these compounds to endocrine disruption. More toxic phthalates such as diethylhexyl phthalate (DEHP) and di-butyl phthalate (DBP) are already being phased out entirely or in some products, e.g., children’s toys in the EU and the US. Few researchers have also shown interest in using bioremediation techniques to study the effectiveness of removing phthalates, as shown in . Composting can degrade multi-pollutants, as shown by Fu, et al. [Citation7]. Tran, et al. [Citation6] also showed that pilot-scale food waste composting removed 98% of DOTP with high concentrations of 11,882 mg kg−1 after only 35 days of composting. This was significantly higher than the single-strain bioremediation study, which had lower concentrations and a scale over 1,000 times smaller.
The microbial degradation of phthalates involves a series of β-oxidation and de-esterification reactions to produce phthalic, terephthalic, or isophthalic acids [Citation73–76]. Boll, et al. [Citation77] detailed the most updated understanding of microbial degradation of the resultant acids. They reported that almost all the aerobic microorganisms convert these compounds into protocatechuate, a TCA intermediate. These reactions involve three steps for phthalic acid; dioxygenation, dehydrogenation and decarboxylation, and two steps for terephthalic and isophthalic acids; dioxygenation and dehydrogenation. The involved enzymes are decarboxylases, dehydrogenases, and dioxygenases. Several species were found in compost [Citation6,Citation33,Citation78], e.g., Microbacterium sp and Rhodococcus erythropolis, Gordonia sp. Pseudomonas sp. Bacillus sp., Rhizobium sp., and Achromobacter sp. can completely metabolize phthalates, even at high concentrations [Citation73]. Therefore, composting is well equipped to degrade phthalates effectively.
3.3. Bioremediation of pesticides
Pesticides, including herbicides, insecticides, and fungicides, have been extensively used in agriculture to boost yield by keeping away pests and weeds. Some of these pesticides from the organochlorine group, e.g., dichlorodiphenyltrichloroethane (DDT), lindane, endrin, dieldrin, endosulfan, and heptachlor, are part of the compounds listed in the Stockholm Convention of POPs and are banned or restricted globally because of the environmental and human health risk. However, these legacy pesticides are highly persistent with incredibly long half-lives and are still found in soil [Citation79] and sediment [Citation80,Citation81]. summarizes the results of some recent studies on bioremediation of organochlorine and organophosphate pesticides by biological treatment approaches. Bioremediation of organochlorine (DDT, aldrin, lindane, α- and β-endosulfan) appears slower than organophosphate (phorate and chlorpyrifos) pesticides, which is attributable to their higher toxicity. Egbe, et al. [Citation82] reported that organochlorine pesticides reduced the number of bacterial and fungal species when added to agricultural soils, implying that these compounds are toxic to some microorganisms. Bioremediation also showed high removal efficiency. Ali, et al. [Citation8] reported that composting could simultaneously degrade multiple legacy pesticides and achieve high degradation efficiencies of 80–87% after 100 days of composting.
Some specific strains found in compost can degrade some pesticides. For example, Kumar and Pannu [Citation83] identified Rhodanobacter lindaniclasticus, Alkaligens faecalis, and Pseudomonas aeruginosa as capable of dechlorinating lindane, Wang, et al. [Citation84] reported that Stroptomyces sp. strain can degrade DDT, and Seralathan, et al. [Citation85] confirmed that Pseudomonas aeruginosa, Ochrobacterium sp, and Achromobacter xylosoxidans degrade endosulfan and use it as a source of sulfur. These organochlorine pesticide-degrading species contain genes, e.g., lin and Esd genes that encode for dehalogenases, hydrolases, dehydrochlorinases, and monooxygenases enzymes that take part in mineralizing these compounds [Citation86]. Hydrolysis, mediated by phosphotriesterase, is the primary step in the bacterial degradation of organophosphate pesticides which causes the cleaving of the P-O/F/S bond separating the two main moieties, which undergo further reactions to produce TCA intermediates [Citation86,Citation87]. Since organophosphates have lower toxicity and less persistence in the environment, many more microbial species documented by Mulla, et al. [Citation87] have been identified to degrade organophosphate pesticides.
3.4. Bioremediation of halogenated biphenyls, dioxins, and furans
These persistent organic pollutants (POPs), including polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and polychlorinated biphenyls (PCBs), stubbornly remain in the soil and sediments and are also carcinogenic and mutagenic. They constitute some of the most toxic compounds known to man and were among the first listed compounds in the Stockholm Convention on POPs. PCBs were manufactured and widely used as coolants for many decades, but like PCDD/Fs, they can also be unintentionally produced during incomplete combustion. Therefore, all combustion sources such as engines, incinerators, and power plants, can produce these compounds. Since they are highly hydrophobic and have a high affinity to particles, they are found mostly in soil and sediments, where they have long half-lives that could last for more than 100 years [Citation88].
documents some studies on the bioremediation of these POPs published in the last ten years. The first observation is that the initial concentrations, especially for PCDD/Fs, are much lower than those used in the bioremediation of the other compounds presented in this review. This is probably because of the high toxicity of these compounds. Successful pilot-scale bioremediation of these POPs via composting has been reported [Citation9,Citation10] with removal efficiencies > 75%. Bacterial species with the dioxygenase encoding genes are capable of degrading PCDD/F and PCDD/F-like compounds. Some of these species that have been found in compost include Sphingomonas sp., Pseudomonas aeruginosa, Acinetobacter sp., Pseudomonas putida, Ralstonia sp., Burkholderia sp., Comamonas testosteroni, Novosphingobium sp., Burkholderia cepacia, and Pseudomonas sp. [Citation9,Citation89,Citation90].
The aerobic bacterial degradation mechanism has been reported to be initiated via either angular or lateral dioxygenation [Citation91]. The degradation mechanisms of these compounds have been carried out using non-chlorinated congeners because they are less stable. The dioxygenation reaction involves the addition of OH to the angular or lateral position. This initial reaction is catalyzed by monooxygenases or hydroxylating dioxygenases. The preceding oxygenation knocks down the planar structure of the molecule in the angular dioxygenation, making the compound less toxic. The series of reactions proceed by opening the aromatic ring producing salicylic acid and catechol, which are further broken down to TCA intermediates. The fungal degradation mechanism involves similar reactions in the degradation of aromatic compounds previously mentioned in Section 3.1.
4. Conclusions and future perspectives
From reviewing the recent publications, we can conclude that bioremediation via composting is still a nascent topic but has great potential to remove recalcitrant organic pollutants in soils and sediments. Some of the organic contaminants that have been successfully treated via composting include diesel, total petroleum hydrocarbons (TPH), polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), diethylhexyl phthalate (DEHP), and polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs). Generally, highly toxic compounds like PCDD/Fs take longer to degrade, and only lower initial concentrations can be successfully treated. Since composting is a biological process, ensuring that key parameters, including particle size, nutrients, oxygen content, and moisture content, are within the suitable ranges of effective composting enhances the bioremediation. Moisture content of 55–65%, C/N ratio of 25–30 and oxygen content of > 10% in the compost gas have been recommended by researchers as optimal. However, the research on the optimal ranges of the other parameters is still inadequate to draw any certain conclusions. Interesting and important research directions that need further investigation include bio-augmenting/inoculating the composting process with specific microbes capable of degrading a contaminant at different composting stages. Another important topic is studying the pairing of composting with other bioremediation technologies, e.g., using mature compost with residual contamination in mycoremediation or phytoremediation. In addition, investigating the ability of composting to degrade multiple organic contaminants simultaneously is another interesting research area. In conclusion, composting is a suitable bioremediation technology that deserves more attention.
Highlights
Composting can successfully bioremediate several recalcitrant organic contaminants
The microbial diversity of composting offers unique bioremediation merits
Production of biosurfactants and the high temperatures promote bioavailability
Ensuring optimal composting conditions enhances the bioremediation
The exact degradation mechanism of contaminants remains complex and unconfirmed
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Lu Y, Zheng G, Zhou W, et al. Bioleaching conditioning increased the bioavailability of polycyclic aromatic hydrocarbons to promote their removal during co-composting of industrial and municipal sewage sludges. Sci Total Environ. 2019;665:1073–1082.
- Guo Y, Rene ER, Wang J, et al. Biodegradation of polyaromatic hydrocarbons and the influence of environmental factors during the co-composting of sewage sludge and green forest waste. Bioresour Technol. 2020;297:122434.
- Ozaki N, Nakazato A, Nakashima K, et al. Loading and removal of PAHs, fragrance compounds, triclosan and toxicity by composting process from sewage sludge. Sci Total Environ. 2017;605:860–866.
- Becarelli S, Chicca I, Siracusa G, et al. Hydrocarbonoclastic Ascomycetes to enhance co-composting of total petroleum hydrocarbon (TPH) contaminated dredged sediments and lignocellulosic matrices. N Biotechnol. 2019;50:27–36.
- Lin C, Sheu D-S, Lin T-C, et al. Thermophilic biodegradation of diesel oil in food waste composting processes without bioaugmentation. Environ Eng Sci. 2012;29(2):117–123.
- Tran HT, Lin C, Bui XT, et al. Bacterial community progression during food waste composting containing high dioctyl terephthalate (DOTP) concentration. Chemosphere. 2021;265:129064.
- Fu J, Pan F, Song S, et al. Biodegradation of phthalic acid esters in sewage sludge by composting with pig manure and rice straw. Environ Earth Sci. 2013;68(8):2289–2299.
- Ali M, Kazmi A, Ahmed N. Study on effects of temperature, moisture and pH in degradation and degradation kinetics of aldrin, endosulfan, lindane pesticides during full-scale continuous rotary drum composting. Chemosphere. 2014;102:68–75.
- Tran HT, Lin C, Hoang HG, et al. Biodegradation of dioxin-contaminated soil via composting: identification and phylogenetic relationship of bacterial communities. Environ Technol Innov. 2020;19:101023.
- Huang W-Y, Ngo -H-H, Lin C, et al. Aerobic co-composting degradation of highly PCDD/F-contaminated field soil. A study of bacterial community. Sci Total Environ. 2019;660:595–602.
- Kapanen A, Vikman M, Rajasärkkä J, et al. Biotests for environmental quality assessment of composted sewage sludge. Waste Manage. 2013;33(6):1451–1460.
- Abena MTB, Li T, Shah MN, et al. Biodegradation of total petroleum hydrocarbons (TPH) in highly contaminated soils by natural attenuation and bioaugmentation. Chemosphere. 2019;234:864–874.
- Tran H-T, Lin C, Bui X-T, et al. Aerobic composting remediation of petroleum hydrocarbon-contaminated soil. Current and Future Perspectives. Sci Total Environ. 2021;753:142250.
- Sayara T, Sánchez A. Bioremediation of PAH-contaminated soils: process enhancement through composting/compost. Appl Sci. 2020;10(11):3684.
- Antizar-Ladislao B, Lopez-Real J, Beck A. Bioremediation of polycyclic aromatic hydrocarbon (PAH)-contaminated waste using composting approaches. Crit Rev Environ Sci Technol. 2004;34(3):249–289.
- Chen M, Xu P, Zeng G, et al. Bioremediation of soils contaminated with polycyclic aromatic hydrocarbons, petroleum, pesticides, chlorophenols and heavy metals by composting: applications, microbes and future research needs. Biotechnol Adv. 2015;33(6):745–755.
- Loick N, Hobbs PJ, Hale MD, et al. Bioremediation of poly-aromatic hydrocarbon (PAH)-contaminated soil by composting. Crit Rev Environ Sci Technol. 2009;39(4):271–332.
- Purnomo AS, Mori T, Kamei I, et al. Basic studies and applications on bioremediation of DDT: a review. Int Biodeterior Biodegradation. 2011;65(7):921–930.
- FAO. Biofertilizer production plant. Myanmar: FAO; 2002.
- Wang X, Selvam A, Wong JW. Influence of lime on struvite formation and nitrogen conservation during food waste composting. Bioresour Technol. 2016;217:227–232.
- Wang X, Cui H, Shi J, et al. Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour Technol. 2015;198:395–402.
- Iqbal M, Nadeem A, Sherazi F, et al. Optimization of process parameters for kitchen waste composting by response surface methodology. Int J Environ Sci Technol. 2015;12(5):1759–1768.
- Li W, Wu C, Wang K, et al. Nitrogen loss reduction by adding sucrose and beet pulp in sewage sludge composting. Int Biodeterior Biodegradation. 2017;124:297–303.
- Meng L, Zhang S, Gong H, et al. Improving sewage sludge composting by addition of spent mushroom substrate and sucrose. Bioresour Technol. 2018;253:197–203.
- Meng L, Li W, Zhang S, et al. Effects of sucrose amendment on ammonia assimilation during sewage sludge composting. Bioresour Technol. 2016;210:160–166.
- Meng L, Li W, Zhang S, et al. Effect of different extra carbon sources on nitrogen loss control and the change of bacterial populations in sewage sludge composting. Ecol Eng. 2016;94:238–243.
- Janczak D, Malińska K, Czekała W, et al. Biochar to reduce ammonia emissions in gaseous and liquid phase during composting of poultry manure with wheat straw. Waste Manage. 2017;66:36–45.
- Agyarko-Mintah E, Cowie A, Van Zwieten L, et al. Biochar lowers ammonia emission and improves nitrogen retention in poultry litter composting. Waste Manage. 2017;61:129–137.
- Yu H, Xie B, Khan R, et al. The changes in carbon, nitrogen components and humic substances during organic-inorganic aerobic co-composting. Bioresour Technol. 2019;271:228–235.
- Zhao G-H, Yu Y-L, Zhou X-T, et al. Effects of drying pretreatment and particle size adjustment on the composting process of discarded flue-cured tobacco leaves. Waste Manage Res. 2017;35(5):534–540.
- Lin CT, Cheruiyot NK, Hoang HG, et al. Benzophenone biodegradation and characterization of malodorous gas emissions during co-composting of food waste with sawdust and mature compost. Environ Technol Innov. 2021;21.
- He XQ, Yin HJ, Sun XX, et al. Effect of different particle-size biochar on methane emissions during pig manure/wheat straw aerobic composting: insights into pore characterization and microbial mechanisms. Bioresour Technol. 2018;268:633–637.
- Wei L, Shutao W, Jin Z, et al. Biochar influences the microbial community structure during tomato stalk composting with chicken manure. Bioresour Technol. 2014;154:148–154.
- Malinowski M. Impact of air-flow rate and biochar addition on the oxygen concentration in waste and emitted gases during biostabilization of undersized fraction from municipal solid waste. J Ecol Eng. 2021;22(6):136–144.
- Cao Y, Wang X, Liu L, et al. Acidification of manure reduces gaseous emissions and nutrient losses from subsequent composting process. J Environ Manage. 2020;264:110454.
- Diaz L, Savage G. Factors that affect the process. Waste Management Series: Elsevier. 2007; 8:49–65.
- Chang R, Li Y, Li N, et al. Effect of microbial transformation induced by metallic compound additives and temperature variations during composting on suppression of soil-borne pathogens. J Environ Manage. 2021;279:111816.
- Yu Z, Tang J, Liao H, et al. The distinctive microbial community improves composting efficiency in a full-scale hyperthermophilic composting plant. Bioresour Technol. 2018;265:146–154.
- Huang Y, Li D, Wang L, et al. Decreased enzyme activities, ammonification rate and ammonifiers contribute to higher nitrogen retention in hyperthermophilic pretreatment composting. Bioresour Technol. 2019;272:521–528.
- Cui P, Chen Z, Zhao Q, et al. Hyperthermophilic composting significantly decreases N2O emissions by regulating N2O-related functional genes. Bioresour Technol. 2019;272:433–441.
- Wen P, Tang J, Wang Y, et al. Hyperthermophilic composting significantly decreases methane emissions: insights into the microbial mechanism. Sci Total Environ. 2021;784:147179.
- McCartney D, Eftoda G. Windrow composting of municipal biosolids in a cold climate. J Environ Eng Sci. 2005;4(5):341–352.
- Zhou H, Shen Y, Meng H, et al. Effect of air temperature and aeration strategy on water removal during sewage sludge composting. Dry Technol. 2018;36(12):1474–1480.
- Stegenta S, Sobieraj K, Pilarski G, et al. Analysis of the spatial and temporal distribution of process gases within municipal biowaste compost. Sustainability. 2019;11(8):2340.
- Tuomela M, Vikman M, Hatakka A, et al. Biodegradation of lignin in a compost environment: a review. Bioresour Technol. 2000;72(2):169–183.
- Zhang L, Sun X. Influence of bulking agents on physical, chemical, and microbiological properties during the two-stage composting of green waste. Waste Manage. 2016;48:115–126.
- Ren GM, Xu XH, Qu JJ, et al. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J Microbiol Biotechnol. 2016;32(6). DOI:10.1007/s11274-016-2059-7
- Wang S-P, Zhong X-Z, Wang -T-T, et al. Aerobic composting of distilled grain waste eluted from a Chinese spirit-making process: the effects of initial pH adjustment. Bioresour Technol. 2017;245:778–785.
- Cerda A, Artola A, Font X, et al. Composting of food wastes: status and challenges. Bioresour Technol. 2018;248:57–67.
- Diaz L, De Bertoldi M. History of composting. Waste Management Series: Elsevier. 2007; 8:7–24.
- Stone GE. Composting at Johnson City: final report on joint USEPA-TVA composting project with operational data, 1967-1971. US Environmental Protection Agency, 1975.
- Willson G, Parr J, Epstein E, et al. Manual for composting sewage sludge by the Beltsville aerated-pile method. US Environmental Protection Agency. 1980; 1–65.
- Epstein E, Willson G, Burge W, et al. A forced aeration system for composting wastewater sludge. J Water Pollut Control Fed. 1976;48:688–694.
- Zheng X, Aborisade MA, Liu S, et al. The history and prediction of composting technology: a patent mining. J Clean Prod. 2020;276:124232.
- Wang S-P, Wang L, Sun Z-Y, et al. Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour Technol. 2021;337:125492.
- Liu X, Hou Y, Li Z, et al. Hyperthermophilic composting of sewage sludge accelerates humic acid formation: elemental and spectroscopic evidence. Waste Manage. 2020;103:342–351.
- Ding Y, Xiong JS, Zhou BW, et al. Odor removal by and microbial community in the enhanced landfill cover materials containing biochar-added sludge compost under different operating parameters. Waste Manage. 2019;87:679–690.
- Andraskar J, Yadav S, Kapley A. Challenges and control strategies of odor emission from composting operation. Appl Biochem Biotechnol. 2021;193(1):1–26.
- Covino S, Fabianova T, Kresinova Z, et al. Polycyclic aromatic hydrocarbons degradation and microbial community shifts during co-composting of creosote-treated wood. J Hazard Mater. 2016;301:17–26.
- Mironov V, Vanteeva A, Merkel A. Microbiological activity during co-composting of food and agricultural waste for soil amendment. Agronomy-Basel. 2021;11.
- Walther E, Ferrier R, Bennacer R, et al. Heat recovery in compost piles for building applications. Therm Sci. 2017;21(2):775–784.
- Smith MM, Aber JD. Energy recovery from commercial-scale composting as a novel waste management strategy. Appl Energy. 2018;211:194–199.
- Yeh CK, Lin C, Shen HC, et al. Optimizing food waste composting parameters and evaluating heat generation. Appl Sci. 2020;10(7):2284.
- Kästner M, Miltner A. Application of compost for effective bioremediation of organic contaminants and pollutants in soil. Appl Microbiol Biotechnol. 2016;100(8):3433–3449.
- Lin CT. Method for removing pollutants in pollutants-contaminated soil. In: USPTO, editor. United States of America. lin Chi Tsan. 2012; 1–7.
- Varjani SJ. Microbial degradation of petroleum hydrocarbons. Bioresour Technol. 2017;223:277–286.
- Abbasian F, Lockington R, Mallavarapu M, et al. A comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. Appl Biochem Biotechnol. 2015;176(3):670–699.
- Mahmoud GA-E, Bagy MMK. Microbial degradation of petroleum hydrocarbons. In: K V, K M, P R, editors. Microbial action on hydrocarbons. Singapore: Springer; 2018. p. 299–320.
- Haritash S, Haritash AK. A comprehensive review of metabolic and genomic aspects of PAH-degradation. Arch Microbiol. 2020;202(8):2033–2058.
- Gupta S, Pathak B. Abatement of Environmental Pollutants. Amsterdam, Netherlands: Elsevier; 2020. Mycoremediation of polycyclic aromatic hydrocarbons; p. 127–149. https://doi.org/10.1016/B978-0-12-818095-2.00006-0.
- Graça J, Murphy B, Pentlavalli P, et al. Bacterium consortium drives compost stability and degradation of organic contaminants in in-vessel composting process of the mechanically separated organic fraction of municipal solid waste (MS-OFMSW). Bioresour Technol Rep. 2021;13:100621.
- Atagana HI. Co‐composting of PAH‐contaminated soil with poultry manure. Lett Appl Microbiol. 2004;39(2):163–168.
- Carmen S. Microbial capability for the degradation of chemical additives present in petroleum-based plastic products: a review on current status and perspectives. J Hazard Mater. 2021;402:123534.
- Phale PS, Sharma A, Gautam K. Microbial degradation of xenobiotics like aromatic pollutants from the terrestrial environments. In: Prasad MNV, Kapley A, and Vithanage M, editors. Pharmaceuticals and personal care products: waste management and treatment technology. Oxford, United Kingdom: Butterworth-Heinemann; 2019. p. 259–278.
- Tang WJ, Zhang LS, Fang Y, et al. Biodegradation of phthalate esters by newly isolated Rhizobium sp. LMB‐1 and its biochemical pathway of di-n-butyl phthalate. J of Appl Microbiol. 2016;121(1):177–186.
- Amir S, Hafidi M, Merlina G, et al. Fate of phthalic acid esters during composting of both lagooning and activated sludges. Process Biochem. 2005;40(6):2183–2190.
- Boll M, Geiger R, Junghare M, et al. Microbial degradation of phthalates: biochemistry and environmental implications. Environ Microbiol Rep. 2020;12(1):3–15.
- Egelkamp R, Schneider D, Hertel R, et al. Nitrile-degrading bacteria isolated from compost. Front Environ Sci. 2017;5:56.
- Zhang Y, Qi S, Xing X, et al. Legacies of organochlorine pesticides (OCPs) in soil of China—a review, and cases in Southwest and Southeast China. In: Benedetto De Vivo H, editor. Environmental geochemistry: site characterization, data analysis and case histories. Amsterdam, Netherlands: Elsevier; 2018. p. 543–565.
- Yang -Y-Y, Toor GS, Williams CF. Pharmaceuticals and organochlorine pesticides in sediments of an urban river in Florida, USA. J Soils Sediments. 2015;15(4):993–1004.
- Ogbeide O, Tongo I, Ezemonye L. Assessing the distribution and human health risk of organochlorine pesticide residues in sediments from selected rivers. Chemosphere. 2016;144:1319–1326.
- Egbe CC, Oyetibo GO, Ilori MO. Ecological impact of organochlorine pesticides consortium on autochthonous microbial community in agricultural soil. Ecotox Environ Safe. 2021;207:111319.
- Kumar D, Pannu R. Perspectives of lindane (γ-hexachlorocyclohexane) biodegradation from the environment: a review. Bioresour Bioprocess. 2018;5(1):1–18.
- Wang B, Liu W, Liu X, et al. Comparative analysis of microbial communities during enrichment and isolation of DDT-degrading bacteria by culture-dependent and -independent methods. Sci Total Environ. 2017;590-591:297–303.
- Seralathan MV, Sivanesan S, Nargunanathan S, et al. Chemotaxis-based endosulfan biotransformation: enrichment and isolation of endosulfan-degrading bacteria. Environ Technol. 2015;36(1):60–67.
- Hussain S, Siddique T, Arshad M, et al. Bioremediation and phytoremediation of pesticides: recent advances. Crit Rev Environ Sci Technol. 2009;39(10):843–907.
- Mulla SI, Ameen F, Talwar MP, et al. Bioremediation of industrial waste for environmental safety. Singapore: Springer; 2020. Organophosphate pesticides: impact on environment, toxicity, and their degradation; 265–290.
- Sinkkonen S, Paasivirta J. Degradation half-life times of PCDDs, PCDFs and PCBs for environmental fate modeling. Chemosphere. 2000;40(9–11):943–949.
- Wu J-H, Chen W-Y, Kuo H-C, et al. Redox fluctuations shape the soil microbiome in the hypoxic bioremediation of octachlorinated dibenzodioxin-and dibenzofuran-contaminated soil. Environ Pollut. 2019;248:506–515.
- Chen W-Y, Wu J-H, Lin -Y-Y, et al. Bioremediation potential of soil contaminated with highly substituted polychlorinated dibenzo-p-dioxins and dibenzofurans: microcosm study and microbial community analysis. J Hazard Mater. 2013;261:351–361.
- Saibu S, Adebusoye SA, Oyetibo GO. Aerobic bacterial transformation and biodegradation of dioxins: a review. Bioresour Bioprocess. 2020;7(1):1–21.
- Qian X, Shen G, Wang Z, et al. Co-composting of livestock manure with rice straw: characterization and establishment of maturity evaluation system. Waste Manage. 2014;34(2):530–535.
- Bai L, Deng Y, Li J, et al. Role of the proportion of cattle manure and biogas residue on the degradation of lignocellulose and humification during composting. Bioresour Technol. 2020;307:122941.
- Islam MS, Kasim S, Alam KM, et al. Changes in chemical properties of banana pseudostem, mushroom media waste, and chicken manure through the co-composting process. Sustainability. 2021;13(15):8458.
- Chai E, H’ng P, Peng S, et al. Compost feedstock characteristics and ratio modelling for organic waste materials co-composting in Malaysia. Environ Technol. 2013;34(20):2859–2866.
- Li J, Xing W, Bao H, et al. Impact of pine leaf biochar amendment on bacterial dynamics and correlation of environmental factors during pig manure composting. Bioresour Technol. 2019;293:122031.
- Awasthi MK, Duan Y, Liu T, et al. Relevance of biochar to influence the bacterial succession during pig manure composting. Bioresour Technol. 2020;304:122962.
- Rihani M, Malamis D, Bihaoui B, et al. In-vessel treatment of urban primary sludge by aerobic composting. Bioresour Technol. 2010;101(15):5988–5995.
- Tabrika I, Azim K, Zaafrani M. Composting of tomato plant residues: improvement of composting process and compost quality by integration of sheep manure. Org Agric. 2020;10(2):229–242.
- Jain MS, Paul S, Kalamdhad AS. Interplay of physical and chemical properties during in-vessel degradation of sewage sludge. Waste Manage. 2019;98:58–68.
- Lim -S-S, Park H-J, Hao X, et al. Nitrogen, carbon, and dry matter losses during composting of livestock manure with two bulking agents as affected by co-amendments of phosphogypsum and zeolite. Ecol Eng. 2017;102:280–290.
- Zhou Y, Awasthi SK, Liu T, et al. Patterns of heavy metal resistant bacterial community succession influenced by biochar amendment during poultry manure composting. J Hazard Mater. 2021;420:126562.
- Awasthi MK, Wang M, Pandey A, et al. Heterogeneity of zeolite combined with biochar properties as a function of sewage sludge composting and production of nutrient-rich compost. Waste Manage. 2017;68:760–773.
- Yousefi K, Mohebbi A, Pichtel J. Biodegradation of weathered petroleum hydrocarbons using organic waste amendments. Appl Environ Soil Sci. 2021;2021:6620294.
- Soleimani M, Farhoudi M, Christensen JH. Chemometric assessment of enhanced bioremediation of oil contaminated soils. J Hazard Mater. 2013;254:372–381.
- Christopher JM, Sridharan R, Somasundaram S, et al. Bioremediation of aromatic hydrocarbons contaminated soil from industrial site using surface modified amino acid enhanced biosurfactant. Environ Pollut. 2021;289:117917.
- Escobar-Alvarado L, Vaca-Mier M, López R, et al. Hydrocarbon degradation and lead solubility in a soil polluted with lead and used motor oil treated by composting and phytoremediation. Bull Environ Contam Toxicol. 2018;100(2):280–285.
- Forján R, Lores I, Sierra C, et al. Bioaugmentation treatment of a PAH-polluted soil in a slurry bioreactor. Appl Sci. 2020;10(8):2837.
- Jun W, Zhang M-Y, Ting C, et al. Isolation and identification of a di-(2-ethylhexyl) phthalate-degrading bacterium and its role in the bioremediation of a contaminated soil. Pedosphere. 2015;25(2):202–211.
- Jariyal M, Jindal V, Mandal K, et al. Bioremediation of organophosphorus pesticide phorate in soil by microbial consortia. Ecotox Environ Safe. 2018;159:310–316.
- Wang B, Wang Q, Liu W, et al. Biosurfactant-producing microorganism Pseudomonas sp. SB assists the phytoremediation of DDT-contaminated soil by two grass species. Chemosphere. 2017;182:137–142.
- Singh P, Saini HS, Raj M. Rhamnolipid mediated enhanced degradation of chlorpyrifos by bacterial consortium in soil-water system. Ecotox Environ Safe. 2016;134:156–162.
- Horváthová H, Lászlová K, Dercová K. Bioremediation of PCB-contaminated shallow river sediments: the efficacy of biodegradation using individual bacterial strains and their consortia. Chemosphere. 2018;193:270–277.
- Delsarte I, Veignie E, Landkocz Y, et al. Bioremediation Performance of Two Telluric Saprotrophic Fungi, Penicillium Brasilianum and Fusarium Solani, in Aged Dioxin-contaminated Soil Microcosms. Soil Sediment Contam. 2021; 30:1–14.
- Kaewlaoyoong A, Cheng C-Y, Lin C, et al. White rot fungus Pleurotus pulmonarius enhanced bioremediation of highly PCDD/F-contaminated field soil via solid state fermentation. Sci Total Environ. 2020;738:139670.
- Kaewlaoyoong A, Chen J-R, Cheng C-Y, et al. Innovative mycoremediation technique for treating unsterilized PCDD/F-contaminated field soil and the exploration of chlorinated metabolites. Environ Pollut. 2021;289:117869.