ABSTRACT
The plateau is a special environment with low air pressure and low oxygen content. The average altitude of Qinghai-Tibet is 3,500 m, and the atmospheric oxygen partial pressure in most areas is lower than 60% of that at sea level. In order to adapt to the plateau low-oxygen environment, the human body has developed corresponding physiological structure and functions adjust. In the present review, the regulation mechanism of cerebral blood flow (CBF) under high-altitude environments was elaborated in eight aspects: the arterial blood gas, endogenous substances in the nerve and blood, the cerebral neovascularization, the hematocrit, cerebral auto-regulation mechanism, cerebrovascular reactivity, pulmonary vasoconstriction, and sympathetic automatic regulation, aiming to further explore the characteristics of changes in brain tissue and cerebral blood flow in a hypoxic environment.
Introduction
The brain tissue is extremely sensitive to the changes in oxygen demand and oxygen partial pressure. Under the condition of acute hypobaric hypoxia, the body can guarantee the brain oxygen supply and energy by strengthening the ventilation, accelerating the heart rate, increasing the blood pressure, and changing the arterial blood gas balance. Acute low pressure and hypoxia can stimulate the nervous and endocrine system may be stimulated and result in the release of cytokines, such as an endothelium-derived nitric oxide (NO), which leads to the contraction of blood vessels (including the cerebral vessels) in the whole body, thus accelerating the speed of cerebral blood flow (CBF) [Citation1]. With the gradual adaptation to the high-altitude environment, the cerebrovascular reactivity and the vasomotor function can gradually be restored, and the CBF may gradually reduce to the level close to the normal range [Citation2]. Although there exist differences in the CBF among individuals, the trend of CBF changes is basically the same after moving into the plateau from the plain [Citation3]. The regulation of CBF is complicated under the hypoxia environment at high altitudes, which include the regulations at the physiological, biochemical, and molecular levels.
It has been proven over years that the change of CBF is controlled by the tension of the vascular smooth muscle, which is affected by many factors such as the nerves, the body fluids, the metabolism, and the physics, thus forming the complex regulatory networks characterized by a number of vasoconstrictors and vasodilators released by the endothelial cells and nerve cells, such as NO [Citation4], the prostaglandins, natriuretic peptide,\and the endothelin-1 [Citation5]. The above endogenous substances are activated under different physiological stimulations and cause contraction or relaxation of the vascular smooth muscle by changing the concentration of the intracellular calcium and regulating the potassium channel. These physiological stimuli include the circulating substances such as PaCO2, PaO2, pH, lactate, glucose, adenosine [Citation6], and postganglionic neurotransmitters such as NO, acetylcholine, vasoactive peptide, calcitonin gene-related peptide, and noradrenaline.
The cerebrovascular response induced by the environmental hypoxia can be roughly divided into two parts: the acute hypoxia response (seconds to hours) and short-term to long-term hypoxia response (days to years). The time-dependent change of CBF has been confirmed in the study of early altitude acclimatization. Severinghause et al. first reported in 1966 that compared with CBF at sea level (CBF = 42 + −2 ml (100 g)−1min−1), CBF increased by 24% (51 + −4 ml (100 g)−1min−1) while engaged in 6–12 hours of abrupt highland advancing (3810 m) [Citation7]. However, the increase in CBF was only 13% 3–5 days later [Citation8]. The ventilatory acclimatization was consistent with the decrease of CBF. The above studies suggested that CBF would peak in 2–3 days after arriving at the plateau, and then return to near what it would be at sea level within 1–3 weeks. The adjustment of CBF is also affected by many factors, which are briefly described as follows:
The effects of arterial blood gas
Hypoxia can cause cerebral vasodilation and hypocapnic cerebral vasoconstriction. The hypobaric and hypoxic environment at high altitude may lead to a decrease of arterial PO2. The individual can reverse the tissue hypoxia by improving the ventilation function. The individual with significantly improved ventilation function may increase the arterial PO2 and decrease the PCO2. Lucas et al. reported that the changes in the arterial blood gas greatly affected the changes of CBF at high altitude (~40%). Hypoxia can cause cerebral vasodilation and hypocapnic cerebrovascular constriction. At the same time, some studies have found that cerebral vasoconstrictor-diastolic response to hypoxemia and hypocapnia is different in different stages of the altitude adaptation. The vasoconstrictor-diastolic response caused by the changes of PCO2 may affect the sensitivity of ventilation [Citation2]. The increase in blood pressure caused by hypoxia and brain auto-regulation may also affect the increase of the initial CBF. In conclusion, under the high-altitude environment, the individuals with a high ventilatory response may manifest as an increased PaO2, decreased PaCO2, and a significantly increased CBF. Individuals with inactive ventilatory responses have significantly reduced CBF [Citation9].
It had also been found that although the CBF was reduced after the initial 3–5 days of adaptation to the high-altitude environment, the brain tissue had sufficient oxygen supply [Citation10]. These indicated that the change of CBF was not the only factor to maintain sufficient oxygen supply in the brain tissue in the long-term under the hypoxia environment at high altitude, and other more complex and precise mechanisms might be involved.
It was proposed by Severinghouse in 2001 that the disorder of the acid-base balance of cerebrospinal fluid (CSF) might be another important factor for the decrease of CBF during the process of adaptation to hypoxia at high altitude. But until now, there has been no study concerning the relationship between the change of CSF index and the change of CBF. It should not be ignored that the CSF is an important body fluid which can directly reflect the brain metabolism. Therefore, the study and analysis of CSF in the high-altitude adaptive and non-adaptive population will provide more direct data supporting the study of the adaptation and injury mechanism of brain tissue under the hypoxia environment at high altitudes in the future.
The effects of the endogenous substances in the nerve and blood
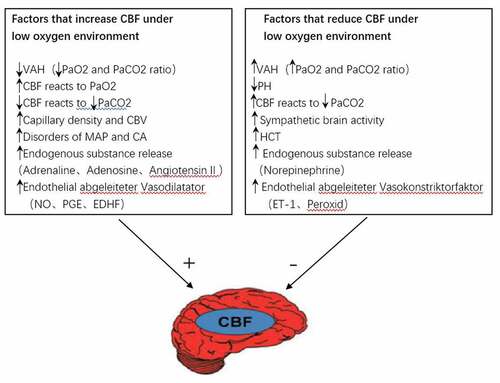
The regulation of CBF caused by hypoxia and the cytokines, endocrine substances, signal substances, proteins and blood oxygen concentration induced by hypoxia constitute a complex regulatory network. Golanov et al. suggested that the increase of CBF was closely correlated with the hypoxia induction initiated and passed by the neural response network and the blood oxygen concentration of the brain stem [Citation11]. There are also many hypoxia-induced cytokines, endocrine substances, signaling substances, and proteins () involved in the formation of a complex regulatory network, see for details. However, due to the particular nature of brain tissue, the molecular mechanism of CBF regulation remains unclear. Current studies have found that the peripheral blood transcriptome can largely reflect brain nerve activity [Citation12]. Therefore, with further study of the transcriptome related to the metabolic ion channels of the peripheral blood and brain, it is possible to reveal the molecular mechanism of cerebral blood flow regulation.
Figure 1. Factors influencing the changes of CBF in a hypoxic environment.
The effects of neovascularization in the brain
Exposure to low oxygen environment causes new capillaries to appear in the brain. In mammalian studies, it was found that after exposure to hypoxia for 1–3 weeks, the density of cerebral capillaries doubled, indicating that neovascularization was a very important regulatory mechanism in adapting to hypoxia [Citation13]. Hypoxia-induced angiogenesis might increase CBF [Citation14]. Despite the lack of human experimental data in this area, it is confirmed that the hypoxia-induced increase of CBF is closely correlated with an increase in capillary density [Citation15].
The effects of hematocrit
High altitude low pressure and low oxygen environment can cause the body’s blood hemoglobin (Hb) and hematocrit (Hct) to increase, resulting in a decrease in cerebral blood flow. The hypobaric and hypoxic environment at high altitudes may lead to the gradual increase of blood Hb and Hct [Citation16]. The interaction mechanism between the CBF and red blood cells may be related to the following reasons: The increase in RBC will lead to the increase of the blood concentration, viscosity, aggregation, and plasma viscosity, together with the changes of viscous friction, which may result in the increased resistance of cerebral vessels [Citation16], and the slowing down in blood flow speed, causing the cerebral blood flow to drop.
The influence of the CA mechanism
Cerebral auto-regulation (CA) is an important mechanism of cerebral blood flow regulation. CA mainly regulates the diameter of the cerebral artery to ensure that CBF can fully supply the metabolic needs of the brain tissue, which is relatively unaffected by the peripheral blood pressure. So far, it can be affirmed that this mechanism is a complex regulatory network that is involved in the myogenic regulation, metabolism-related regulation, and neurogenic regulation at the same time. The injury in the CA was found in individuals who moved to the plateau, lived at an altitude of more than 4000 meters, and suffered from acute mountain sickness [Citation17–20]. The injury of CA may lead to over perfusion and angioedema, which will result in the damage of the blood-brain barrier [Citation21]. Under the condition of CA injury, the increase of blood pressure will lead to an increase of pressure-dependent CBF. It is very important to clarify the regulatory mechanism of CA for screening the susceptible population of acute mountain sickness.
The influence of the cerebrovascular reactivity
Cerebral vascular reactivity (CVR) changes significantly under high altitude and hypoxic conditions, and is an important factor in the regulation of cerebral blood flow. The cerebrovascular reactivity (CVR) refers to the ability of the cerebral vessels to relax or contract under the influence of various relaxing or contracting factors. Some studies have shown that the response of CBF to hypercapnia is weakened or even disappeared at high altitude, while the response to hypocapnia is abnormally increased [Citation22–25]. It has also been reported that compared with that of the healthy population at the same altitude, the response of CBF to hypocapnia was increased, and the response to hypercapnia was decreased significantly [Citation26]. It might be concluded that the degree of the changes in CBF is correlated with the intensity of vasodilation caused by hypoxia and vasoconstriction caused by hypocapnia. Therefore, CVR has a great influence on CBF under the hypoxia environment at high altitudes.
The influence of the pulmonary vasoconstriction
The change of pulmonary vascular tension will affect the function of cerebral vasoconstriction and vasodilatation under the condition of hypoxia at high altitudes, thereby affecting cerebral blood flow. Hypoxia may lead to pulmonary vasoconstriction, increased pulmonary tissue system pressure [Citation27], and increased pulmonary vascular resistance, which may result in cardiac dysfunction, such as increased ventricular and atrial pressure at the end of the diastolic period of the right ventricle, and eventually lead to reducing the left atrial filling [Citation27]. At the same time, the increased right atrial pressure may result in venous return obstruction, cerebral venous return obstruction, and brain edema [Citation28], which reduces cerebral blood flow. Pulmonary vasoconstriction may affect the left ventricular filling, resulting in left ventricular dysfunction and reduced cardiac output. It is found that there is a positive correlation between the change of cardiac output and CBF [Citation29]. Thus, the pulmonary vasoconstriction and the impairment of cardiac function will change the function of the cerebral vasoconstriction and relaxation at high altitude, and affect the regulation of CBF.
The influence of the sympathetic auto-regulation
The regulation of sympathetic nerves in cerebral blood vessels may protect the blood-brain barrier and reduce the possibility of cerebral edema. The function of the sympathetic nerve in vascular regulation is mainly to promote the contraction of arterioles, but it is relatively weak in the central nervous system. Some studies have found an increased sympathetic activity at high altitude [Citation30]. In the study of animals and human beings, it is suggested that when the CA regulation is out of control, the sympathetic nerve in cerebral circulation may regulate the vasoconstriction and has a protective effect on the blood-brain barrier [Citation29]. This indicates that the regulation of the sympathetic nerve may protect the blood-brain barrier and possibly reduce the occurrence of brain edema in the early stage of mountain sickness. However, there are many disagreements concerning the role of sympathetic activity in the CBF regulation. The enhancement of the sympathetic activity in the resting state has a great limitation on the regulation of CBF [Citation31]. The regulation of the sympathetic activity may be covered by other more direct and obvious factors. At present, the regulation of the sympathetic nerve on CBF at high altitude is still lacking experimental data that can explain its effect.
Limitations
The regulation mechanism of CBF is complex, and there are many intervention factors under the hypobaric hypoxic condition at high altitudes. In recent years, the research concerning CBF is mainly focused on the small sample study of the people who moved into the plateau and the people who lived in the plateau for a long time [Citation1]. This research included the study of simulated hypoxia environments, hypobaric hypoxic environments, and field research at high altitude areas. The duration of the hypoxic stimulation varied from several minutes to several weeks [Citation10,Citation32,Citation33]. At the same time, the CBF evaluation methods and the types of hypoxia exposure were inconsistent, and the sample size was relatively small. The discrepancy of the results might be caused by the above reasons [Citation1,Citation34].
Conclusion
To sum up, in the high altitude hypoxic environment, the body passes through the decrease of arterial oxygen content and the increase of arterial CO2, the increase of capillary density and cerebral blood volume, the increase of central arterial pressure, and endogenous substances such as epinephrine and adenosine, Angiotensin II, etc. The release of vasodilator factors such as nitric oxide, prostaglandin, endothelium-dependent hyperpolarizing factor, etc. can relax the cerebral blood vessels, thereby increasing cerebral blood flow. On the contrary, the increase in arterial oxygen content and the decrease of arterial CO2, the decrease of blood PH, the increase of cerebral sympathetic nerve excitability, the increase of hematocrit, the release of norepinephrine, and the vasoconstriction factors such as endothelin-1 and peroxide can contract cerebral blood vessels and reduce cerebral blood flow, thereby achieving regulation of cerebral blood flow.
In view of the limitations of previous studies, we look forward to more research on cerebral blood flow in high altitude hypoxic environment in the future, such as research on altitude hypoxic adaptation in a natural high altitude experimental environment. So as to explore the regulatory characteristics of the brain tissue and blood flow under the hypoxic environment at high altitude, explore the regulatory mechanism from the molecular level to the physiological level, and provide a theory for disease prevention of high-altitude practitioners together with the prevention and treatment of chronic mountain sickness in the future.
Highlights
This paper summarizes the regulatory mechanisms of cerebral blood flow in the special environment of low pressure and low oxygen at plateau.
It is found that the human body adapts to the low oxygen environment of the plateau, and the corresponding habitual regulation occurs.
The adjustment of cerebral blood flow is affected by many factors.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wu S, Hao G, Zhang S, et al. Cerebral vasoconstriction reactions and plasma levels of ETBR, ET-1, and eNOS in patients with chronic high altitude disease. Mol Med Rep. 2016 Sep;14(3):2497–2502. Epub 2016 Jul 27. PMID: 27485004; PMCID: PMC4991730.
- Lucas SJE, Burgess KR, Thomas KN, et al. Alterations in cerebral blood flow and cerebrovascular reactivity during 14 days at 5050 m. J Physiol. 2011;589:741–753.
- Hoiland RL, Howe CA, Coombs GB, et al. Ventilatory and cerebrovascular regulation and integration at high-altitude. Clin Auton Res. 2018 Aug;28(4):423–435. Epub 2018 Mar 24. PMID: 29574504.
- Otis SM, Rossman ME, Schneider PA, et al. Regulation of cerebral blood flow in mammals during chronic hypoxia: a matter of balance. Exp Physiol. 2010;95:251–262. Available at: http://ep.physoc.org/cgi/doi/10.1113/expphysiol.2008.045575
- Bailey DM, Taudorf S, Berg RMG, et al. Transcerebral exchange kinetics of nitrite and calcitonin gene-related peptide in acute mountain sickness: evidence against trigeminovascular activation? Stroke. 2009;40:2205–2208.
- Henze D, Menzel M, Soukup J, et al. Endothelin-1 and cerebral blood flow in a porcine model. J Clin Neurosci. 2007;14:8.
- Edvinsson L, Krause DN. Cerebral blood flow and metabolism. 2002.
- Severinghaus JW, Chiodi H, Eger EI, et al. Cerebral blood flow in man at high altitude. Role of cerebrospinal fluid pH in normalization of flow in chronic hypocapnia. Circ Res. 1966;19:274–282.
- Milledge JS, Sorensen SC. Cerebral arteriovenous oxygen difference in man native to high altitude. J Appl Physiol. 1972;32:687–689.
- Smith ZM, Krizay E, Guo J, et al. Sustained high-altitude hypoxia increases cerebral oxygen metabolism. J Appl Physiol. 2012;114:11–18.
- Lafave HC, Zouboules SM, James MA, et al. Steady-state cerebral blood flow regulation at altitude: interaction between oxygen and carbon dioxide. Eur J Appl Physiol. 2019 Dec;119(11–12):2529–2544. Epub 2019 Sep 26. PMID: 31559499.
- Golanov EVE, Christensen JRJ, Reis DJD. Neurons of a limited subthalamic area mediate elevations in cortical cerebral blood flow evoked by hypoxia and excitation of neurons of the rostral ventrolateral medulla. Trans IRE Prof Group Audio. 2001;21:4032–4041.
- Sarkar S, Chakraborty D, Bhowmik A, et al. Cerebral ischemic stroke: cellular fate and therapeutic opportunities. Front Biosci (Landmark Ed). 2019;24:435–450.
- Ren C, Yao Y, Han R, et al. Cerebral ischemia induces angiogenesis in the peri-infarct regions via Notch1 signaling activation. Exp Neurol. 2018 Jun;304:30–40.
- Halder SK, Milner R. The impact of chronic mild hypoxia on cerebrovascular remodelling; uncoupling of angiogenesis and vascular breakdown. Fluids Barriers CNS. 2021;18(1):50.
- Zhang H, Rzechorzek W, Aghajanian A, et al. Hypoxia induces de novo formation of cerebral collaterals and lessens the severity of ischemic stroke. J Cereb Blood Flow Metab. 2020;40(9):1806–1822.
- Howe CA, Ainslie PN, Tremblay JC, et al. UBC-Nepal expedition: haemoconcentration underlies the reductions in cerebral blood flow observed during acclimatization to high altitude. Exp Physiol. 2019 Dec;104(12):1963–1972.
- Levine BD, Zhang R, Roach RC. Dynamic cerebral autoregulation at high altitude. Adv Exp Med Biol. 1999;474:319–322.
- Jansen GFA, Krins A, Basnyat B, et al. Cerebral autoregulation in subjects adapted and not adapted to high altitude. Stroke. 2000;31:2314–2318.
- Jansen GFA, Krins A, Basnyat B, et al. Role of the altitude level on cerebral autoregulation in residents at high altitude. J Appl Physiol. 2007;103:518–523.
- Curtelin D, Morales-Alamo D, Torres-Peralta R, et al. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J Cereb Blood Flow Metab. 2018 Jan;38(1):136–150.
- Kunze R, Marti HH. Angioneurins - key regulators of blood-brain barrier integrity during hypoxic and ischemic brain injury. Prog Neurobiol. 2019 Jul;178:101611.
- Fan JL, Subudhi AW, Duffin J, et al. AltitudeOmics: resetting of cerebrovascular CO2 reactivity following acclimatization to high altitude. Front Physiol. 2016 Jan 8;6:394.
- Lafave HC, Zouboules SM, James MA, et al. Steady-state cerebral blood flow regulation at altitude: interaction between oxygen and carbon dioxide. Eur J Appl Physiol. 2019;119(11–12):2529–2544.
- Querido JS, Jordan S, Philip N, et al. Dynamic cerebral autoregulation during and following acute hypoxia: role of carbon dioxide. J Appl Physiol. 2013;114:1183–1190.
- Hoiland RL, Howe CA, Carter HH, et al. UBC-Nepal expedition: phenotypical evidence for evolutionary adaptation in the control of cerebral blood flow and oxygen delivery at high altitude. J Physiol. 2019 Jun;597(12):2993–3008.
- Liu C, Chen X, Guo G, et al. Effects of intermittent normoxia on chronic hypoxic pulmonary hypertension and right ventricular hypertrophy in rats. High Alt Med Biol. 2021 Jun;22(2):184–192.
- Wang N, Shen X, Zhang G, et al. Cerebrovascular disease in pregnancy and puerperium: perspectives from neuroradiologists. Quant Imaging Med Surg. 2021 Feb;11(2):838–851.
- Ogoh SS, Brothers RM, Barnes Q, et al. The effect of changes in cardiac output on middle cerebral artery mean blood velocity at rest and during exercise. J Physiol. 2005;569:697–704.
- Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–929.
- Ainslie PNP, Duffin JJ. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1473–R1495.
- Teppema LJL, Balanos GM, Steinback CD, et al. Effects of acetazolamide on ventilatory, cerebrovascular, and pulmonary vascular responses to hypoxia. Am J Respir Crit Care Med. 2007;175:277–281.
- Chan CWMC, Hoar H, Pattinson K, et al. Effect of sildenafil and acclimatization on cerebral oxygenation at altitude. Clin Sci (Lond). 2005;109:319–324.
- Claydon VE, Gulli G, Slessarev M, et al. Cerebrovascular responses to hypoxia and hypocapnia in Ethiopian high altitude dwellers. Stroke. 2008;39:336–342.
