ABSTRACT
Long-chain non-coding RNAs are reported to be involved in cartilage damage. However, less research on the role of actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1) in osteoarthritis. To investigate AFAP1-AS1 function in osteoarthritis development, AFAP1-AS1 and miR-512-3p expression levels in osteoarthritis cartilage and cells were evaluated using RT-qPCR. The downstream target genes of AFAP1-AS1 and miR-512-3p were predicted and validated using luciferase reporter assays. Moreover, a knee osteoarthritis model was established by injecting monoiodoacetate into the knee joints of mice. The effects of AFAP1-AS1 and miR-512-3p on osteoarthritis chondrocyte proliferation and MMP-13, collagen II, and collagen IV expressions were detected in vivo using CCK-8 assay and Western blotting and RT-qPCR, respectively. AFAP1-AS1 expression was upregulated in osteoarthritis cartilage and cells. MiR-512-3p expression was downregulated in osteoarthritis cartilage. AFAP1-AS1 overexpression inhibited miR-512-3p expression in chondrocytes. Furthermore, AFAP1-AS1 over-expression promoted chondrocyte proliferation, and miR-512-3p mimic inhibited chondrocyte proliferation in vivo. AFAP1-AS1 overexpression reduced type II and type IV collagen expression, while miR-512-3p overexpression promoted type II and type IV collagen in vivo. AFAP1-AS1 overexpression enhanced MMP-13 expression in vivo. AFAP1-AS1 overexpression regulated chondrocyte proliferation by inhibiting miR-512-3p expression in vivo. AFAP1-AS1 could be a potential target to treat osteoarthritis by inhibiting miR-512-3p and subsequently inducing chondrocyte proliferation and regulating matrix synthesis.
Introduction
Osteoarthritis is a chronic degenerative musculoskeletal disease characterized by dysfunction of joints and chronic pain [Citation1,Citation2]. It seriously affects patients’ quality of life and imposes severe burdens on patients’ families and society. Osteoarthritis is caused by multiple factors, including genetic factors, metabolic factors, biochemical factors, and their combined effects, leading to unbalanced synthesis and degradation of chondrocytes, subchondral bone, and extracellular matrix and abnormal joint metabolism, which in turn causes articular cartilage degeneration. With the continuous damage of chronic inflammation and gradual structural changes of joint tissues, the condition continues to progress, further causing synovial joint damages, including articular cartilage damage, meniscus damage, ligament laxity, osteophyte formation, subchondral bone damage, irreversible loss of joint function, and pain [Citation3,Citation4]. Many methods have been used to treat osteoarthritis. Early-stage patients are often treated with conservative treatment with limited efficacy. It can only relieve the pain symptoms and improve joint function in a short period of time and cannot prevent the pathological process of osteoarthritis, let alone regenerate the damaged articular cartilage [Citation5]. Surgical treatment is used to treat patients with severe joint degeneration and poor outcomes after conservative treatment, but the curative effect is limited [Citation6]. Although the curative effect improves with prosthesis design and surgical techniques advancing, surgical treatment still faces the risk of revision even multiple revision surgeries due to complications such as aseptic prosthesis loosening, infection, and periprosthetic fracture [Citation7,Citation8]. Therefore, studying the specific pathogenesis of osteoarthritis and finding its targets are urgent problems to be solved.
Research on long non-coding RNAs (lncRNAs) has drawn more attention [Citation9,Citation10]. Their abnormal expression is associated with genetic diseases, tumors, and metabolic diseases [Citation11] by epigenetic, transcriptional, and post-transcriptional regulation of related genes [Citation12,Citation13]. LncRNAs can regulate their associated protein-coding genes, and improper expression of lncRNAs may lead to diseases. Studies have found lncRNAs play important roles in osteoarthritis [Citation14,Citation15]. Among them, lncRNA AFAP1-AS1 is abnormally overexpressed in tumors [Citation16,Citation17]. Whether and how AFAP1-AS1 participates in osteoarthritis occurrence and development is still unclear.
Studies have found that lncRNAs may regulate downstream target genes [Citation18] via sponging target miRNAs, exhibiting developmental stage or tissue-specific specificity. Besides orthopedic diseases, viral infections, lymphomas, and tumors [Citation19,Citation20], miRNAs are related to osteochondral catabolism and synthesis [Citation21] by regulating single or multiple target genes in chondrocytes, thereby affecting their metabolism, proliferation, and differentiation and regulating cartilage extracellular matrix (ECM) synthesis and metabolism [Citation22]. MiR-512-3p is involved in tumor metastasis, cell programmed death, and other life activities [Citation23,Citation24], but its role in osteoarthritis remains unclear.
The function of matrix metalloproteinases (MMPs) (mainly MMP-13) is mainly to degrade type I, II, and III collagen and proteoglycans. MMPs overexpression can promote the destruction of articular cartilage during osteoarthritis [Citation25]. Therefore, we hypothesized that lncRNA AFAP1-AS1 might participate in osteoarthritis via regulating miR-512-3p/MMP-13 and be a new target for osteoarthritis treatment.
Methods and materials
Tissue samples
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Bengbu Medical College and was in line with the Helsinki Declaration. The patient’s diagnosis was in accordance with the standards of the American College of Rheumatology. A written informed consent form was signed by all patients.
Osteoarthritis was diagnosed if patients met the following ①+②+③ or ①+②+④+⑥+⑦ or ①+②+⑤+⑥+⑦ criteria: ① Meet the diagnostic criteria of Chinese and Western medicine; ② Recurring knee joint pain in the past 1 month; ③ X-rays showed joint space narrowing, subchondral bone sclerosis or cystic degeneration, and osteophyte formation at the joint edges; ④ Joints fluid (at least 2 times) is clear and viscous and WBC < 2000/mL; ⑤ at the age of 40–70 years; ⑥ Morning stiffness ≤30 min; and ⑦ Bone friction sound during activities. Patients were excluded if they did not meet the above-mentioned knee joint inclusion criteria and had major systemic diseases, such as diabetes, heart disease, infectious diseases, mental illness, and secondary knee joint osteoarthritis.
Knee cartilage samples were collected from 12 healthy subjects and 12 osteoarthritis patients who underwent knee replacement surgery. According to the Kellgren/Lawrence criteria (KL), these samples were divided into 1) KL grade O normal cartilage, 2) KL grade I and II mild osteoarthritis cartilage, and 3) KL grade III and IV moderate/severe advanced osteoarthritis cartilage.
Cell culture and transfection
Human chondrocyte cell line C28/I2 was obtained from ATCC and cultured in DMEM with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 0.1 mg/mL streptomycin, 0.25 µg/mL amphotericin-B, and 50 µg/mL ascorbic acid (Sigma‐Aldrich). MiR-512-3p mimic and scramble and AFAP1-AS1 and control vectors were from RiboBio. Transient transfection was performed using Lipofectamine 3000 Plus Reagent (Thermo Fisher Scientific). At 48 h of post-transfection, cells were harvested and subjected to subsequent analyses.
Preparation of knee osteoarthritis model mice
Knee osteoarthritis model mice were constructed using monoiodoacetate. In brief, mice were anesthetized by intraperitoneally injecting 3.0% sodium pentobarbital at a dose of 1.0 mL/kg. Under anesthesia, 40 mg/L monoiodoacetate was injected into the joint cavity of the right knee. At 2 weeks after injection, knee arthritis model mice were judged using the arthritis index scoring method: 0 points for normal joints; 1–5 points for mild swelling or red spots at joints; 6–10 points for joints showing general redness and swelling; 11–15 points for massive redness and swelling; extremely severe redness, and swelling on the joints, and 16–20 points for not being able to bear weight. In this study, mice with an arthritis point of 11–15 were selected for follow-up experiments.
Quantitative real-time-PCR (qRT-PCR)
Total RNAs were extracted from cartilage tissue and cells using TRIzol reagent. Approximately 1.2 μg RNAs were reversely transcribed into cDNA using the Prime Script RT Master Mix (TaKaRa, Japan) and then subject to qRT-PCR using SYBR Premix Ex Taq II kit (Takara, Otsu, Japan) on a Quantstudio™ DX system (Applied Biosystems, Singapore) at conditions of 5 min at 95°C followed by 40 cycles of 95°C for 30s and 65°C for 45s. GAPDH and U6 were used as the internal references [Citation26]. Gene expression levels were normalized using the 2−ΔΔCt method. The primers were 5′-TGCTTCCTGATGACGATGTAC-3′ and 5’-TCCTCGGAGACTGGTAATGG-3′ for MMP-13, 5′-CTTTGGGTGCGACTTGACG-3′ and 5′-GTCGACCCCGCTCCTTTT-3′ for Ki-67, 5′-AATGGTGGTAGGAGGGAGG-3′ and 5′-CACACAGGGGAATGAAGAGG-3′ for AFAP1-AS1, 5′-CACTCAGGCCTTGAGGGCACTTTC-3′ and 5′- AAGTGCTGTCATAGCTGAGGTC-3′ for MiR-512-3p, and 5′-GACTAATGACAACAGTCCATGC-3′ and 5′-AGAGGCAGGGATGATGGTCGG-3′ for GAPDH.
Western blot analysis
After cells were transfected and collected, total cellular proteins were extracted using RIPA Lysis Buffer (Beyotime, Beijing, China) containing 1.0 mmol/L PMSF, 0.1 mmol/L leupeptin, and 1.0 μg/mL aprotinin according to the manufacturer’s instructions. Protein concentration was quantified using BCA Protein Assay Kit. Approximately equal amounts of total proteins were separated by 12% SDS-PAGE and transferred onto PVDF membranes. After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies against MMP-13 and GAPDH (1:1000, Sigma). After being washed in TBST, the membranes were incubated with HRP-conjugated secondary antibodies. Protein signals were visualized using ECL (enhanced chemiluminescence) [Citation27].
Cell proliferation assay
Transfected cells were resuspended as 2 × 105 cells/mL solutions and seeded into 96-well plates. Cell proliferation was measured using Cell Counting Kit-8, and the absorbance values at 450 nm were determined with a microplate reader.
Luciferase reporter assay
AFAP1-AS1 or MMP-13 3ʹUTR (WT/Mut) or mutated AFAP1-AS1 luciferase vectors were constructed by cloning the amplified DNA sequences into pmiR-RB-REPORTTM vector. MiR-512-3p mimics and AFAP1-AS1 or MMP-13 3ʹUTR (WT) or mutant AFAP1-AS1 or MMP-133ʹUTR (Mut) were co-transfected into cells approximately 1 × 105 cells seeded in 24-well plates using Lipofectamine 2000. At 48 h of post-transfection, luciferase activity was measured using a dual luciferase assay system (Promega) [Citation25].
Statistical analyses
Data analyses were expressed as mean ± standard deviation (SD) and analyzed using SPSS19.0 software. Differences among multiple groups were analyzed using one-way ANOVA and LSD test. P < 0.05 was considered statistically significant.
Results
AFAP1-AS1 expression was upregulated in osteoarthritis cartilage and cells, while MiR-512-3p expression was downregulated in osteoarthritis cartilage. AFAP1-AS1 overexpression inhibited miR-512-3p expression in chondrocytes and promoted chondrocyte proliferation. MiR-512-3p mimic inhibited chondrocyte proliferation in vivo; AFAP1-AS1 overexpression reduced type II and type IV collagen levels, while miR-512-3p overexpression promoted type II and type IV collagen in vivo; AFAP1-AS1 overexpression enhanced MMP-13 expression in vivo; AFAP1-AS1 overexpression regulated chondrocyte proliferation by inhibiting miR-512-3p expression in vivo.
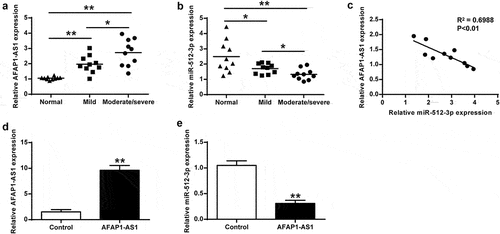
AFAP1-AS1 and miR-512-3p expression in osteoarthritis cartilage
Compared with the normal cartilage and mild osteoarthritis cartilage, AFAP1-AS1 expression was significantly increased in moderate and severe osteoarthritis cartilage tissues (), p < 0.01), Moreover, miR-512-3p expression was significantly downregulated in moderate and severe osteoarthritis cartilage tissues (), p < 0.01). AFAP1-AS1 was negatively correlated with miR-512-3p expression in osteoarthritis tissues (P < 0.01) ()). Introduction of AFAP1-AS1 markedly enhanced AFAP1-AS1 expression compared with the corresponding negative control (P < 0.01) ()). Moreover, AFAP1-AS1 overexpression inhibited miR-512-3p expression in chondrocytes (P < 0.01) ()).
Figure 1. AFAP1-AS1 and miR-512-3p expression in osteoarthritis cartilage. (a) AFAP1-AS1 expression in normal cartilage and osteoarthritis cartilage. (b) MiR-512-3p expression in osteoarthritis cartilage and normal cartilage. (c) MiR-512-3p correlated with AFAP1-AS1 expression in osteoarthritis cartilage. (d) AFAP1-AS1 expression was measured in chondrocytes after treated with pcDNA-AFAP1-AS1. (e) AFAP1-AS1 overexpression inhibited miR-512-3p expression in chondrocytes. * P < 0.05, ** P < 0.01.
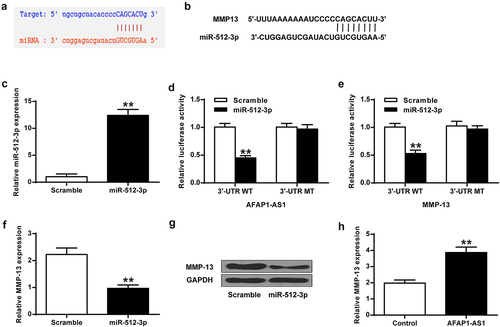
MMP-13 expression was suppressed by miR-512-3p in chondrocytes
The potential binding sites of miR-512-3p on the 3’-UTR of AFAP1-AS1 and MMP-13 were predicted using Starbase 2.0 software (http://starbase. sysu.edu.cn/starbase2/index.php) and TargetScan (www.targetscan.org/vert_71/) database (). The results revealed putative binding sites of miR-512-3p to MMP-13 and AFAP1-AS1, suggesting that MMP-13 and AFAP1-AS1 might be a target of miR-512-3p. MiR-512-3p mimics transfection indeed elevated miR-512-3p expression in chondrocytes ()). Furthermore, miR-512-3p mimics transfection decreased the luciferase activity of AFAP1-AS1-WT and MMP-13-WT, but not AFAP1-AS1-mut and MMP-13-mut ()), indicating that AFAP1-AS1 and MMP-13 were direct targets of miR-512-3p. In addition, miR-512-3p overexpression inhibited MMP-13 expression in chondrocytes ( < 0.01), while AFAP1-AS1 overexpression promoted MMP-13 mRNA expression (P < 0.01) ()).
Figure 2. MiR-512-3p inhibited MMP-13 expression in chondrocytes. (a) MiR-512-3p binds to the 3’ UTR of AFAP1-AS1. (b) MiR-512-3p binds to the 3ʹUTR of MMP-13. (c) MiR512-3p expression in chondrocytes. (d) Luciferase activity of AFAP1-AS1 3’-UTR and miR-512-3p. (e) Luciferase activity of miR-512-3p and MMP-13 3’-UTR. (f, g) MMP-13 expression at mRNA and protein levels. (h) AFAP1-AS1 overexpression improved MMP-13 mRNA level. ** P < 0.01.
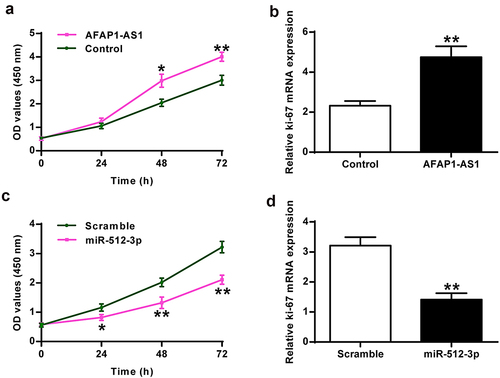
Roles of AFAP1-AS1 and miR-512-3p in chondrocyte cell proliferation
Compared to the control group, AFAP1-AS1 overexpression enhanced chondrocyte proliferation (P <0.01) in vivo ()) and promoted ki-67 expression in chondrocytes (P < 0.01) in vivo ()). In addition, miR-512-3p overexpression inhibited chondrocyte proliferation (P < 0.01) ()) in vivo and ki-67 expression in chondrocytes in vivo (P < 0.01) ()).
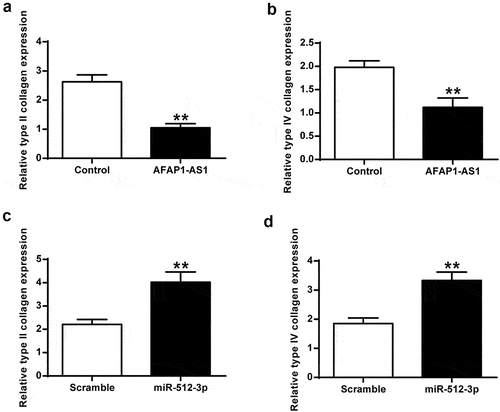
AFAP1-AS1 overexpression decreased type II collagen and type IV collagen expression
As shown in ) and 4B, AFAP1-AS1 overexpression significantly inhibited type II and type IV collagen expression in chondrocytes (P < 0.01) in vivo. Furthermore, miR-512-3p overexpression increased type II collagen and type IV collagen expression (), p < 0.01) in vivo.
Figure 4. AFAP1-AS1 overexpression inhibited type II and type IV collagen expression. (a) AFAP1-AS1 overexpression inhibited type II collagen expression. (b) AFAP1-AS1 overexpression inhibited type IV collagen expression. (c) miR-512-3p overexpression promoted type II collagen expression. (d) miR-512-3p overexpression promoted type IV collagen expression. ** P < 0.01.
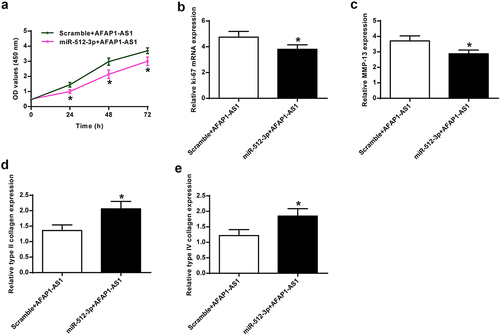
AFAP1-AS1 regulated collagen expression and chondrocyte cell proliferation through inhibiting miR-512-3p expression
To further investigate whether miR-512-3p mediated AFAP1-AS1 function, AFAP1-AS1 and miR-512-3p were co-transfected into chondrocytes. AFAP1-AS1 overexpression induced chondrocyte proliferation with prolonged duration of action, which was reversed by co-transfecting with miR-512-3p (), p < 0.01) in vivo. AFAP1-AS1 overexpression promoted ki-67 mRNA expression in chondrocytes, which was reversed by co-transfecting with miR-512-3p (P < 0.01) ()) in vivo. AFAP1-AS1 overexpression raised MMP-13 expression in chondrocytes, which was reversed by co-transfecting with miR-512-3p (P < 0.01). ()) in vivo. Furthermore, AFAP1-AS1 overexpression reduced type II and type IV collagen mRNA expression, which was reversed by co-transfecting with miR-512-3p mRNA (), p <0.01) in vivo.
Figure 5. AFAP1-AS1 regulated chondrocyte proliferation and collagen expression by inhibiting miR-512-3p. (a) Proliferation under different treatment conditions. (b) Ki-67 levels were detected under different treatment conditions. (c) MMP-13 mRNA levels were detected under different treatment conditions. (d) Type II collagen mRNA levels were detected under different treatment conditions. (e) Type IV collagen mRNA levels were detected under different treatment conditions. * P < 0.05.
Discussion
Osteoarthritis is one of the most common joint diseases [Citation28]. It mainly manifests as chronic and degenerative changes of bones and joints and later increased pain and dysfunction, affecting patients’ quality of life and leading to disability in severe cases. With the recent findings in enzymology, inflammatory cytokines, and cell apoptosis, our understanding of the degeneration mechanism of cartilage has changed [Citation29,Citation30]. Studies have shown that the main pathological process of osteoarthritis is mediated by inflammatory cytokines, pathogenic factors-promoted expression of various proteases in articular cartilage and synovium, decomposed collagen fiber network and proteoglycan in articular cartilage matrix, and increased degenerative changes of articular cartilage [Citation31]. Therefore, developing more effective osteoarthritis treatments has become the focus of molecular biology.
LncRNAs have been reported to sponge miRNA and regulate gene transcription [Citation32]. They participate in many pathological processes, cell differentiation, and apoptosis [Citation33]. With the development of genetic testing methods, more and more evidence indicates that lncRNAs can regulate the expression of their related protein-coding genes, and improper expression of lncRNAs may lead to diseases [Citation34]. Studies have found that lncRNA CIR plays an important regulatory role in osteoarthritis by promoting extracellular matrix degradation [Citation35]. LncRNA AFAP1-AS1 is associated with lung cancer. However, its biological role in osteoarthritis development and occurrence is still unclear [Citation36]. Our study found that AFAP1-AS1 expression was upregulated in osteoarthritis chondrocyte cells and tissues, and AFAP1-AS1 overexpression induced osteoarthritis chondrocyte cell proliferation. Studies have found that collagen metabolism is closely related to the pathological grade of articular cartilage degeneration during osteoarthritis. Collagen content is positively correlated with pathological osteoarthritis grade before pathological level 11 [Citation37]. In addition, studies have found that serum type II and IV collagen levels are higher in patients with osteoarthritis [Citation38]. Our study found that AFAP1-AS1 overexpression reduces type II and type IV collagen expression, indicating that AFAP1-AS1promotes the development of osteoarthritis.
MiRNAs play major roles in regulating gene expression in the development of diseases [Citation39]. MiR-9, miR-98, and miR-146 inhibit interleukin-1β in primary chondrocytes [Citation40]. MiR-512-3p expression is downregulated in breast cancer tissues than in normal breast tissues [Citation23]. Our experiment showed that miR-512-3p is a target of AFAP1-AS1, and its expression in osteoarthritis cartilage is reduced. Moreover, miR-512-3p overexpression inhibits osteoarthritis chondrocyte proliferation. Furthermore, AFAP1-AS1 overexpression inhibits miR-512-3p in chondrocytes. In osteoarthritis cartilage, miR-512-3p expression is negatively correlated with AFAP1-AS1, and co-transfection of AFAP1-AS1 with miR-512-3p reverses AFAP1-AS1-mediated chondrocyte proliferation. These results indicate AFAP1-AS1 may promote osteoarthritis via modulating miR-512-3p.
MMP-13 is an extracellular matrix degradation-related enzyme produced at the site of bone and joint [Citation41,Citation42]. It causes the collapse of cartilage scaffold structure, destroys the structural basis of chondrocytes, accelerates the destruction of extracellular matrix balance, and causes a vicious circle of inflammation and cartilage destruction [Citation43]. Consistent with this study, we found that miR-512-3p overexpression inhibits MMP-13, and AFAP1-AS1 overexpression increases MMP-13 expression in chondrocytes. Moreover, co-transfection of AFAP1-AS1 with miR-512-3p reverses AFAP1-AS1-mediated MMP-13 expression in chondrocytes.
Although this study identified changes in the expression of LncRNA at different stages of osteoarthritis progression, the fact that LncRNA can be developed as a biomarker and therapeutic target. However, research on the pathogenesis of cartilage formation, osteogenesis and osteoarthritis is still in its infancy, and more in-depth research is needed. Although it was determined that a large amount of LncRNA was differentially expressed during these treatments, only a small part of LncRNA was elucidated. A single LncRNA is unlikely to cause cartilage destruction or the clinical symptoms observed in synovial joints. Instead, a combination of LncRNA and their targets may be required. Therefore, it is very important to verify other LncRNAs involved in the development of osteoarthritis.
Conclusion
LncRNA AFAP1-AS1 is upregulated in osteoarthritis cartilage. AFAP1-AS1 overexpression suppresses miR-512-3p expression and enhances MMP-13 expression in chondrocytes. This study provides new insight into the function of lncRNAs in cartilage damage and the possibility of miR-512-3p/MMP-13 as the therapeutic target for osteoarthritis treatment.
Highlights
AFAP1-AS1 expression was upregulated in osteoarthritis cartilage and cells.
AFAP1-AS1 overexpression induces chondrocyte proliferation and regulates matrix synthesis via inhibiting miR-512-3p.
AFAP1-AS1 is a potential target for osteoarthritis treatment.
List of abbreviations
long-chain non-coding RNAs: lncRNAs
actin filament associated protein 1 antisense RNA 1: AFAP1-AS1
matrix metallopeptidase 13: MMP-13
Declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the First Affiliated Hospital of Bengbu Medical College (Approval No. 633,211) and performed in line with the Helsinki Declaration. All patients signed a written informed consent form.
Availability of Data and Materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors’ contributions
Zhi Zhao designed the study. Zhi Zhao carried out experiments and wrote the manuscript, Zhi Zhao revised the paper, Zhiyan Wang, Jianzhong Guan, Lijia Pei, Xinshe Zhou and Pinghui Zhou collected patient specimens and related information. Zhiyan Wang, Jianzhong Guan, Lijia Pei, Xinshe Zhou and Pinghui Zhou contributed to analysing the data. All authors reviewed the results and approved the final version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Altman R, Alarcón G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 2010;34(5):505–514.
- Hart CPVD, Bekerom MPVD, Patt TW. The occurrence of osteoarthritis at a minimum of ten years after reconstruction of the anterior cruciate ligament. J Orthop Surg Resr. 2008;3(1):1–9.
- Silverstein FE, Faich G, Goldstein JL. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis. The CLASS study: a randomized controlled trial. Jama. 2000;284(10):1247–1255.
- Snijders G, Den Broeder AA, van Riel PL, et al. Evidence-based tailored conservative treatment of knee and hip osteoarthritis: between knowing and doing. Scand J Rheumatol. 2011;18(3):S249–S250.
- Parkes MJ, Maricar N, Lunt M, et al. Lateral wedge insoles as a conservative treatment for pain in patients with medial knee osteoarthritis. Jama. 2013;310(7):722–730.
- Gomoll AH, Filardo G, de Girolamo L, et al. Surgical treatment for early osteoarthritis. Part I: cartilage repair procedures. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):450–466.
- Xi-Hai LI, Liang W-N, LIU X-X. Clinical observation on curative effect of dissolving phlegm-stasis on 50 cases of knee osteoarthritis. J Traditional Chin Med. 2010;30(2):108–112.
- Yang XC, He S-F, Wang R-C, et al. [Observation on curative effect of thermal acupuncture needle muscular stimulation therapy for knee osteoarthritis patients]. Acupuncture Res. 2012;37(3):237–241.
- Yang G, Lu X, Yuan L. LncRNA: a link between RNA and cancer. Biochim Biophys Acta. 2014;1839(11):1097–1109.
- Kallen AN, Zhou X-B, Xu J, et al. The imprinted H19 LncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52(1):101–112.
- Necsulea A, Soumillon M, Warnefors M, et al. The evolution of lncRNA repertoires and expression patterns in tetrapods. Nature. 2014;505(7485):635–640.
- Loewen G, Jayawickramarajah J, Zhuo Y, et al. Functions of lncRNA HOTAIR in lung cancer. J Hematol Oncol. 2014;7(1):90.
- Zhu M, Chen Q, Liu X, et al. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2015;281(16):3766–3775.
- Li X, Tang C, Wang J, et al. Methylene blue relieves the development of osteoarthritis by upregulating LncRNA MEG3. Exp Ther Med. 2018;15(4):3856–3864.
- Wang Q, Wu Y, Li F, et al. Association of genetic polymorphisms in immune-related lncRNA with osteoarthritis susceptibility in Chinese Han population. Per Med. 2018;15(2):103–110.
- Hao B, Gong Z, Zhang W, et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget. 2015;6(24):20404–20418.
- Lu X, Zhou C, Li R, et al. Critical role for the long non-coding RNA AFAP1-AS1 in the proliferation and metastasis of hepatocellular carcinoma. Tumour Biol J Int Soc Oncodevelop Biol Med. 2016;37(7):9699–9707.
- Liu X-H, Sun M, Nie F-Q, et al. Lnc RNA HOTAIR functions as a competing endogenous RNA to regulate HER2 expression by sponging miR-331-3p in gastric cancer. Mol Cancer. 2014;13(1):92.
- Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331(6017):550.
- Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16(3):223–229.
- Si H-B, Zeng Y, Liu SY, et al. Intra-articular injection of microRNA-140 (miRNA-140) alleviates osteoarthritis (OA) progression by modulating extracellular matrix (ECM) homeostasis in rats. Osteoarthr Cartil. 2017;S1063458417310476.
- Iliopoulos D, Malizos KN, Oikonomou P, et al. Integrative microRNA and proteomic approaches identify novel osteoarthritis genes and their collaborative metabolic and inflammatory networks. Plos One. 2008;3(11):e3740.
- Chen F, Zhu HH, Zhou LF, et al. Inhibition of c-FLIP expression by miR-512-3p contributes to taxol-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep. 2010;23(5):1457–1462.
- Zhu X, Gao G, Chu K, et al. Inhibition of RAC1-GEF DOCK3 by miR-512-3p contributes to suppression of metastasis in non-small cell lung cancer. Int J Biochem Cell Biol. 2015;61:103–114.
- Blaney Davidson EN, Remst DFG, Vitters EL, et al. Increase in ALK1/ALK5 ratio as a cause for elevated MMP-13 expression in osteoarthritis in humans and mice. J Iimmunol. 2009;182(12):7937–7945.
- Guenin S, Mauriat M, Pelloux J, et al. Normalization of qRT-PCR data: the necessity of adopting a systematic, experimental conditions-specific, validation of references. J Exp Bot. 2009;60(2):487–493.
- Sanchez-Campillo M, Bini L, Comanducci M, et al. Identification of immunoreactive proteins ofChlamydia trachomatis by Western blot analysis of a two-dimensional electrophoresis map with patient sera. Electrophoresis. 2015;20(11):2269–2279.
- Goldring MB. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 2010;43(9):1916–1926.
- Zheng W, Feng Z, You S, et al. Fisetin inhibits IL-1?2-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int Immunopharmacol. 2017;45(Complete):135–147.
- Raghu H, Lepus CM, Wang Q, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation, and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76(5):914.
- Corr EM, Cunningham CC, Helbert L, et al. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res Ther. 2017;19(1):23.
- Peng W-X, Koirala P, Mo -Y-Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661.
- Xiong H, Ni Z, He J, et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene. 2017;36(25):3528–3540.
- Li C, Wang S, Xing Z, et al. A ROR1–HER3–lncRNA signalling axis modulates the Hippo–YAP pathway to regulate bone metastasis. Nat Cell Biol. 2017;19(2):106–119.
- Li Y-F, Li S-H, Liu Y, et al. Long noncoding RNA CIR promotes chondrocyte extracellular matrix degradation in osteoarthritis by acting as a sponge for Mir-27b. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2017;43(2):602.
- Feng Y, Zhang Q, Wang J, et al. Increased lncRNA AFAP1-AS1 expression predicts poor prognosis and promotes malignant phenotypes in gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(17):3842.
- Chan P, Wu C. A peptide fragment of type II collagen induces the OA phenotype. Matrix Biol. 2006;25(2):S44–S44.
- Nummenmaa E, Hämäläinen M, Moilanen T, et al. Effects of FGF-2 and FGF receptor antagonists on MMP enzymes, aggrecan, and type II collagen in primary human OA chondrocytes. Scand J Rheumatol. 2015;44(4):321–330.
- Paul P, Chakraborty A, Sarkar D, et al. Interplay between miRNAs and human diseases: a review. J Cell Physiol. 2017;233:2007–2018.
- Makki MS, Haseeb A, Haqqi TM. MicroRNA-9 promotes IL-6 expression by inhibiting MCPIP1 expression in IL-1β-stimulated human chondrocytes. Arthritis Rheumatol. 2015;67(8):2117.
- Tchetina EV, Kobayashi M, Yasuda T, et al. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biol. 2007;26(4):247–258.
- Tang LP, Ding J-B, Liu Z-H, et al. LncRNA TUG1 promotes osteoarthritis-induced degradation of chondrocyte extracellular matrix via miR-195/MMP-13 axis. Eur Rev Med Pharmacol Sci. 2018;22(24):8574–8581.
- Piecha D, Weik J, Kheil H, et al. Novel selective MMP-13 inhibitors reduce collagen degradation in bovine articular and human osteoarthritis cartilage explants. Inflammation Res. 2010;59(5):379–389.