ABSTRACT
Diabetic retinopathy (DR) remains a major cause of blindness among diabetes mellitus patients. C1q/tumor necrosis factor-related protein-3 (CTRP3) is a novel adipokine which is associated with multiple types of metabolism. Nevertheless, little is known about the role of CTRP3 in high glucose (HG)-induced human retinal pericytes (HRPs). This study set out to assess the influence of CTRP3 on HG-induced HRPs and elucidate the latent regulatory mechanism. RT-qPCR and Western blot were to analyze CTRP3 and forkhead box O4 (FOXO4) expression. Western blot was also utilized to detect the protein levels of apoptosis-related factors and nuclear factor erythroid 2-related factor 2 (Nrf2)/Nuclear factor-kappaB (NF-κB) signaling-related factors. CCK-8 was to measure cell proliferation while TUNEL assay was to estimate cell apoptosis. Levels of oxidative stress biomarkers including manganese (MnSOD), catalase (CAT) and malonedialdehyde (MDA) were evaluated by the corresponding kits. JASPAR database, ChIP and luciferase reporter assay were to verify the interaction between FOXO4 and CTRP3 promoter. The experimental results uncovered that CTRP3 expression was decreased in HG-stimulated HRPs. Moreover, CTRP3 overexpression strengthened the viability while abrogated the apoptosis and oxidative stress of HG-induced HRPs. Furthermore. FOXO4 was up-regulated in HG-induced HRPs. Besides, FOXO4 bond to CTRP3 promoter and inhibited CTRP3 transcription to modulate the Nrf2/NF-κB signaling pathway. FOXO4 up-regulation reversed the influence of CTRP3 elevation on the proliferation, apoptosis and oxidative stress of HG-induced HRPs. To be summarized, CTRP3 negatively modulated by FOXO4 prevented HG-induced oxidative damage in DR via modulation of Nrf2/NF-κB signaling.
Graphical abstract
Introduction
It is widely identified that diabetic retinopathy (DR) is a kind of ocular disease due to retina damage induced by diabetes [Citation1]. As a frequent and specific microvascular complication of diabetes, DR is deemed as a leading cause of irreversible blindness in working-age adults [Citation2]. Long diabetes duration, high blood pressure, poor metabolic control, sleep apnea syndrome are considered to be main risk factors for DR [Citation3]. According to the World Health Organization, there will be more than 190 million diabetics worldwide by 2030. Among them, the incidence of DR in patients with diabetes over 20 years is 80%. At present, the clinical treatment of DR is mainly drug therapy, hormone therapy and surgical treatment [Citation4]. However, very little is currently known about the pathogenesis of DR. Pericytes are vascular mural cells which regulate multiple pathological processes including angiogenesis, vascular remodeling, wound healing [Citation5]. Unlike pericytes in many other organs, retinal pericytes (RPCs) play a critical role in the neurovascular unit [Citation6]. More importantly, the dysfunction or loss of RPCs is one of the primary features of early stage of DR [Citation7]. Hence, it is essential to explore the altered functions of RPCs to get a better understanding of the pathogenesis of DR.
CTRP3, an important member of CTRP family, is one kind of adipokine with a large quantity of biological effects including inflammation, angiogenesis, glucose and lipid metabolism [Citation8]. Numerous reports have manifested that CTRP3 is determined as a crucial participator in cerebral ischemic stroke [Citation9], severe acute pancreatitis [Citation10], gestational diabetes mellitus [Citation11] and so on. In the literature, CTRP3 has been clarified to mitigate the oxidative stress and apoptosis of retinal pigment epithelial cells under HG conditions [Citation12]. Also, Yan et al. have proposed that serum CTRP3 level is down-regulated in type 2 diabetes mellitus patients and suppresses VCAM-1 production stimulated by high glucose and high lipid [Citation13]. However, the specific regulatory mechanism of CTRP3 in DR has not been reported so far.
FOXO4, a member of human Forkhead-box (FOX) gene family, is known as a common transcription factor and implicated in the regulation of metabolism, oxidative stress resistance, cell proliferation and apoptosis [Citation14]. It is well documented that FOXO4 plays the promoting role in myocardial ischemia-reperfusion injury [Citation15] and acts as a suppressor in colorectal cancer [Citation16], gastric cancer [Citation17] and Cholangiocarcinoma [Citation18]. However, whether FOXO4 interplays with CTRP3 in DR needs more exploration.
Nrf2/NF-κB signaling represents a functional cross-talk between two key transcription factors Nrf2 and NF-κB [Citation19]. In addition, Nrf2/NF-κB signaling has been validated to exert influence on HG-induced oxidative stress and apoptosis of PRCs [Citation20–22]. Thus Nrf2/NF-κB signaling is emerged as a key player in the pathogenesis of DR.
According to the above content, it is reasonable to assume that CTRP3 is regulated by FOXO4, thus regulating the oxidative stress damage of RPCs in DR. High glucose (HG) has been demonstrated to impair pericyte proliferation and induce pericyte apoptosis [Citation23]. Therefore, HG was utilized to establish DR model in HRPs in this study. This study is designed to uncover the biological role of CTRP3 in the dysfunction of HRPs under HG conditions and illuminate the relationship among CTRP3, FOXO4 and Nrf2/NF-κB signaling.
Materials and methods
Cell culture
Primary HRPs (ACBRI 183) obtained from Cell Systems Corporation (Kirkland, WA, USA) were inoculated in Dulbecco’s modified Eagle’s medium (DMEM; Gibco). The medium was supplemented with 10% fetal bovine serum (FBS; Gibco, 11,573,397) and 1% antibiotics (streptomycin-penicillin) under the condition of 5% CO2 and 37°C.
To explore the damage to HRPs in DR, we constructed an in vitro DR model. HRPs were respectively maintained in 5.6 mmol/L of glucose as normal control (Control group), 5.6 mmol/L of glucose and 24.6 mmol/L of mannitol as osmotic pressure control (MA group), 30 mmol/L of glucose as high glucose group (HG group) [Citation24].
Cell transfection
Sangon Biotech Co., Ltd. (Shanghai, China) synthesized the overexpression lentivirus plasmids carrying CTRP3 gene (Ov-CTRP3) or FOXO4 gene (Ov-FOXO4) as well as the overexpression empty vectors (Ov-NC). Stable transfection cells were selected with puromycin for 2 weeks. Above plasmids were transfected into cells for gene overexpression for another 48 h using Lipofectamine 2000 (Invitrogen, Waltham, USA) in line with the manufacturer’s protocols.
Reverse transcription-quantitative PCR (RT-qPCR)
With the adoption of RNA Extraction Kit (Omega Bio-tek Inc, Norcross, GA, U.S.A.), total RNA was extracted from indicated cells, followed by reverse transcription into cDNA by Primescript™ RT reagent kit (Takara, Dalian, China). PCR reactions were prepared through SYBRGreen Master Mix (Bio-Rad, USA) on the 7500HT Fast Real Time PCR system (Applied Biosystems, Foster City, CA, USA) with GAPDH as the endogenous reference. The primer sequence were as follows: CTRP3, forward, 5’-ATGCTTTGGAGGCAGCTCAT-3’, reverse, 5’-TCACCTTTGTCGCCCTTCTC-3’; FOXO4, forward, 5’-CCAGAGATCGCTAACCAGCC-3’, reverse, 5’-TTTCAATGGCCTTTTCCCCCA-3’. Relative mRNA levels of CTRP3 and FOXO4 were measured with the adoption of 2−ΔΔCt methods [Citation25].
Cell Counting Kit-8 (CCK-8) assay
HRPs were inoculated into 96-well plates at a density of 5,000 cells per well and incubated at 37°C. After indicated treatment, 10 μl CCK-8 solution (40203ES60, Yeasen Biotechnology, Shanghai, China) was added to each well. After being maintained at 37°C for 2 h, the measurement of absorbance at 450 nm was carried out with a microplate reader (Bio-Tek Technologies, Winooski, VT, United States).
Terminal-deoxynucleotidyl Transferase Mediated Nick End Labeling (TUNEL) assay
TUNEL assay was performed by the in situ cell death detection kit (Roche) in compliance with the manufacturer’s instructions. In short, HRPs were fixed using 4% paraformaldehyde (Sigma-Aldrich), after which cells were washed with PBS and 0.1% Triton X-100 was utilized to permeabilize these washed cells. DAPI was used to label the nuclei staining for 10 min. Finally, the apoptotic cells were observed under a fluorescence microscope (Nikon Instruments Inc., Melville, NY, USA).
Detection of MnSOD, CAT and MDA
Cells were decomposed in 300 μl lysis buffer and total protein was quantified by BCA kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). A commercially available Ransod kit (Randox, Laboratories Ltd. Ardmore, UK), Catalytic enzymes activity kit and MDA assay kit from Nanjing Jiancheng Bioengineering Co. Ltd. were employed to incubate with total protein to respectively determine the MnSOD activity, CAT activity and MDA activity in accordance with the supplier’s guidance. The absorbance was determined at 450 nm using a microplate reader (Thermo, Minneapolis, MN, USA).
Chromatin immunoprecipitation (ChIP)
A commercially available kit for DNA gel extraction (Beyotime, Shanghai, China) was utilized in the ChIP assay [Citation26]. After cells were cross-linked with 1% formaldehyde, they were randomly fragmented by ultrasonic. Stained chromatin was incubated with FOXO4 antibody (Abcam, 1:1000, ab128908) or IgG antibody (Abcam, 1:1000, ab109489) as control in magnetic beads. The enrichment of precipitated chromatin DNA was subjected to PCR analysis.
Luciferase reporter assay
After the construction of the wild type and mutant sequences of CTRP3 promoter region into the pGL3 vector (Promega Corporation, Madison, WI, USA), they were co-transfected along with Ov-FOXO4 plasmids or the empty vector into cells. After 48 h, the luciferase activity was subjected to the detection from Luciferase Reporter Assay System (Promega, Madison, WI) [Citation27].
Western blot
Protein samples collected in RIPA lysis buffer (Sigma-Aldrich; Merck KGaA) were added to 10% SDS-PAGE for electrophoresis and moved to PVDF membranes (Millipore, USA). The membranes were impeded by 5% nonfat milk and incubated with the following primary antibodies: anti-CTRP3 (Abcam, ab36870), anti-B cell lymphoma-2 (Bcl-2) (Abcam, 1:1000, ab32124), anti-BCL-2 associated X (Bax) (Abcam, 1:1000, ab32503), anti-cleaved caspase 3 (Abcam, 1:500, ab32042), anti-caspase 3 (Abcam, 1:5000, ab32351), anti-cleaved Poly (ADP-ribose) polymerase (PARP) (Abcam, 1:1000, ab32064), PARP (Abcam, 1:1000, ab191217), anti-FOXO4 (Abcam, 1:1000, ab128908), anti-Nrf2 (Abcam, 1:1000, ab62352), anti- phosphorylated (p-)NF-κB p65 (Abcam, 1:1000, ab76302), anti-NF-κB p65 (Abcam, 1:1000, ab32536) and anti-GAPDH (Abcam, 1:2500, ab9485) at 4°C overnight. Afterward, the membranes were incubated with HRP‐conjugated goat anti-rabbit IgG (Abcam, 1:1000, ab109489) at room temperature for 1 h. Then, the blots were detected by the ECL detection kit (Roche) and analyzed with the help of Image J software (NIH, Bethesda, MD, USA).
Bioinformatics tools
With the application of JASPAR database (https://jaspar.genereg.net/), the possible binding sites between FOXO4 and CTRP3 promoter were predicted [Citation28].
Statistical analyses
Statistical analysis in this study were completed using GraphPad Prism 8.0 software (GraphPad Software, Inc.) with the employment of Student’s t-test and one-way analysis of variance (ANOVA) as well as Tukey’s post hoc test. All experimental data were exhibited as the mean ± SD and the data was determined to be statistically significant when p < 0.05.
Results
Overexpression of CTRP3 enhances the viability of HG-induced HRPs
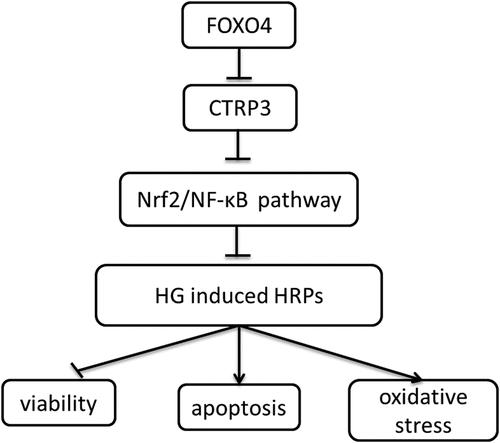
First of all, CTRP3 expression was detected with the employment of RT-qPCR and Western blot analysis. The results indicated that after induced by HG, CTRP3 expression was discovered to be decreased in HRPs in comparison with the MA group ()). For the subsequent experiments, CTRP3 expression was enhanced by transfection of Ov-CTRP3 plasmid and the transfection efficiency was tested by RT-qPCR and Western blot analysis ()). As demonstrated in , the results from CCK-8 assay revealed that HRPs viability was attenuated under HG conditions while this effect was restored by up-regulation of CTRP3. In a word, CTRP3 prevented the loss of cell viability of HRPs under HG conditions.
Figure 1. Overexpression of CTRP3 enhances the viability of HG-induced HRPs. (a) CTRP3 expression in control group, MA group and HG group was examined through RT-qPCR and Western blot. ***P < 0.001 vs. MA. (b) The transfection efficiency of Ov-CTRP3 plasmid was tested by RT-qPCR and Western blot. ***P < 0.001 vs. Ov-NC. (c) Cell proliferation was evaluated by CCK-8 assay. ***P < 0.001 vs. MA. ##P < 0.01 vs. HG+Ov-NC. CTRP3, C1q/tumor necrosis factor-related protein-3. HG, high glucose. MA, mannitol.
CTRP3 hampers HG-stimulated HRPs apoptosis
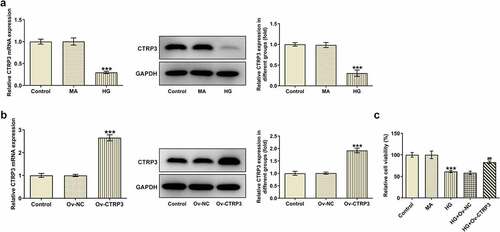
To detect the effect of CTRP3 on HRPs apoptosis induced by HG, the apoptotic ability of HRPs was assessed by TUNEL assay and Western blot analysis. From TUNEL assay, it was clearly observed that HG-induced HRPs apoptosis was rescued by CTRP3 elevation ()). Similarly, the protein levels of apoptosis-related factors including Bcl-2, Bax, cleaved caspase 3, caspase 3, cleaved PARP and PARP were analyzed by Western blot. It was observed that the decrease in Bcl-2 protein level and the increase in Bax, cleaved caspase 3, cleaved PARP protein levels triggered by HG exposure were all offset by up-regulation of CTRP3 while no apparent changes were noticed in the protein levels of caspase 3 and PARP ()). In short, CTRP3 played the suppressive role in the apoptosis of HRPs exposed to HG.
Figure 2. CTRP3 hampers HG-stimulated HRPs apoptosis. (a) Cell apoptosis was appraised by TUNEL assay. (b) The protein levels of apoptosis-related factors were analyzed by Western blot. ***P < 0.001 vs. MA. ##P < 0.01, ###P < 0.001 vs. HG+Ov-NC. CTRP3, C1q/tumor necrosis factor-related protein-3. HG, high glucose. Bcl-2, B cell lymphoma-2. Bax, BCL-2 associated X. cleaved PARP, cleaved Poly (ADP-ribose) polymerase. PARP, Poly (ADP-ribose) polymerase.
CTRP3 ameliorates HG-mediated oxidative stress of HRPs
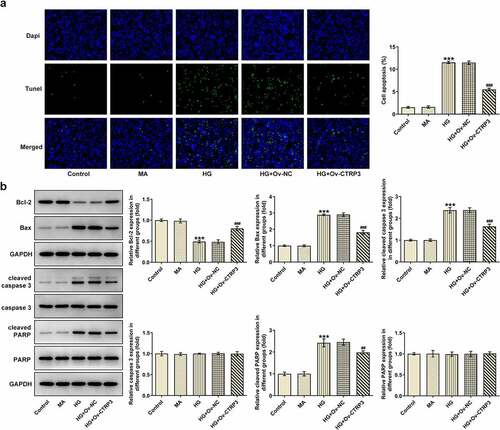
Oxidative stress is a key driver in the progression of DR [Citation29]. Thereafter, the levels of oxidative stress markers including MnSOD, CAT and MDA were examined by the corresponding kits. As a result, HG treatment improved MDA level and lessened MnSOD and CAT levels, while this result was countervailed after CTRP3 was overexpressed (). The results reveled that CTRP3 ameliorates HG-mediated oxidative stress of HRPs.
Figure 3. CTRP3 ameliorates HG-mediated oxidative stress of HRPs. Activities of MnSOD, CAT and MDA were measured by kits. ***P < 0.001 vs. MA. ###P < 0.001 vs. HG+Ov-NC. CTRP3, C1q/tumor necrosis factor-related protein-3. HG, high glucose. MA, mannitol. MnSOD, manganese. CAT, catalase. MDA, malonedialdehyde.
Transcription factor FOXO4 inhibits the transcription of CTRP3
Subsequently, we further explored the regulation mechanism of CTRP3 on HG-induced HRPs in DR. Intriguingly, with the employment of JASPAR database, the potent binding site between FOXO4 and CTRP3 promoter was predicted and displayed in . Moreover, RT-qPCR and Western blot detected elevated FOXO4 expression at mRNA and protein level in HRPs after HG treatment relative to the MA group ()). Subsequently, HG-induced HRPs were transfected with Ov-FOXO4 to successfully up-regulate FOXO4 expression and the transfection efficiency was tested by RT-qPCR and Western blot ()). Meanwhile, luciferase reporter assay verified that elevation of FOXO4 dramatically decreased the luciferase activity of CTRP3 promoter ()), which implied the possible interaction between FOXO4 and CTRP3 promoter. Similarly, ChIP assay confirmed that CTRP3 was enriched in FOXO4 antibody (), suggesting the strong affinity of FOXO4 with CTRP4 promoter. Eventually, it turned out that overexpression of FOXO4 cut down the mRNA and protein level of CTRP3 (). To sum up, FOXO4 functioned as a transcription inactivator of CTRP3.
Figure 4. Transcription factor FOXO4 inhibits the transcription of CTRP3. (a) Binding motif of FOXO4 with CTRP4 promoter was predicted by JASPAR database. (b) FOXO4 expression in Control group, MA group and HG group was examined through RT-qPCR and Western blot. ***P < 0.001 vs. MA. (c) The transfection efficiency of Ov-FOXO4 plasmid was tested by RT-qPCR and Western blot. ***P < 0.001 vs. Ov-NC. (d) The luciferase activities of CTRP3 WT promoter and CTRP3 MUT promoter after transfection of Ov-FOXO4 were detected by luciferase reporter assay. (e) RIP assay confirmed the abundance of CTRP3 in FOXO4 antibody. ***P < 0.001 vs. IgG. (f) CTRP3 expression in Control group, Ov-NC group and Ov-FOXO4 group was examined through RT-qPCR and Western blot. ***P < 0.001 vs. Ov-NC. CTRP3, C1q/tumor necrosis factor-related protein-3. HG, high glucose. MA, mannitol. FOXO4, forkhead box O4.
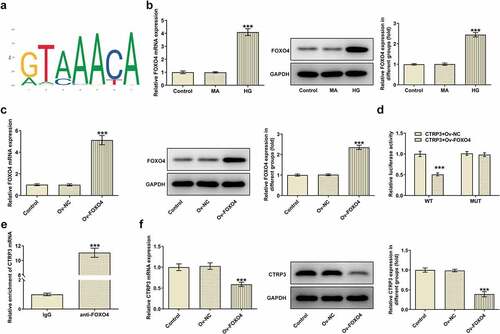
CTRP3 inactivated by FOXO4 modulates the Nrf2/NF-κB signaling pathway
Further, the protein levels of Nrf2, p-NF-κB p65 and t-NF-κB p65 were analyzed with the application of Western blot. As a result, HG treatment decreased Nrf2 protein level and elevated p-NF-κB p65 protein level. Under this condition, overexpression of CTRP3 increased Nrf2 protein level and reduced p-NF-κB p65 protein level, whereas this effect was restored again by up-regulation of FOXO4 (). Collectively, overexpression of FOXO4 inhibited the regulatory role of CTRP3 in HG-induced Nrf2 signaling inactivation and NF-κB signaling activation.
Figure 5. CTRP3 inactivated by FOXO4 modulates the Nrf2/NF-κB signaling pathway. The protein levels of Nrf2, p-NF-κB p65 and NF-κB p65 were analyzed by Western blot. ***P < 0.001 vs. MA. ###P < 0.001 vs. HG+Ov-NC. $$$P < 0.001 vs. HG+Ov-CTRP3+ Ov-NC. CTRP3, C1q/tumor necrosis factor-related protein-3. HG, high glucose. MA, mannitol. FOXO4, forkhead box O4. Nrf2, nuclear factor erythroid 2-related factor 2. p-NF-κB p65, phosphorylated-Nuclear factor-kappaB p65. NF-κB p65, Nuclear factor-kappaB p65.
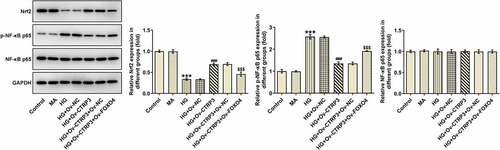
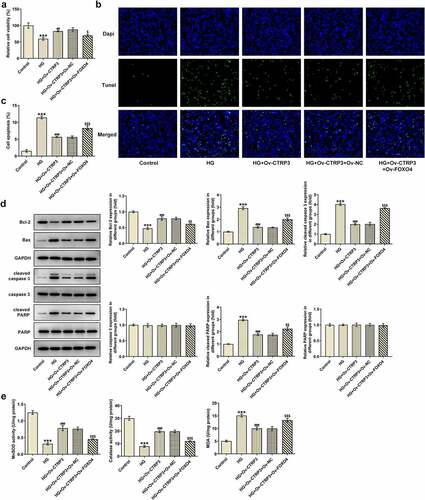
CTRP3 mediated by FOXO4 regulates HRPs viability, apoptosis and oxidative stress under HG conditions through Nrf2/NF-κB signaling
For the purpose of the verification of the interaction between CTRP3 and FOXO4 in HG-induced HRPs, rescue assays were carried out. CCK-8 assay substantiated that CTRP3 exacerbated the inhibited HRPs proliferation caused by HG, while this result was counteracted by FOXO4 overexpression ()). On the contrary, the protective role of CTRP3 against HG-induced HRPs apoptosis was repressed by overexpression of FOXO4 ()). The similar result could also be seen in Western blot analysis, accompanied by the finding that the reduced protein level of Bcl-2 and the elevated protein levels of Bax, cleaved caspase 3 and cleaved PARP in HG-stimulated HRPs were all restored by CTRP3 up-regulation and reversed again by FOXO4 elevation ()). Besides, the inhibitory effect of CTRP3 on HG-induced oxidative stress was impeded after FOXO4 was overexpressed, as evidenced by the result that FOXO4 cut down the enhanced MnSOD and CAT levels while augmented the decreased MDA level due to CTRP3 overexpression ()). Collectively, the influence of CTRP3 on the viability, apoptosis and oxidative stress of HRPs upon exposure to HG was all abrogated by FOXO4 up-regulation.
Figure 6. CTRP3 mediated by FOXO4 regulates HRPs viability, apoptosis and oxidative stress under HG conditions through Nrf2/NF-κB signaling. (a) Cell proliferation was evaluated by CCK-8 assay. (b) Cell apoptosis was appraised by TUNEL assay and (c) quantification. (d) The protein levels of apoptosis-related factors were analyzed by Western blot. (e) Activities of MnSOD, CAT and MDA were measured by kits. ***P < 0.001 vs. Control. ##P < 0.01, ###P < 0.001 vs. HG. $P < 0.005, $$P < 0.01, $$$P < 0.001 vs. HG+Ov-CTRP3+ Ov-NC. CTRP3, C1q/tumor necrosis factor-related protein-3. HG, high glucose. FOXO4, forkhead box O4. Bcl-2, B cell lymphoma-2. Bax, BCL-2 associated X. cleaved PARP, cleaved Poly (ADP-ribose) polymerase. PARP, Poly (ADP-ribose) polymerase. MnSOD, manganese. CAT, catalase. MDA, malonedialdehyde.
Discussion
Early and selective death of pericytes is considered to be one of the main features of DR pathogenesis [Citation30]. What’s more, it is believed that pericytes are susceptible to the metabolic abnormalities, thereby contributing to the development of DR even diabetes mellitus [Citation31]. The most important early manifestations of DR are the reduction or disappearance of HRPs, pathological capillary endothelial cell proliferation, basement membrane thickening, blood-retinal barrier function destruction, retinal ischemia and hypoxia, resulting in pathological neangiogenesis [Citation32]. And late proliferative DR appears, which is also an important cause of blindness in diabetic patients. Therefore, we chose HRPs for experiment Oxidative stress is recognized as a metabolic condition derived from an imbalance between the production of oxygen radicals and their antioxidant capacity [Citation33]. In detail, oxidative stress, a consequence of overproduction of reactive oxygen species (ROS) and the imbalance of the antioxidant defense systems for ROS elimination, plays a central role in a wide variety of biological processes of DR [Citation29]. Moreover, during that process, the break of the balance leads to retinal cell injury for the reason that the retina is sensitive to ROS due to high-energy demands and light exposure [Citation34]. As one of the antioxidant enzymes, MnSOD serves as a main defense against oxidative stress [Citation35]. Also, CAT is a kind of natural antioxidase capable of scavenging ROS [Citation36]. MDA is the most commonly measured indicator of oxidative stress [Citation37]. Therefore, MnSOD, CAT and MDA activities were estimated for detection of the oxidative stress levels in HRPs in this study. HG condition mediating connexin expression and gap junction intercellular communication is implicated in the initiation and development of DR, a glucose-related disease [Citation23]. Hence, HRPs were treated with HG to establish a DR model in this study and the experimental results disclosed that after stimulated by HG, the viability of HRPs was alleviated while the apoptosis and oxidative stress of HRPs were both exacerbated.
Current researches have certified that CTRP3 exerts an enormous function on human diseases, such as severe acute pancreatitis [Citation10], cerebral ischemic stroke [Citation9], gestational diabetes mellitus [Citation11] and cancer [Citation38]. A previous study has shown that CTRP3 reduces the proliferation and ECM production of glomerular mesangial cells induced byHG through inactivation of JAK2/STAT3 signaling pathway [Citation39]. More importantly, Zhang et al. have elaborated that CTRP3 plays an inhibitory role in the apoptosis and oxidative stress of retinal pigment epithelial cells exposed to HG [Citation12]. CTRP3 can protect human umbilical vein endothelial cell injury induced by high glucose [Citation40]. Yan et al. have illustrated that CTRP3 exhibits low serum levels in type 2 diabetes mellitus patients and could be viewed as a novel hallmark for DR [Citation13]. Consistent with these findings, CTRP3 was discovered to be lowly expressed in HRPs under HG conditions. Meanwhile, gain-of-function experiments testified that up-regulation of CTRP3 aggravated the viability of HG-stimulated HRPs while attenuated the apoptosis and oxidative stress of HG-induced HRPs.
Transcription factor FOXO4 has been regarded as a mediator of cellular gene regulation. For instance, Arg1 transcription is activated by FOXO4 in myocardial infarction [Citation41]. FOXO4 suppresses the transcription of USP10 to boost acute myocardial infarction [Citation42]. Functionally, it is also well documented that FOXO4 chiefly serves as a suppressor in different types of tumors [Citation43]. At the same time, FOXO4 overexpression has been exposed to drive the process of DR, and its overexpression abrogates the antioxidant and anti-apoptotic effects of α-melanocyte-stimulating hormone in HG-stimulated retinal vascular endothelial cells [Citation44,Citation45]. It was noticed in our experiments that FOXO4 expression was increased in HG-stimulated HRPs. In addition, FOXO4 had a strong affinity with CTRP3 promoter and hindered the transcription of CTRP3. Rescue assays eventually verified that the enhanced viability, the inhibited apoptosis and oxidative stress of HG-mediated HRPs on account of overexpression of CTRP3 were all reversed by up-regulation of FOXO4.
The understanding of the regulatory mechanism of Nrf2 activity and its downstream signaling pathways have great significance in multiple cellular processes, such as inflammation, autophagy and metabolism [Citation46]. Additionally, it turns out that Nrf2 plays a protective role against oxidative stress [Citation20,Citation47]. NF-κB is also a pivotal determinant in various biological processes and phosphorylation of IκBs could release NF-κB to result in nuclear translocation and activation of gene transcription [Citation48]. The interplay between Nrf2 and NF-κB constitutes Nrf2/NF-κB signaling in which Nrf2 deficiency can augment NF-κB activity, whereas NF-κB can modulate Nrf2 transcription and activity [Citation19]. The combination of the two is involved in the development of a variety of diseases. For example, protocatechuic acid impacts cerebral aneurysm through TNF-α/NF-κB/Nrf-2 signaling [Citation49]. Egg yolk oils eases inflammatory response via Nrf2/NF-κB pathway [Citation50]. Furthermore, NF-κB pathway modulates pericyte apoptosis in DR [Citation21]. Activation of NF-κB stimulates retinal pericyte apoptosis [Citation22]. As reported, CTRP3 is an activator in Nrf2 pathway in HG-induced retinal pigment epithelial cells [Citation12]. More intriguingly, Lv et al. have demonstrated that CTRP3 decreases p-NF-κB p65 expression and p53 acetylation [Citation10]. Sang et al. have validated that FOXO4 cuts down NF-κB and p-NF-κB p65 expressions and inactivates NF-κB signaling in alcohol-induced chronic liver injury [Citation51]. The experimental results in this study confirmed that after stimulated by HG, Nrf2 protein level was reduced while p-NF-κB p65 protein level was enhanced in HRPs. Under this condition, CTRP3 elevation increased Nrf2 protein level while decreased NF-κB p65 protein level. As a consequence, after FOXO4 was overexpressed, the impacts of CTRP3 on Nrf2 and NF-κB p65 protein levels were both restored again.
Conclusion
To be concluded, CTRP3 negatively modulated by FOXO4 could enhance the viability while attenuate the apoptosis and oxidative damage of HRPs upon exposure to HG via mediating Nrf2/NF-κB pathway. All these findings implies that CTRP3 might exert protective effects in HG-induced HRPs damage, which might provide effective therapeutic strategies for DR from a brand-new perspective.
Highlights
CTRP3 is activated by FOXO4 down-regulation in DR
CTRP3 inhibites HG-induced oxidative damage of HRPs in DR
CTRP3 can regulate Nrf2/NF-κB signaling pathway
Consent for publication
All the authors agreed to be published.
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Henriques J, Vaz-Pereira S, Nascimento J, et al. [Diabetic eye disease]. Acta Med Port. 2015;28(1):107–113.
- Lightman S, Towler HM. Diabetic retinopathy. Clin Cornerstone. 2003;5(2):12–21.
- Moreno A, Lozano M, Salinas P. Diabetic retinopathy. Nutr Hosp. 2013;28 Suppl 2:53–56.
- Terasaki H. Multimodal approaches for the analysis of retinal functional disorders-focusing on retinal detachment. Nippon Ganka Gakkai Zasshi. 2017;121(3):185–231.
- Motiejūnaite R, Kazlauskas A. Pericytes and ocular diseases. Exp Eye Res. 2008;86(2):171–177.
- Pfister F, Przybyt, E, Harmsen , MC, et al. Pericytes in the eye. Pflugers Arch. 2013;465(6):789–796.
- Trost A, Bruckner , D, Rivera , FJ, et al. Pericytes in the retina. Adv Exp Med Biol. 2019;1122:1–26.
- Zhang CL, Wu LL, Li L. [Research progress of complement-C1q/tumor necrosis factor-related protein 3]. Sheng Li Xue Bao. 2017;69(5):666–676.
- Gao J, Qian T, Wang W. CTRP3 activates the AMPK/SIRT1-PGC-1α pathway to protect mitochondrial biogenesis and functions in cerebral ischemic stroke. Neurochem Res. 2020;45(12):3045–3058.
- Lv C, He Y, Wei M, et al. CTRP3 ameliorates cerulein-induced severe acute pancreatitis in mice via SIRT1/NF-κB/p53 axis. Biosci Rep. 2020;40(10). DOI:10.1042/BSR20200092.
- Xia L, Huijuan , Z, Qiang , S, et al. Protective role of CTRP3 and CTRP9 in the development of gestational diabetes mellitus. Clin Lab. 2020;66(11). DOI:10.7754/Clin.Lab.2020.200247.
- Zhang J, He J. CTRP3 inhibits high glucose-induced oxidative stress and apoptosis in retinal pigment epithelial cells. Artif Cells Nanomed Biotechnol. 2019;47(1):3758–3764.
- Yan Z, Zhao J, Gan L, et al. CTRP3 is a novel biomarker for diabetic retinopathy and inhibits HGHL-induced VCAM-1 expression in an AMPK-dependent manner. PLoS One. 2017;12(6):e0178253.
- Link W. Introduction to FOXO biology. Methods Mol Biol. 2019;1890:1–9.
- Yu L, Zhang W, Huang C, et al. FoxO4 promotes myocardial ischemia-reperfusion injury: the role of oxidative stress-induced apoptosis. Am J Transl Res. 2018;10(9):2890–2900.
- Bian L, Zhi X, Ma L, et al. Hsa_circRNA_103809 regulated the cell proliferation and migration in colorectal cancer via miR-532-3p/FOXO4 axis. Biochem Biophys Res Commun. 2018;505(2):346–352.
- Wang XH, Jiang Z-H, Yang H-M, et al. Hypoxia-induced FOXO4/LDHA axis modulates gastric cancer cell glycolysis and progression. Clin Transl Med. 2021;11(1):e279.
- Intuyod K, Chomwong S, Thongpon P, et al. Expression of FOXO4 inhibits cholangiocarcinoma cell proliferation in vitro via induction of G(0)/G(1) arrest. Anticancer Res. 2020;40(12):6899–6905.
- Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43(4):621–626.
- Wang W, Zhao H, Chen B. DJ-1 protects retinal pericytes against high glucose-induced oxidative stress through the Nrf2 signaling pathway. Sci Rep. 2020;10(1):2477.
- Yun JH. Interleukin-1β induces pericyte apoptosis via the NF-κB pathway in diabetic retinopathy. Biochem Biophys Res Commun. 2021;546:46–53.
- Kim J, Kim C-S, Sohn E, et al. KIOM-79 protects AGE-induced retinal pericyte apoptosis via inhibition of NF-kappaB activation in vitro and in vivo. PLoS One. 2012;7(8):e43591.
- Roy S, Kim D, Lim R. Cell-cell communication in diabetic retinopathy. Vision Res. 2017;139:115–122.
- Gan J, Huang M, Lan G, et al. High glucose induces the loss of retinal pericytes partly via NLRP3-Caspase-1-GSDMD-mediated pyroptosis. Biomed Res Int. 2020;2020:4510628.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408.
- Chen X, Xu M, Xu X, et al. METTL14-mediated N6-methyladenosine modification of SOX4 mRNA inhibits tumor metastasis in colorectal cancer. Mol Cancer. 2020;19(1):106.
- Yao J, Xu G, Zhu L, et al. circGFRA1 enhances NSCLC progression by sponging miR-188-3p. Onco Targets Ther. 2020;13:549–558.
- Fornes O, Castro-Mondragon JA, Khan A, et al. JASPAR 2020: update of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2020;48(D1):D87–D92.
- Kang Q, Yang C. Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol. 2020;37:101799.
- Beltramo E, Porta M. Pericyte loss in diabetic retinopathy: mechanisms and consequences. Curr Med Chem. 2013;20(26):3218–3225.
- Arboleda-Velasquez JF, Valdez CN, Marko CK, et al. From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr Diab Rep. 2015;15(2):573.
- Cai X, McGinnis JF. Diabetic retinopathy: animal models, therapies, and perspectives. J Diabetes Res. 2016;2016:3789217.
- Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183.
- Calderon GD, Juarez OH, Hernandez GE, et al. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 2017;31(8):1122–1130.
- Becer E, Çırakoğlu A. Association of the Ala16Val MnSOD gene polymorphism with plasma leptin levels and oxidative stress biomarkers in obese patients. Gene. 2015;568(1):35–39.
- Kim JL, Reader BF, Dumond C, et al. Pegylated-catalase is protective in lung ischemic injury and oxidative stress. Ann Thorac Surg. 2021;111(3):1019–1027.
- Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem. 2017;524:13–30.
- Zhang R, Guo C, Liu T, et al. MicroRNA miR-495 regulates the development of Hepatocellular Carcinoma by targeting C1q/tumor necrosis factor-related protein-3 (CTRP3). Bioengineered. 2021;12(1):6902–6912.
- Hu TY, Li LM, Pan YZ. CTRP3 inhibits high glucose-induced human glomerular mesangial cell dysfunction. J Cell Biochem. 2019;120(4):5729–5736.
- Wang F, Zhao L, Shan Y, et al. CTRP3 protects against high glucose-induced cell injury in human umbilical vein endothelial cells. Anal Cell Pathol (Amst). 2019;2019:7405602.
- Zhu M, Goetsch SC, Wang Z, et al. FoxO4 promotes early inflammatory response upon myocardial infarction via endothelial Arg1. Circ Res. 2015;117(11):967–977.
- Huang J, Liu, Y, Wang, M, et al. FoxO4 negatively modulates USP10 transcription to aggravate the apoptosis and oxidative stress of hypoxia/reoxygenation-induced cardiomyocytes by regulating the Hippo/YAP pathway. J Bioenerg Biomembr. 2021;53(5):541–551.
- Coomans de Brachène A, Demoulin JB. FOXO transcription factors in cancer development and therapy. Cell Mol Life Sci. 2016;73(6):1159–1172.
- Zhang J, Wu, L, Chen, J, et al. Downregulation of MicroRNA 29a/b exacerbated diabetic retinopathy by impairing the function of Müller cells via Forkhead box protein O4. Diab Vasc Dis Res. 2018;15(3):214–222.
- Zhang L, Dong L, Liu X, et al. α-Melanocyte-stimulating hormone protects retinal vascular endothelial cells from oxidative stress and apoptosis in a rat model of diabetes. PLoS One. 2014;9(4):e93433.
- Tonelli C, Chio IIC, Tuveson DA. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745.
- Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2. Curr Cancer Drug Targets. 2018;18(6):538–557.
- Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–468.
- Xiao G, Zhang M, Peng X, et al. Protocatechuic acid attenuates cerebral aneurysm formation and progression by inhibiting TNF-alpha/Nrf-2/NF-kB-mediated inflammatory mechanisms in experimental rats. Open Life Sci. 2021;16(1):128–141.
- Xiao N, Zhao, Y, He, W, et al. Egg yolk oils exert anti-inflammatory effect via regulating Nrf2/NF-κB pathway. J Ethnopharmacol. 2021;274:114070.
- Sang L, Kang K, Sun Y, et al. FOXO4 ameliorates alcohol-induced chronic liver injury via inhibiting NF-κB and modulating gut microbiota in C57BL/6J mice. Int Immunopharmacol. 2021;96:107572.
