ABSTRACT
Age-related cataract (ARC) is one of the most common causes of vision loss in aging people. This research analyzed the functions and mechanism of long noncoding RNA KCNQ1 overlapping transcript 1 (KCNQ1OT1) in hydrogen peroxide (H2O2)-stimulated human lens epithelial cells (SRA01/04 cells) in ARC. SRA01/04 cells were stimulated with 200 µM H2O2 to establish oxidative damage in the ARC model. A MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay and flow cytometry analysis were conducted to evaluate cell growth and apoptosis. The relevance between KCNQ1OT1 and microRNA (miR)-124-3p or miR-124-3p and BCL-2-like 11 (BCL2L11) was measured through Starbase and a dual luciferase reporter gene assay. The levels of KCNQ1OT1 and miR-124-3p were assessed via quantitative real-time polymerase chain reaction (qRT-PCR). We observed that KCNQ1OT1 was over-expressed and miR-124-3p was low-expressed in H2O2-stimulated SRA01/04 cells. KCNQ1OT1 interacted with miR-124-3p and negatively mediated its levels. In addition, KCNQ1OT1-siRNA reversed the effects of H2O2 on SRA01/04 cells, evidenced by enhanced cell viability, inhibited apoptotic cells, promoted Bcl-2 expression, and reduced Bax levels. Nevertheless, these observations were inverted after miR-124-3p inhibitor treatment. Likewise, miR-124-3p mimic had a protective effect on H2O2-stimulated SRA01/04 cells. Our data suggested that BCL2L11 targeted miR-124-3p directly. In summary, the data indicated that lncRNA KCNQ1OT1 down-regulation protected SRA01/04 cells from oxidative stress stimulated damage via the miR-124-3p/BCL2L11 pathway.
GRAPHICAL ABSTRACT
Introduction
Age-related cataract (ARC), considered as senile cataract, is the most common type of cataract among middle-aged and older adults over 50 years old [Citation1,Citation2]. The incidence rate, related with the degeneration of slow metabolism, increases with age. However, most cases are slow and do not affect vision. The diagnosis of age-related cataract, which affects vision due to lens clouding, is of clinical significance [Citation3]. Although surgery is the most effective treatment method, it also includes complications, which may inevitably lead to irreversible blindness [Citation4,Citation5]. Therefore, further research on the pathogenesis of cataract and new treatment methods is the key to improving its therapeutic effect.
Oxidative stress (OS), an imbalance between oxidation and antioxidation in the body, tends to oxidize cells and leads to neutrophil inflammatory infiltration and the production of extensive oxidative intermediates [Citation6,Citation7]. OS, a side effect of free radicals in an organism, is a supposed key factor which results in aging and pathema [Citation8]. It is associated with the mechanisms of cataract, which are mainly caused by oxygen free radicals induced lens epithelial cells injury, change in protein conformation, and protein cross-linking and accumulation [Citation9]. This stress can cause lens epithelial cells (LECs) apoptosis, leading to cataract, the common cellular basis of ARC. Nevertheless, the concrete mechanisms of oxidative stress-induced LECs apoptosis and ARC are still unclear.
Long noncoding RNA (LncRNA), a kind of ribonucleic acid (RNA) transcript, containing more than 200 nucleotides yet lacks protein coding potential [Citation10]. It is associated with gene regulation and maintaining genome stability, and plays a significant role in cell growth, migration, invasion, apoptosis, and differentiation [Citation11]. According to recent studies, lncRNA expression is closely related with a variety of tumors, which regulates genes expressions and associates with abnormal proliferation and chemotherapy resistance. Yi et al. suggested that lncRNA KCNQ1OT1 accelerated the chemoresistance of oxaliplatin in colorectal carcinoma through the miR-34a/autophagy related 4B pathway [Citation12]. Meanwhile, Wang et al. reported that KCNQ1OT1 accelerated gastric cancer development via miR-4319/DNA damage regulated autophagy modulator 2 axis [Citation13]. However, the function of KCNQ1OT1 in ARC remains unclear, and its mechanism requires further research.
microRNAs (miRNAs), a group of endogenous, small non-coding RNAs, which regulate gene expression post-transcriptionally by binding with 3’-untranslated regions of their target mRNAs [Citation14,Citation15]. Recent research has indicated that miRNAs play an important role in the pathogenesis of cataracts [Citation16–18]. miR-124-3p has been proved to be down-regulated in the cataractous lens samples [Citation19]. Several studies have shown that miR-124-3p plays an important role in the regulation of oxidative stress and inflammatory responses [Citation20,Citation21]. These researches suggested that miR-124-3p may participates in ARC via regulating oxidative stress. Excitingly, bioinformatics analysis revealed the existence of binding sites for KCNQ1OT1 and miR-124-3p.
In the present study, we hypothesized that KCNQ1OT1 plays a role in ARC development through regulating oxidative stress via miR-124-3p. Therefore, Therefore, our study aimed to reveal the underlying functions of KCNQ1OT1 in SRA01/04 cells after oxidative stress and explain its latent mechanisms.
Materials and methods
Specimens
20 cases anterior lens capsules were collected from 20 ARC patients (10 male, 10 female; age 52–74 years old) undergoing phacoemulsification surgery at the Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science. Besides, another 20 anterior lens capsules of transparent lens (10 male, 10 female; age 55–71 years old) obtained from the Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science were served as the Healthy control. All specimens were immediately stored in liquid nitrogen at the time of collection. This study was approved by the Ethics Committee of the Xiangyang Central Hospital, Affiliated Hospital of Hubei University of Arts and Science, and signed informed consent was obtained from each patient.
Cell cultivation and oxidative stress model establishment
The SRA01/04 cells were obtained from the American Type Culture Collection (ATCC), cultivated in Dulbecco’s modified easy medium (DMEM), which contained 10% FBS (Invitrogen), 1% penicillin, and streptomycin, and maintained at 37°C with 5% CO2 in an incubator. The cells were stimulated with 200 µM H2O2 for 24 h to conduct the oxidative stress model in vitro [Citation22].
Dual-luciferase reporter assay [Citation23]
TargetScan (StarBase, Microrna) was used to investigate the latent target genes of KCNQ1OT1. We built the reporter vector KCNQ1OT1 wild-type (KCNQ1OT1-WT) or KCNQ1OT1 mutated-type (KCNQ1OT1-MUT) 3’-UTR luciferase reporter gene plasmids using genomic PCR and pMIR vectors (Ambion, USA). Next, miR-124-3p wild-type or mutant portion bound to miR-124-3p, mimic or mimic control, were added into 293 cells using Lipofectamine 2000 (Invitrogen) according to the instructions. Luciferase activity was assessed via a dual-luciferase reporter assay (Promega).
qRT-PCR analysis
After treatment, KCNQ1OT1 and miR-124-3p levels were examined using qRT-PCR. The separation of RNA from SRA01/04 was conducted by TRIzol reagent (TaKara, Shiga, Japan) according to the instructions. Next, the total RNA was reversed to cDNA in accordance with the instructions of the PrimeScript RT Reagent Kit (TaKaRa, China). Furthermore, qRT-PCR analysis was performed by the SYBR PrimeScript RT-PCR Kit (Vazyme, Nanjing, Jiangsu) with ABI PRISM 7900 sequence detection system (Vazyme, Nanjing, Jiangsu). The target gene expressions were assessed by 2−ΔΔCt formula [Citation24].
Western blot analysis [Citation25]
The total proteins were harvested from the SRA01/04 cells with a radioimmunoprecipitation (RIPA) lysis buffer (Beyotime). The protein consistencey was evaluated by NanoDrop. The specimens were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and diverted to poly(vinylidene fluoride) (PVDF) membrane. After they were treated with 5% nonfat milk for 1 h, the membranes were cultivated in primary antibodies against β-actin, Bcl2, and Bax (1: 1000 dilution) overnight at 4°C. Following that, the membranes were treated with secondary antibodies for 1 h. Finally, the protein signals were detected by the enhanced chemiluminescence (ECL) detection system reagents (Pierce, USA) and quantified using Image J Software.
Cell transfection
The control-siRNA, KCNQ1OT1-siRNA, inhibitor control, miR-124-3p inhibitor, mimic control or miR-124-3p mimic were synthesized by GenePharma (Shanghai, China) and transfected into the SRA01/04 cells by Lipofectamine™3000 (Invitrogen) according to the instructions. After 48 hours of treatment, RNA was extracted for qRT-PCR analysis, and the Western blot was used to evaluate the protein expression.
MTT assay [Citation26]
The SRA01/04 cells were cultured in 96-well plates at 37°C. Next, the cells were mixed with 10 μl MTT (5 mg/ml) solution and cultivated for 4 h. The culture medium was removed and 150 ul of dimethyl sulfoxide (DMSO) was added to dissolve the formazan product in darkness for 10 min. Finally, the OD570nm wavelength was measured by a microplate reader (BioTek, USA) after vibration mixing according to the instructions.
Flow cytometry analysis [Citation27]
After treatment, SRA01/04 cells apoptosis was checked by an annexin V-FITC/PI apoptosis detection kit (BD Bioscience) according to the instructions. Finally, the cells were assessed by the BD FACSCalibur flow cytometer (BD Technologies) and analyzed using FlowJo Analysis software.
Statistical analysis
All results were expressed as mean ± SD from three independent experiments. A one-way analysis of variance (ANOVA) or Student’s t-test was used to estimate the mean diversity between the groups. Statistical significance was set as p < 0.05.
Results
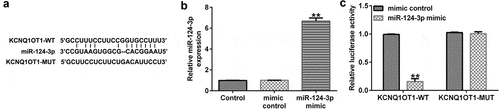
miR-124-3p directly interacted with KCNQ1OT1
Previous reports confirmed that lncRNAs and miRNAs were involved in cataracts. In addition, KCNQ1OT1 was significantly up-regulated in cataracts. Thus, we first investigated the correlation between KCNQ1OT1 and miRNAs using bioinformatics tools (Starbase) which revealed the binding sites between miR-124-3p and KCNQ1OT1 (). Furthermore, it was revealed that miR-124-3p mimic significantly enhanced miR-124-3p levels in 293 T cells compared to the mimic control group (). Furthermore, the dual-luciferase reporter assay suggested that miR-124-3p mimic significantly reduced KCNQ1OT1-WT luciferase activity, while KCNQ1OT1-MUT luciferase activity had no change (), which suggested that miR-124-3p directly interacted with KCNQ1OT1.
Figure 1. miR-124-3p directly targeted KCNQ1OT1.
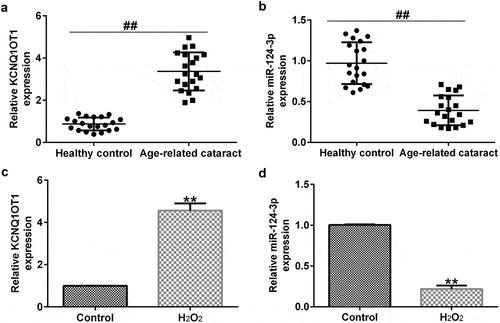
KCNQ1OT1 was significantly over-expressed and miR-124-3p was low-expressed in H2O2-stimulated SRA01/04 cells
To confirm the expression of miR-124-3p and KCNQ1OT1 in ARC, the level of miR-124-3p and KCNQ1OT1 in anterior lens capsules of ARC patients were determind using RT-qPCR. Results indicated that compared with the healthy control group, KCNQ1OT1 was significantly enhanced, while miR-124-3p was down-regulated in the anterior lens capsules of ARC patients (). SRA01/04 cells were exposed to 200 µM H2O2 to generate oxidative stress model in vitro. The miR-124-3p and KCNQ1OT1 levels in the H2O2-induced SRA01/04 cells were assessed via qRT-PCR analysis. As displayed in , KCNQ1OT1 levels were higher in the H2O2-treated SRA01/04 cells compared to the control group. Our findings demonstrated that miR-124-3p was significantly down-regulated after H2O2 treatment, which indicated that KCNQ1OT1/miR-124-3p played a regulatory role in H2O2-stimulated SRA01/04 cells.
Figure 2. Effects of H2O2 on miR-124-3p and KCNQ1OT1 level in SRA01/04 cells.
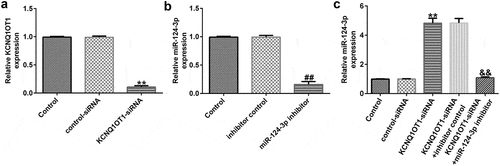
KCNQ1OT1 negatively regulated miR-124-3p expression in SRA01/04 cells
To further explore the regulatory functions of KCNQ1OT1 and miR-124-3p in the H2O2-stimulated SRA01/04 cells, they were exposed to control-siRNA, KCNQ1OT1-siRNA, inhibitor control or miR-124-3p inhibitor. Our data suggested that KCNQ1OT1-siRNA reduced KCNQ1OT1 levels in SRA01/04 cells (). Moreover, our findings (as shown in ) revealed that miR-124-3p was down-regulated after miR-124-3p inhibitor transfection compared to the inhibitor control group. In addition, compared to the control-siRNA group, KCNQ1OT1-siRNA significantly promoted miR-124-3p expression in SRA01/04 cells. However, this promotion was eliminated by miR-124-3p inhibitor (), which suggested that KCNQ1OT1 negatively regulated miR-124-3p expression in SRA01/04 cells.
Figure 3. Down-regulation of miR-124-3p abolished the influence of KCNQ1OT1-siRNA on miR-124-3p level in SRA01/04 cells.
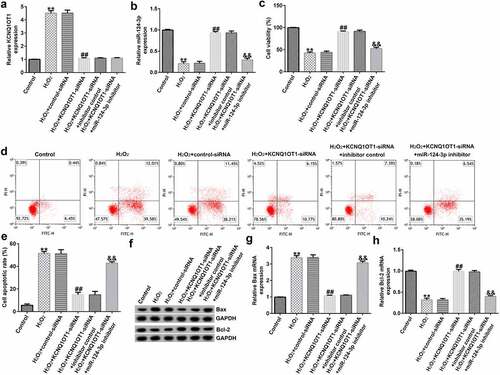
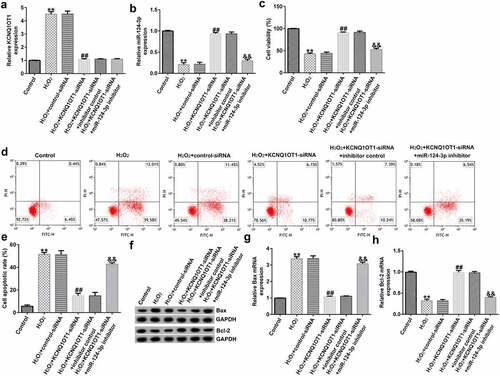
Down-regulation of miR-124-3p reversed the roles of KCNQ1OT1-siRNA in H2O2-stimulated oxidative stress in SRA01/04 cells
To further study the roles of miR-124-3p in H2O2-stimulated SRA01/04 cells, the SRA01/04 cells were induced by control-siRNA, KCNQ1OT1-siRNA, inhibitor control or miR-124-3p inhibitor, followed by 200 µM H2O2. Next, we conducted a qRT-PCR analysis to evaluate the miR-124-3p and KCNQ1OT1 levels in different groups. We found that the level of KCNQ1OT1 was higher and miR-124-3p was lower in H2O2-stimulated SRA01/04 cells than that in control group (). Compared to H2O2+ control-siRNA group, the expression of KCNQ1OT1 was reduced and miR-124-3p expression was fortified in KCNQ1OT1-siRNA transfected cells (), while the increased miR-124-3p level which resulted from KCNQ1OT1-siRNA transfection was reversed by down-regulating miR-124-3p (). Further, the MTT and flow cytometry assay suggested that KCNQ1OT1-siRNA promoted growth in H2O2-stimulated SRA01/04 cells, and this increase was eliminated by down-regulating miR-124-3p (). In addition, KCNQ1OT1-siRNA stimulation resulted in reduced apoptotic cells, increased Bcl-2 expression and reduced Bax level. Furthermore, these data were eliminated by miR-124-3p inhibitor, which suggested that KCNQ1OT1 relieved H2O2-stimulated oxidative stress via regulating miR-124-3p ().
Figure 4. Down-regulation of miR-124-3p abolished the influence of KCNQ1OT1-siRNA on cells growth and apoptosis in H2O2-stimulated SRA01/04 cells.
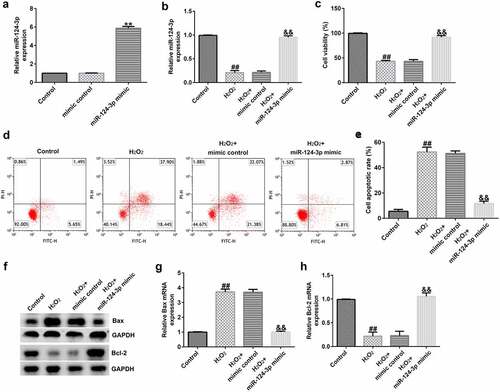
miR-124-3p relieved H2O2-stimulated cell injury in SRA01/04 cells
To lucubrate the roles of miR-124-3p in H2O2-stimulated SRA01/04 cells, SRA01/04 cells were induced by mimic control or miR-124-3p mimic and 200 µM H2O2 for 24 h. Results from a qRT-PCR suggested that up-regulation of miR-124-3p enhanced the messenger RNA (mRNA) level of miR-124-3p in SRA01/04 cells compared to mimic control (). The results (as shown in ) confirmed that H2O2 led to inhibited miR-124-3p expression, depressed cell growth, as well as promoted apoptotic cells. Furthermore, H2O2 decreased Bcl-2 expression and increased Bax level (). However, all these findings were reversed by miR-124-3p mimic, which suggested that miR-124-3p alleviated H2O2-induced cells injury in SRA01/04 cells.
Figure 5. Influence of miR-124-3p on H2O2-stimulated SRA01/04 cells viability and apoptosis.
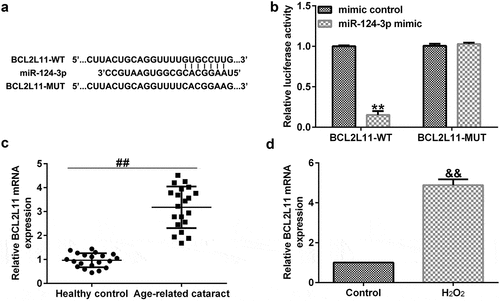
BCL2L11 directly targeted miR-124-3p
As we had already explored the relevance between KCNQ1OT1 and miR-124-3p, we explained the latent mechanisms of miR-124-3p in SRA01/04 cells. TargetScan analysis revealed that BCL2L11 was a candidate target of miR-124-3p (). Further, the dual luciferase reporter gene system verified that BCL2L11 was a sponge for miR-124-3p (). Moreover, we found that compared with the healthy control group, BCL2L11 was significantly enhanced in the anterior lens capsules of ARC patients (). And BCL2L11 level was higher in the H2O2-treated SRA01/04 cells compared to the control group (). All the abovementioned findings revealed that down-regulation of lncRNA KCNQ1OT1 protected LECs from oxidative stress stimulated apoptosis through mediating the miR-124-3p/BCL2L11 pathway in ARC.
Figure 6. BCL2L11 directly targeted miR-124-3p.
Discussion
The novel breakthroughs of this research included: i) Down-regulation of lncRNA KCNQ1OT1 alleviated oxidative stress induced injury in SRA01/04 cells; ii) the protective effects of KCNQ1OT1 on H2O2-stimulated cells were regulated through the miR-124-3p/BCL2L11 pathway. To our knowledge, we are the first study to explained the functions of KCNQ1OT1 on H2O2-stimulated cells apoptosis and investigate the latent underlying mechanisms.
There are three major forms of ARC, which include the cortical cataract, nuclear cataract, and posterior subcystic cataract [Citation28]. Regardless of how they differ, they are associated with abnormal growth or apoptotic cell death in the lens. Recently, studies on the pathogenesis of cataract found that LECs apoptosis was closely associated with the pathogenesis of cataract, which has attracted widespread attention [Citation29,Citation30]. Li et al. suggested that microRNA-15a regulated LECs apoptosis and viability by targeting Bcl-2 and E2F3 in ARC [Citation31]. Furthermore, Lu et al. suggested that miR-211 accelerated LECs apoptosis by targeting SIRT1 in ARC. In addition, previous studies have shown that oxidative stress was identified as one of the prime reasons for cataract [Citation32]. Exposure of lens epithelial cells to ultraviolet light, calcium ions, or H2O2 may lead to an increase in the abundance of apoptotic proteins, thereby inducing cell death, which is a familiar cellular basis for ARC [Citation33]. In our research, SRA01/04 cells were stimulated with 200 µM H2O2 to conduct the ARC model in vitro.
Various lncRNAs, which included lncRNA PLCD3-OT1 and lncRNA H19, have been evidenced to be associated with ARC [Citation34,Citation35]. LncRNA KCNQ1OT1, has also been evidenced to be related to the advance of multiple diseases, including cancer [Citation11–13] and cataract [Citation36–38]. Jin et al. reported that KCNQ1OT1 silencing significantly inhibited H2O2-induced SRA01/04 cell pyroptosis, which is the critical step in cataract formation [Citation36]. Liu et al indicated that knockdown of KCNQ1OT1 inhibits cell viability, migration and epithelial-mesenchymal transition in high glucose-treated lens epithelial cells in diabetic cataract [Citation38]. However, the functions of KCNQ1OT1 in ARC have not been fully explained. Based on Starbase and the dual-luciferase reporter analysis, we observed that miR-124-3p was a sponge of KCNQ1OT1. MiR-124-3p was a tumor regulator in many cancers, such as angiotensin II-dependent hypertension [Citation39], gastric cancer [Citation40], and peripheral arterial disease [Citation41]. Besides, miR-124-3p has been revealed to play important roles in the regulation of oxidative stress, which is the critical factor in ARC [Citation20,Citation21]. Nevertheless, miR-124-3p functions in ARC are largely indistinct. Consistent with previous studies [Citation36,Citation37], we further verified that KCNQ1OT1 was remarkably over-expressed in the anterior lens capsules of ARC patients and in H2O2-stimulated SRA01/04 cells. While miR-124-3p was down-expressed in anterior lens capsules of ARC patients and in H2O2-stimulated SRA01/04 cells. Therefore, we hypothesized that KCNQ1OT1 participated in ARC through regulating miR-124-3p. And we confirmed that KCNQ1OT1 negatively regulated miR-124-3p expression in SRA01/04 cells.
To better illustrate the KCNQ1OT1/miR-124-3p functions in H2O2-treated SRA01/04 cells, they were exposed to control-siRNA, KCNQ1OT1-siRNA, inhibitor control, or miR-124-3p inhibitor, and 200 µM H2O2 for another 24 h. Results from the MTT and flow cytometry revealed that KCNQ1OT1-siRNA promoted SRA01/04 cells viability and decreased apoptotic cells. Further analysis suggested that Bax was down-expressed and Bcl-2 was up-regulated in lens epithelial cells from cataract patients, which were reversed via miR-124-3p inhibitor.
To further explore the roles of miR-124-3p in ARC, the SRA01/04 cells were treated with mimic control, miR-124-3p mimic, or H2O2 for 24 h. Our data indicated that miR-124-3p mimic enhanced the mRNA level of miR-124-3p in SRA01/04 cells compared to mimic control. Meanwhile, H2O2 led to inhibited miR-124-3p expression, depressed cell growth, as well as promoted apoptotic cells, decreased Bcl-2 expression, and increased Bax levels. However, all these observations were reversed through miR-124-3p mimic, which indicated that miR-124-3p alleviated H2O2-induced SRA01/04 cells growth and apoptosis. BCL2L11, a novel target of miR-9, was found to provoke apoptosis [Citation42,Citation43]. Our data further implied that BCL2L11 was a direct target of miR-124-3p, and it was significantly enhanced in the anterior lens capsules of ARC patients and in the H2O2-treated SRA01/04 cells.
Conclusion
The finding revealed that down-regulation of lncRNA KCNQ1OT1 protected LECs from oxidative stress stimulated apoptosis via regulating the miR-124-3p/BCL2L11 pathway in age-related cataract. Therefore, it is expected to be a promising avenue for ARC therapy. However, further investigations are required to illustrate the mechanisms of how BCL2L11 expression affects age-related cataract.
Research highlights
KCNQ1OT1 was significantly over-expressed and miR-124-3p was low-expressed in H2O2-stimulated SRA01/04 cells;
Down-regulation of miR-124-3p reversed the roles of KCNQ1OT1-siRNA in H2O2-stimulated oxidative stress in SRA01/04 cells;
miR-124-3p relieved H2O2-stimulated cell injury in SRA01/04 cells.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hugosson M, Ekstrom C. Prevalence and risk factors for age-related cataract in Sweden. Ups J Med Sci. 2020;125(4):311–315.
- Zhu Z, Wang L, Scheetz J, et al. Age-related cataract and 10-year mortality: the Liwan eye study. Acta Ophthalmol. 2020;98(3):e328–e332.
- Wang Y, Liu Z, Huang C, et al. Analysis of lens epithelium telomere length in age-related cataract. Exp Eye Res. 2020;201:108279.
- Jain S, Rajshekar K, Aggarwal A, et al. Effects of cataract surgery and intra-ocular lens implantation on visual function and quality of life in age-related cataract patients: a systematic review protocol. Syst Rev. 2019;8(1):204.
- Wang Y, Du J, Yang M, et al. Distinct macular thickness changes after femtosecond laser-assisted cataract surgery of age-related cataract and myopia with cataract. Sci Rep. 2018;8(1):3279.
- Wang H, Wang N, Xu D, et al. Oxidation of multiple MiT/TFE transcription factors links oxidative stress to transcriptional control of autophagy and lysosome biogenesis. Autophagy. 2020;16(9):1683–1696.
- Thomas MM, Haghiac M, Grozav C, et al. Oxidative stress impairs fatty acid oxidation and mitochondrial function in the term placenta. Reprod Sci. 2019;26(7):972–978.
- Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148–160.
- Ma Z, Liu J, Li J, et al. Klotho ameliorates the onset and progression of cataract via suppressing oxidative stress and inflammation in the lens in streptozotocin-induced diabetic rats. Int Immunopharmacol. 2020;85:106582.
- Liu J, Liu ZX, Wu QN, et al. Long noncoding RNA AGPG regulates PFKFB3-mediated tumor glycolytic reprogramming. Nat Commun. 2020;11(1):1507.
- Liu C, Wang L, Li YW, et al. Long noncoding RNA LUCAT1 promotes migration and invasion of prostate cancer cells by inhibiting KISS1 expression. Eur Rev Med Pharmacol Sci. 2020;24(13):7213.
- Li Y, Li C, Li D, et al. lncRNA KCNQ1OT1 enhances the chemoresistance of oxaliplatin in colon cancer by targeting the miR-34a/ATG4B pathway. Onco Targets Ther. 2019;12:2649–2660.
- Wang J, Wu F, Li Y, et al. KCNQ1OT1 accelerates gastric cancer progression via miR-4319/DRAM2 axis. Int J Immunopathol Pharmacol. 2020;34:2058738420954598.
- Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, et al. An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234(5):5451–5465.
- Lu TX, Rothenberg ME. MicroRNA. J Allergy Clin Immunol. 2018;14(4):1202–1207.
- Zhou CY, Huang XQ, Li X, et al. Circular RNA erythrocyte membrane protein band 4.1 assuages ultraviolet irradiation-induced apoptosis of lens epithelial cells by stimulating 5’-bisphosphate nucleotidase 1 in a miR-24-3p-dependent manner. Bioengineered. 2021;12(1):8953–8964.
- Yu X, Zheng H, Chan MT, et al. MicroRNAs: new players in cataract. Am J Transl Re. 2017;9:3896–3903.
- Kumar S, Vijayan M, Bhatti JS, et al. MicroRNAs as peripheral biomarkers in aging and age-related diseases. Prog Mol Biol Transl Sci. 2017;146:47–94.
- Wu C, Liu Z, Ma L, et al. MiRNAs regulate oxidative stress related genes via binding to the 3’ UTR and TATA-box regions: a new hypothesis for cataract pathogenesis. BMC Ophthalmol. 2017;17(1):142.
- Geng L, Liu W, Chen Y. miR-124-3p attenuates MPP+-induced neuronal injury by targeting STAT3 in SH-SY5Y cells. Exp Biol Med (Maywood). 2017;242(18):1757–1764.
- Wei YJ, Wang JF, Cheng F, et al. miR-124-3p targeted SIRT1 to regulate cell apoptosis, inflammatory response, and oxidative stress in acute myocardial infarction in rats via modulation of the FGF21/CREB/PGC1alpha pathway. J Physiol Biochem. 2021;77(4):577–587.
- Tu Y, Li L, Qin B, et al. Long noncoding RNA glutathione peroxidase 3-antisense inhibits lens epithelial cell apoptosis by upregulating glutathione peroxidase 3 expression in age-related cataract. Mol Vis. 2019;25:734–744.
- Zhou XQ, Chang YZ, Zhu LR, et al. LINC00839/miR-144-3p/WTAP (WT1 associated protein) axis is involved in regulating hepatocellular carcinoma progression. Bioengineered. 2021;12(2):10849–10861.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408.
- Han D, Yu ZJ, Zhang H, et al. Microenvironment-associated gene HSD11B1 may serve as a prognostic biomarker in clear cell renal cell carcinoma: a study based on TCGA, RT‑qPCR, western blotting, and immunohistochemistry. Bioengineered. 2021;12(2):10891–10904.
- Kumar P, Nagarajan A, and Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc. 2018;2018 (6) 469–471.
- Telford WG. Multiparametric analysis of apoptosis by flow cytometry. Methods Mol Biol. 2018;1678:167–202.
- Pendergrass W, Zitnik G, Tsai R, et al. X-ray induced cataract is preceded by LEC loss, and coincident with accumulation of cortical DNA, and ROS; similarities with age-related cataracts. Mol Vis. 2010;16:1496–1513.
- Kronschläger M, Ruiß M, Dechat T, et al. Single high-dose peroral caffeine intake inhibits ultraviolet radiation-induced apoptosis in human lens epithelial cells in vitro. Acta Ophthalmol. 2021;99(4):e587–e593.
- Zhou W, Xu J, Wang C, et al. miR-23b-3p regulates apoptosis and autophagy via suppressing SIRT1 in lens epithelial cells. J Cell Biochem. 2019;120(12):19635–19646.
- Li Q, Pan H, Liu Q. MicroRNA-15a modulates lens epithelial cells apoptosis and proliferation through targeting B-cell lymphoma-2 and E2F transcription factor 3 in age-related cataracts. Biosci Rep. 2019;39(12). DOI:10.1042/BSR20191773
- Lu B, Christensen IT, Ma LW, et al. Yan QC. miR-211 promotes lens epithelial cells apoptosis by targeting silent mating-type information regulation 2 homolog 1 in age-related cataracts. Int J Ophthalmol. 2018;11(2):201–207.
- Gu XL. MicroRNA-124 prevents H2O2-induced apoptosis and oxidative stress in human lens epithelial cells via inhibition of the NF-kappaB signaling pathway. Pharmacology. 2018;102(3–4):213–222.
- Xiang J, Chen Q, Kang L, et al. LncRNA PLCD3-OT1 functions as a CeRNA to prevent age-related cataract by sponging miR-224-5p and regulating PLCD3 expression. Invest Ophthalmol Vis Sci. 2019;60(14):4670–4680.
- Cheng T, Xu M, Qin B, et al. lncRNA H19 contributes to oxidative damage repair in the early age-related cataract by regulating miR-29a/TDG axis. J Cell Mol Med. 2019;23(9):6131–6139.
- Jin X, Jin H, Shi Y, et al. Long non-coding RNA KCNQ1OT1 promotes cataractogenesis via miR-214 and activation of the caspase-1 pathway. Cell Physiol Biochem. 2017;42(1):295–305.
- Yao L, Yang L, Song H, et al. MicroRNA miR-29c-3p modulates FOS expression to repress EMT and cell proliferation while induces apoptosis in TGF-β2-treated lens epithelial cells regulated by lncRNA KCNQ1OT1. Biomed Pharmacother. 2020;129:110290.
- Liu J, Dong Y, Wen Y, et al. LncRNA KCNQ1OT1 knockdown inhibits viability, migration and epithelial-mesenchymal transition in human lens epithelial cells via miR-26a-5p/ITGAV/TGF-beta/Smad3 axis. Exp Eye Res. 2020;200:108251.
- Lv L, Shen J, Xu J, et al. MiR-124-3p reduces angiotensin II-dependent hypertension by down-regulating EGR1. J Hum Hypertens. 2021;35(8):696–708.
- Wu Q, Zhong H, Jiao L, et al. MiR-124-3p inhibits the migration and invasion of gastric cancer by targeting ITGB3. Pathol Res Pract. 2020;216(1):152762.
- Shi Y, Xu X, Luan P, et al. miR1243p regulates angiogenesis in peripheral arterial disease by targeting STAT3. Mol Med Rep. 2020;22(6):4890–4898.
- Huang J, Huang Y, Feng Z, et al. MiR-1247-3p protects rat cardiomyocytes against hypoxia/reoxygenation-induced injury via targeting BCL2L11 and caspase-2. J Recept Signal Transduct Res. 2021;41(1):6–14.
- Chen J, Lin Y, Jia Y, et al. LncRNA HAND2-AS1 exerts anti-oncogenic effects on ovarian cancer via restoration of BCL2L11 as a sponge of microRNA-340-5p. J Cell Physiol. 2019;234(12):23421–23436.
