ABSTRACT
This study explored the role and potential molecular mechanism of phillyrin in cerebral ischemia/reperfusion (I/R) injury. The rat middle cerebral artery occlusion (MCAO)/R model was constructed, and cerebral infarction volume, brain water content, and neurological score were measured. Neuron morphological structures in brain tissues and primary neuron apoptosis were detected using hematoxylin and eosin (H&E) staining and Hoechst 33258 staining, respectively. In MCAO/R rats, phillyrin markedly reduced cerebral infarction volume, neurological score, and brain water content and inhibited neuron apoptosis. In vitro experiments showed that phillyrin remarkably increased viability and decreased lactate dehydrogenase (LDH) release of H2O2-injured neurons. Moreover, phillyrin remarkably downregulated the proportion of apoptosis-related protein B-associated X (Bax)/B-cell lymphoma protein 2 (Bcl-2) and reduced procaspase-3, phospho-Akt (p-Akt-1), and phosphorylation-mammalian target of rapamycin (p-mTOR) levels in H2O2-injured neurons. Furthermore, phosphatidylinositol-3 kinase (PI3K) inhibitor ZSTK474 weakened the effects of phillyrin on p-mTOR, p-Akt-1, characteristic proteins of autophagy 3-II (LC3-II) and beclin-1 levels, and H2O2-induced neuronal apoptosis and autophagy. Taken together, phillyrin alleviates I/R injury by inhibiting neuronal cell apoptosis and autophagy pathway, which may provide a new treatment strategy for cerebral I/R injury.
Graphical Abstract
Background
Cerebral ischemia reperfusion (I/R) injury is a disease caused by restoring blood flow after cerebral ischemia for a certain period of time, leading to the aggravation of ischemic injury of brain tissues, with a high incidence and disability rate [Citation1,Citation2]. Oxidative stress is a major complication of cerebral I/R injury, which could result in the maladjustment of the body’s antioxidant defense system, toxicity, cell damage, and apoptosis [Citation3]. Therefore, many researchers have suggested that oxidative stress is one of the primary mechanisms that aggravates injury in the cerebral ischemia pathological process [Citation4,Citation5]. The main pathological features of cerebral I/R injury are neuron injury and apoptosis, with the latter being the major modality of neuron damage after cerebral I/R injury [Citation6,Citation7]. Neuronal apoptosis is a dynamic process. Apoptotic neuron number increases with the reperfusion time extending, further promoting nerve injury occurrence and development [Citation8]. Autophagy is a complex physiological process in which damaged intracellular proteins and organelles are degraded into cell proliferating materials [Citation9]. Moderate autophagy has a protective effect on cell growth, while excessive autophagy will lead to apoptosis [Citation10]. Studies have shown that oxidative stress induces apoptosis and activates autophagy [Citation11,Citation12]. Thus, the relationship between apoptosis and autophagy is complex, involving multiple signal transduction pathways in cells [Citation13].
Phillyrin is an important active ingredient in Forsythia suspensa, a traditional Chinese medicine [Citation14]. Recent studies have found that phillyrin has anti-viral, anti-inflammation, anti-oxidation, and many other physiological functions. Phillyrin plays a protective role against H2O2 in PC12 cells, a human brain neuroblastoma cell line by activating mitochondria-dependent apoptosis and reducing Bax/Bcl-2 ratio [Citation15]. In addition, phillyrin could inhibit LPS-induced pulmonary inflammation in acute lung injury mice via suppressing mitogen-activated protein kinase (MAPK) and nuclear factor κB (NF-κB) activation [Citation16]. Nevertheless, how phillyrin involves in cerebral I/R injury, neuronal cell apoptosis, and autophagy induced by oxidative stress remains unclear. Therefore, to explore the role and potential molecular mechanism of phillyrin in cerebral ischemia/reperfusion (I/R) injury, we established a rat MCAO/R model using an improved MCAO method and an oxidative stress injury cell model induced by H2O2 and further investigated whether and how phillyrin involves in cerebral I/R injury.
Methods
Materials
From the Experimental Animal Center of Leshan People’s Hospital, male Sprague Dawley (SD) rats (220–250 g weight, 5–7 weeks old) were purchased and used to establish MCAO/R model. Primary cortical neurons prepared from SD rats (2 days old) were obtained from the Experimental Animal Center of Leshan People’s Hospital. The Animal Research Committee of Leshan People’s Hospital in China approved this study (No. LS753), which was conducted strictly following the guidelines of the National Institutes of Health for the care and use of experimental animals. Phillyrin with purity greater than 98% (molecular weight: 576.85) was obtained from Yuanye Biotechnology Company, Limited (Shanghai, China), and its chemical structure is shown in . Using Neurobasal medium (Invitrogen, Carlsbad, CA, USA), phillyrin was dissolved as 10 mM DMSO and diluted to the required concentration as indicated.
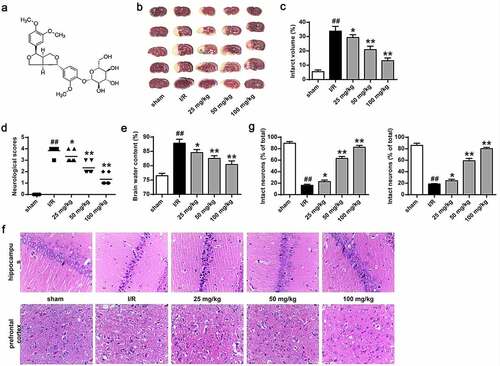
Figure 1. Phillyrin improved the MCAO/R rat brain injury. (a) Schematic diagram of chemical structure of phillyrin. (b) The cerebral infarction analyzed by TTC staining. (c) Percentage of cerebral infarction volume. (d) Neurological deficits of rats. (e) Brain water content of rats. (f) Surviving neuron number in the prefrontal cortex and hippocampus analyzed by H&E staining. (g) Percentage of infarct neurons in prefrontal cortex and hippocampus of rats. Note: n = 6; *P < 0.05 and **P < 0.01 compared with I/R group, ## P < 0.01 compared with the sham group.
MCAO/R model establishment
MCAO/R model was established as previously reported [Citation17]. The rat core temperature was maintained at 37°C throughout the test. First, 10% (w/v) chloral hydrate (350 mg/kg) was intraperitoneally injected into anesthetized rats. Next, after finding the left common carotid artery (CCA) and separating the external carotid artery (ECA), CCA and ECA were blocked with a hemostat. Subsequently, to avoid damaging the middle cerebral artery, the tip of a heparin-coated nylon monofilament (0.25–0.28 mm in diameter) was inserted into the internal carotid artery (ICA) from CCA. To detect the local cerebral blood flow (rCBF), a laser Doppler flowmeter was used. The criterion for judging the success of arterial occlusion was that the rCBF dropped below 20% of the baseline. After removing Nylon monofilaments 2 h after MCAO, rats were reperfused for 24 h. MCAO was conducted on the sham operation group, but no nylon monofilament was inserted.
The rats were divided into I/R group, sham operation group, and three phillyrin groups (25, 50, and 100 mg/kg). Three days before modeling, the rats in phillyrin treatment groups were given different doses of phillyrin daily via gavage, while rats in sham operation and I/R group were simultaneously given the same volume of saline. The last injection was given 30 min before the operation.
TTC staining
The cerebral infarction volume in rats was measured using TTC staining [Citation18]. First, the brain tissue was obtained and cut into slices with 1.5 mm in thickness. After fixed in 4% paraformaldehyde, the brain sections were stained with 1% TTC for 30 min. The normal tissues were stained red, and the infarct tissues were kept white. The infarct volume was quantified using Bio-Rad Quantity One software. The infarct volume/total volume percentage was calculated.
Neurological score
The effect of phillyrin on nerve injury rats induced by I/R was assessed based on the neurological score [Citation19]. The neurobehavior of rats in each group was scored 24 h after reperfusion based on the specific scoring criteria: 4 for rats lost consciousness, 3 for rats fell to the left side, 2 for rats turned around, 1 for rats unable to fully extend the left foreleg, and 0 for rats with normal performance. Neurological deficit scores were evaluated by an examiner blinded to the experimental groups.
Brain water content determination
Wet and dry weight of the infarcted cerebral hemisphere in rats 24 h after reperfusion was measured before and after drying at 105°C as previously reported [Citation20]. The total brain water content = (wet weight – dry weight)/wet weight × 100%.
H&E staining
After fixed overnight with 4% paraformaldehyde, brain tissues in the prefrontal cortex and hippocampus were embedded with paraffin and cut into 6-μm-thick coronal sections using a microtome. Sections were selected for H&E staining [Citation21] in each rat, and the neuron survival was analyzed.
Culture of primary cortical neurons
Primary cortical neurons were isolated and cultured as previously reported [Citation22]. In brief, rat brain cortex tissues were digested using 0.125% trypsin in Hank’s buffer at 37°C for 10 min. The digested cortical tissues were ground. In Neurobasal medium containing 100 U/mL streptomycin, 0.5 mm glutamine, 2% B27, and 100 U/mL penicillin, the isolated monocytes were cultured for 7 days to obtain mature neurons. The obtained neuron purity examined using MAP2 antibody (Chemicon, CA, USA) was over 98%. The phillyrin with different concentrations was used to treat primary cortical neurons for 2 h and then with H2O2 of different concentrations to induce neuron injury.
Cell viability assay
Cell viability was measured by MTT assay [Citation23]. In brief, cortical neurons with a density of 5 × 104 were inoculated in each well of 96-well plates for 7 days. Some cells were treated with different concentrations of H2O2 for 30 min. Some cells were treated with H2O2 (100 μM) for different times. Some cells were treated with different concentrations of phillyrin for 2 h and then with H2O2 (100 μM) for 30 min. After treatment, the cells in each well were incubated with MTT solution (200 μL of 0.5 mg/mL) for 4 h. The formazan in each well was solubilized in 150 μL DMSO and determined by optical density at 570 nm on a microplate reader to represent neuron cell viability. The amount of LDH released from neurons was determined using an LDH kit following the manufacturer’s instructions to reflect cell damage degree.
Hoechst 33258 staining
The neuron cell apoptosis was detected by staining the apoptotic nuclei with Hoechst 33258 staining [Citation24] to generate blue fluorescence. Briefly, neuron cells with a density of 600 cells/mL were inoculated into each well. After cultured for 24 h, the cells were incubated with phillyrin for 2 h followed by 30 min treatment with H2O2 (100 μM). The cells were then stained with Hoechst 33258 (10 μg/mL) and fixed with 4% paraformaldehyde. Cells with bright blue nuclei in 30 random fields of vision were counted under a fluorescence microscope.
Western blot
Western blot was performed as previously reported [Citation25]. In brief, primary neuron cells were incubated with 20, 40, and 80 μM phillyrin for 2 h followed by 30 min treatment with 100 μM H2O2. Total proteins were extracted from these cells and transferred onto cellulose acetate membranes after separated by SDS-PAGE. After blocking with PBS solution for 1 h, the membranes were incubated with Bax (1:1000, Chemicon, Temecula, CA, USA), Bcl-2 (1:1000), and procaspase-3 (1:1000) primary antibodies from Chemicon, Temecula, CA, USA; pAkt-1 (1:1000), Akt-1 (1:1000), mTOR (1:1000), and LC3-II (1:1000) from Cell Signaling Technology, Danvers, MA, USA; and beclin-1 (1:1000) and β-actin (1:1000) primary antibodies from Sigma-Aldrich, St. Louis, MO, USA, at 4°C for overnight. Then, secondary antibody was added for 1 h at 25°C. ECL chemiluminescence solution was used to visualize protein bands and analyzed using Image J software.
Statistical analysis
All data are expressed as mean ± standard deviation (SD) and analyzed using one-way ANOVA and postmortem LSD tests. P < 0.05 indicated a significant difference.
Results
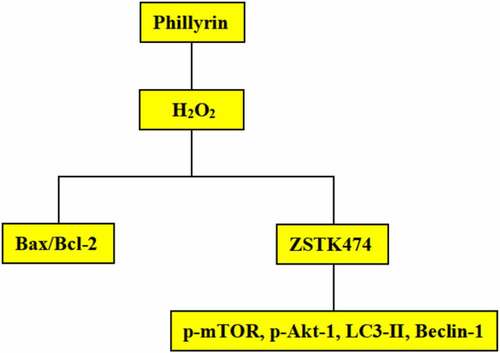
In this study, we explored the role and potential molecular mechanism of phillyrin in cerebral ischemia/reperfusion (I/R) injury. The rat MCAO/R model was constructed, and cerebral infarction volume, brain water content, and neurological score were measured. In MCAO/R rats, phillyrin markedly reduced cerebral infarction volume, neurological score, and brain water content and inhibited neuron apoptosis. Phillyrin remarkably increased viability and decreased LDH release of H2O2-injured neurons. Moreover, phillyrin remarkably downregulated the proportion of apoptosis-related protein Bax/Bcl-2 and reduced procaspase-3, p-Akt-1, and p-mTOR levels in H2O2-injured neurons. Furthermore, PI3K inhibitor ZSTK474 weakened the effects of phillyrin on p-mTOR, p-Akt-1, LC3-II, and beclin-1 levels and H2O2-induced neuronal apoptosis and autophagy. Our results suggested that phillyrin protects neurons by inhibiting neuron cell apoptosis and autophagy pathway, which provided new ideas for treating cerebral I/R injury.
Phillyrin improves the brain injury of MCAO/R rats
The rat MCAO/R model was used to measure phillyrin () function in the brain injury of MCAO/R rats (). Results (, P < 0.05, P < 0.01) revealed that the cerebral infarction volume (33.77 ± 2.74%), neurological score (3.83 ± 0.37%), and cerebral water content (87.80 ± 1.15%) in I/R group were remarkably higher than those in sham group but were significantly reduced in groups treated with phillyrin (P < 0.01). The mortality rate of rats was about 19% in all ischemic groups (data not shown).
In addition, H&E staining found obvious nuclear pyknosis and disordered arrangement in the neurons in the prefrontal cortex and hippocampus of rats in I/R group (). Moreover, intact neuron number was remarkably decreased in the I/R group, but phillyrin treatment increased intact neuron number in rat prefrontal cortex and hippocampus in a dose-dependent manner in the I/R group (, P < 0.05, P < 0.01). These results confirmed that phillyrin could remarkably alleviate rat cerebral I/R injury.
Phillyrin protects primary cortical neurons against H2O2-induced injuries
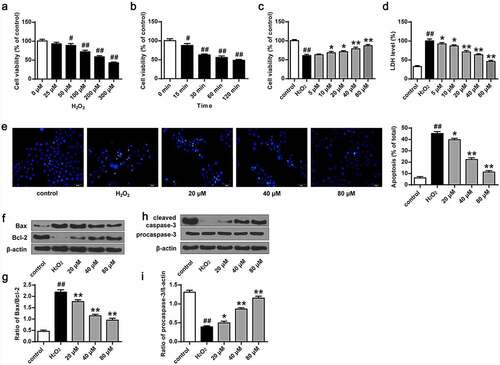
To explore phillyrin function in primary cortical neuron activity damaged by H2O2, we first treated primary cortical neuron cells with H2O2 at different doses for different times to establish an oxidative damage model. MTT assay showed that H2O2 treatment remarkably reduced the activity of primary cortical neuron cells and increased LDH release (P < 0.05, P < 0.01) in a dose- () and time-dependent manner (). In addition, H2O2 treatment also increased Bcl-2 and Bax expression (, P < 0.05) and increased cleaved caspase-3 level (, P < 0.05). To examine the effect of phillyrin treatment, we treated primary cortical neuron cells with 100 μM H2O2 for 30 min. We found that phillyrin treatment, in a dose-dependent manner, improved the viability (, P < 0.05) and reduced apoptosis rate of the H2O2-treated primary cortical neurons (, P < 0.01) and decreased LDH release from the H2O2-treated primary cortical neurons (, P < 0.01). Moreover, phillyrin treatment reduced Bax and Bcl-2 expression (, P < 0.01) and enhanced cleaved caspase-3 expression in the H2O2-induced primary cortical neuron cells in a dose-dependent manner (, P < 0.01). These indicated that phillyrin protected primary cortical neuron cells against H2O2-induced injuries.
Figure 2. Phillyrin improved the activity of primary neurons damaged by H2O2. (a) Cell viability treated with different concentrations of H2O2. (b) Cell viability treated with H2O2 (100 μM) at different time. (c) Effects of phillyrin at different concentrations on H2O2-treated neuron cell viability. (d) Effects of phillyrin with different concentrations on LDH release in H2O2- treated neuron cells. (e) Images of Hoechst staining of neurons and apoptosis rate of neurons in each group. (f, g) Effects of phillyrin with different concentrations on Bax and Bcl-2 expression in H2O2- treated neurons. (h, i) Effects of phillyrin with different concentrations on pro-caspase-3 expression in H2O2- treated neurons. Note: n = 6; *P < 0.05 and **P < 0.01 compared with the H2O2 group; # P < 0.05 and ## P < 0.01 compared with the control group.
Phillyrin promotes Akt-1 phosphorylation in primary neurons
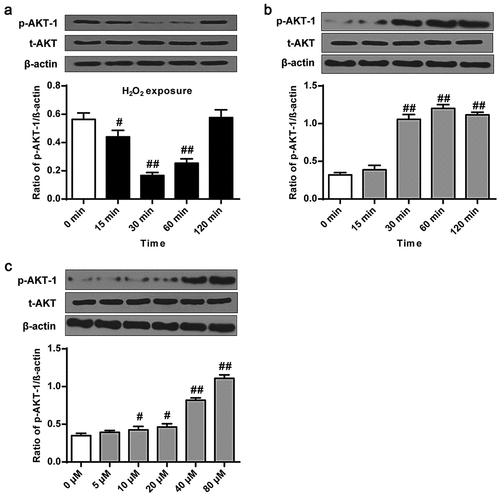
We then determined the effect of phillyrin on Akt-1 phosphorylation in primary neuron cells. First, we examined changes of p-Akt-1 level in primary cortical neuron cells after H2O2 treatment. Western blot results showed that p-Akt-1 level was significantly decreased in primary cortical neuron after H2O2 exposure for 15 min and 30 min and 60 min (, P < 0.05, P < 0.01, P < 0.01), indicating that H2O2 exposure decreased p-Akt-1 in a time-dependent manner. The results showed that phillyrin pretreatment increased p-Akt-1 level in primary cortical neuron cells treated with H2O2 for more than 30 min in a time- (, P < 0.01) and dose- (, P < 0.05, P < 0.01) dependent manner. These demonstrated that phillyrin promotes p-Akt-1 level in primary neuron cells in a dose- and time-dependent manner.
Figure 3. Phillyrin promoted Akt-1 phosphorylation in primary neurons. (a) Western blot showing (a) the effects of H2O2 (100 μM) at different treatment time on Akt and p-Akt-1 levels, (b) the effects of phillyrin at different treatment time on Akt and p-Akt-1 levels in H2O2-treated rat cortical neurons, and (c) the effects of phillyrin at different concentrations on Akt and p-Akt-1 levels in H2O2-treated rat cortical neurons. Note: n = 6; #P < 0.05 and ##P < 0.01 compared with control group.
Phillyrin improves autophagy of H2O2-treated primary neurons
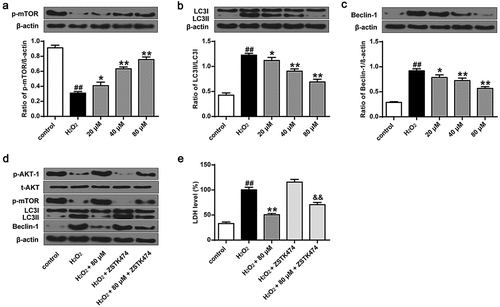
We further investigated the role of phillyrin in the autophagy of primary neurons after H2O2 exposure by detecting mTOR, LC3-II, and beclin-1 expression. The results showed that phillyrin reversed H2O2-induced decreases in p-mTOR and p-Akt-1 and increases in beclin-1 and LC3-II in primary cortical neuron cells in a dose-dependent manner (, P < 0.05, P < 0.01). These effects were blocked by PI3K inhibitor ZSTK474 (). Moreover, ZSTK474 treatment also blocked the effects of phillyrin on H2O2-induced increased LDH release from primary cortical neuron cells (, P < 0.01). It is possible that the dose of ZSTK474 is insufficient to totally block the effects of phillyrin. Altogether, these indicated that phillyrin protects primary cortical neuron cells against oxidative stress-induced injuries via the PI3K/Akt-1/mTOR pathway.
Figure 4. Phillyrin enhanced autophagy of H2O2-treated primary neuron cells. Western blot analysis showing the effects of phillyrin with different concentrations on mTOR (a), LC3 (b), and beclin-1 (c) in H2O2-treated neurons, and (d) the effects of ZSTK474 on the effects of phillyrin in H2O2-treated neurons. (e) LDH release from H2O2-treated neurons with different treatments. Note: n = 6; * P < 0.05 compared with the H2O2 group; **P < 0.01 compared with the H2O2 group; &&P < 0.01 compared with the phillyrin group; ##P < 0.01 compared with the control group.
Discussion
It has been shown that phillyrin could decrease neutrophil infiltration, reduce tissue necrosis, and increase survival rate in the LPS-stimulated zebrafish model [Citation16]. Nevertheless, the mechanism underlying the roles of phillyrin in oxidative stress-induced neuronal cell apoptosis and autophagy and cerebral I/R injury remains unclear. Here, we found that phillyrin treatment improved the cerebral water content, neurologic score, and cerebral infarction of rats in the I/R group. Zhang et al. reported that dendrobium polysaccharides alleviated focal cerebral I/R injury through decreasing neurological function, brain water content, and cerebral infarction volume in rats [Citation26]. Yang et al. reported that ginsenoside Rg1 alleviated cerebral I/R injury via PPAR/HO-1 pathway [Citation25]. Here, we showed that phillyrin mitigated MCAO/R-induced brain injury in rats and had certain neuroprotective effects.
Currently, oxidative stress is considered to be one of the factors causing nerve cell apoptosis in cerebral I/R injury [Citation27]. H2O2 is a strong oxidant able to produce a large amount of oxygen free radicals through Haber–Weiss or Fenton reactions in cells, resulting in oxidative damage and apoptosis of cells [Citation28,Citation29]. In this study, we established an oxidative stress injury model by treating rat primary cortical neurons with H2O2. Previous studies have used the ratio of Bax to Bcl-2 to reflect the cell viability [Citation30,Citation31] and revealed that activation of PI3K/Akt signaling pathway inhibits cell apoptosis [Citation32]. Sun et al. showed that luteolin markedly decreased myocardial infarction area, myocardial apoptosis rate, and LDH release in rats with myocardial I/R injury by upregulating Bcl-2 and p-Akt, thereby improving cardiac function [Citation33]. In this study, we found that phillyrin increased the activity of H2O2-injured primary neurons and dramatically inhibited H2O2-induced LDH release and the apoptosis of neuron cells. Furthermore, phillyrin markedly downregulated the ratio of Bax/Bcl-2 and significantly upregulated pAkt-1 level in H2O2-injured neurons. These results suggest that phillyrin antagonizes H2O2-induced neuron cell apoptosis by activating PI3K/Akt signaling pathway and increasing the ratio of Bax and Bcl-2.
Studies have shown that oxidative stress not only induces apoptosis but also activates autophagy [Citation34]. PI3K/AKT/m-TOR signaling pathways are one of the important intracellular pathways widely present in cells and are involved in the regulation of cell growth, migration, differentiation, apoptosis and metabolism, and other biological processes [Citation35]. PI3K is a lipid kinase regulating the activation of numerous intracellular signal cascades and functioning as various inflammatory mediators [Citation36]. In cerebral ischemia/reperfusion injury, PI3K can not only cause the recruitment of a variety of inflammatory cytokines but also regulate the activation of neutrophils, eosinophils, and macrophages [Citation37]. There are a variety of biological molecules downstream of PI3K. AKT is not only a direct target downstream of PI3K but also the most critical target enzyme responsible for transmitting the biological information of PI3K initiation. m-TOR, a downstream effector molecule of AKT, is a serine-threonine kinase regulating various anabolic and catabolic cellular processes [Citation38]. Studies have reported that the PI3K/AKT/m-TOR signaling pathway is involved in the development of inflammatory responses, such as the onset of temporal lobe mesenchymal epilepsy and the occurrence of inflammatory bowel disease [Citation39]. Here, phillyrin markedly upregulated m-TOR expression and downregulated the levels of beclin-1 and LC3-II in H2O2-injured neuron cells. Moreover, PI3K inhibitor ZSTK474 significantly inhibited the effects of phillyrin on p-Akt-1, p-mTOR, LC3-II, and beclin-1 levels in H2O2-injured neuron cells and the effects of phillyrin on H2O2-induced neuron apoptosis and autophagy. Ye et al. reported that brusatol induced liver cancer cell apoptosis by inhibiting autophagy-related PI3K/Akt/mTOR signaling pathway [Citation40]. Yu et al. found that tetrandrine activated autophagy of HSC-3 cells by activating the caspases/beclin-1/LC3-I/II signaling pathway and inducing cell apoptosis [Citation41]. Consistently, our results suggested that phillyrin exerted its protective effects in H2O2-injured neuron cells by activating PI3K/Akt/m-TOR signaling pathway and inhibiting autophagy via affecting beclin-1 and LC3-II expression. However, this study has some limitations. We established the MCAO/R model through SD rats (weight 220–250 g and 5–7 weeks old) and applied phillyrin to the MCAO/R model rats. In future studies, we hope to include some I/R injury volunteers to further accelerate the clinical process.
Conclusion
A rat MCAO/R model was constructed to explore the role and potential molecular mechanism of phillyrin in cerebral ischemia/reperfusion (I/R) injury. Cerebral infarction volume, brain water content, and neurological score were measured. H&E staining was used to detect neuron morphological structures in brain tissues. Hoechst 33258 staining was used to determine the apoptosis of primary neurons. Our results as graphical abstract suggested that phillyrin protects neurons by inhibiting neuron cell apoptosis and autophagy pathways, which provided new ideas for treating cerebral I/R injury.
Availability of data and material
Data are available upon reasonable request.
Author’s contribution
NDZ supervised the whole study and provided the material source. SC, SZ, HGW, DBZ, GLY, and JY performed the experiments, analyzed the data, and prepared the manuscript.
Ethics approval and consent to participate
This study was approved by the Animal Research Committee of Leshan People’s Hospital in China (No. LS753) and conducted strictly following the guidelines of the National Institutes of Health for the care and use of experimental animals.
Supplemental Material
Download JPEG Image (850.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Hua F, Tang H, Wang J, et al. TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J Cereb Blood Flow Metab. 2015;35(4):536–542.
- Wang YQ, Tang YF, Yang MK, et al. Dexmedetomidine alleviates cerebral ischemia-reperfusion injury in rats via inhibition of hypoxia-inducible factor-1α. J Cell Biochem. 2019;120(5):7834–7844.
- Liu P, Zhao H, Wang R, et al. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke. 2015;46(2):513–519.
- Sun J, Ling Z, Wang F, et al. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett. 2016;613:30–35.
- Liu Y, Zhang L, Liang J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J Neurol Sci. 2015;351(1–2):88–92.
- Fang L, Gao H, Zhang W, et al. Resveratrol alleviates nerve injury after cerebral ischemia and reperfusion in mice by inhibiting inflammation and apoptosis. Int J Clin Exp Med. 2015;8(3):3219.
- X-g Y, B-h C, Wang X, et al. Lateral intracerebroventricular injection of Apelin-13 inhibits apoptosis after cerebral ischemia/reperfusion injury. Neural Regen Res. 2015;10(5):766.
- J-f Z, L-l S, Zhang L, et al. MicroRNA-25 negatively regulates cerebral ischemia/reperfusion injury-induced cell apoptosis through Fas/FasL pathway. J Mol Neurosci. 2016;58(4):507–516.
- Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20(3):460–473.
- Kim YC, Guan K-L. mTOR: a pharmacologic target for autophagy regulation. J Clin Invest. 2015;125(1):25–32.
- Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22(3):377.
- Mei Y, Thompson MD, Cohen RA, et al. Autophagy and oxidative stress in cardiovascular diseases. Biochim Biophys Acta Mol Basis Dis. 2015;1852(2):243–251.
- Nakka VP, Prakash-babu P, Vemuganti R. Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol Neurobiol. 2016;53(1):532–544.
- Han B, Jiang P, Liu W, et al. Role of daucosterol linoleate on breast cancer: studies on apoptosis and metastasis. J Agric Food Chem. 2018;66(24):6031–6041.
- Chung MJ, Lee S, Park YI, et al. Neuroprotective effects of phytosterols and flavonoids from Cirsium setidens and Aster scaber in human brain neuroblastoma SK-N-SH cells. Life Sci. 2016;148:173–182.
- L-h J, X-l Y, N-y Y, et al. Daucosterol protects neurons against oxygen–glucose deprivation/reperfusion-mediated injury by activating IGF1 signaling pathway. J Steroid Biochem Mol Biol. 2015;152:45–52.
- Sui R, Zang L, Bai Y. Administration of troxerutin and cerebroprotein hydrolysate injection alleviates cerebral ischemia/reperfusion injury by down-regulating caspase molecules. Neuropsychiatr Dis Treat. 2019;15:2345–2352.
- Tanaka J, Kiyoshi K, Kadokura T, et al. Elucidation of the enzyme involved in 2,3,5-triphenyl tetrazolium chloride (TTC) staining activity and the relationship between TTC staining activity and fermentation profiles in Saccharomyces cerevisiae. J Biosci Bioeng. 2021;131(4):396–404.
- Amelot A, Terrier LM, Lot G. Predictive factors of neurological recovery after chronic craniovertebral brainstem compression. Acta Neurochir (Wien). 2018;160(6):1243–1250.
- Glaser NS, Wootton-Gorges SL, Kim I, et al. Regional brain water content and distribution during diabetic ketoacidosis. J Pediatr. 2017;180:170–176.
- Blank A, Schenker C, Dawson H, et al. Evaluation of tumor budding in primary colorectal cancer and corresponding liver metastases based on H&E and pancytokeratin staining. Front Med (Lausanne). 2019;6:247.
- Bingqiang H, Chunshuai S, Hui L, et al. Primary culture of adult cortical neurons from reptile Gekko japonicus. J Anat. 2021;239(4):913–919.
- Ates G, Vanhaecke T, and Rogiers V, et al. Assaying cellular viability using the neutral red uptake assay Methods Mol Biol . 2017;1601:19–26.
- Xiang D, Zhai K, Sang Q, et al. Highly sensitive fluorescence quantitative detection of mercury in soil based on non-labeled molecular beacon and fluorescent dye Hoechst 33258. Anal Sci: Int J Jpn Soc Anal Chem. 2017;33(3):275–279
- Yang Y, Li X, Zhang L, et al. Ginsenoside Rg1 suppressed inflammation and neuron apoptosis by activating PPARγ/HO-1 in hippocampus in rat model of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol. 2015;8(3):2484–2494.
- Zhan J, Xiaoqiong L, Hao R. Effect of dendrobium nobile polysaccharides on focal cerebral ischemia/reperfusion rats. Chin J Cereb Dis. 2017;14:25–31.
- El Khashab IH, Abdelsalam RM, Elbrairy AI, et al. Chrysin attenuates global cerebral ischemic reperfusion injury via suppression of oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2019;112:108619.
- Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619.
- Ma T, Chen T, Li P, et al. Heme oxygenase-1 (HO-1) protects human lens epithelial cells (SRA01/04) against hydrogen peroxide (H2O2)-induced oxidative stress and apoptosis. Exp Eye Res. 2016;146:318–329.
- Zheng JH, Follis AV, Kriwacki RW, et al. Discoveries and controversies in BCL‐2 protein‐mediated apoptosis. FEBS J. 2016;283(14):2690–2700.
- Adams JM, Cory S. The BCL-2 arbiters of apoptosis and their growing role as cancer targets. Cell Death Differ. 2018;25(1):27.
- Zhu H, Zhang Y, Shi Z, et al. The neuroprotection of liraglutide against ischaemia-induced apoptosis through the activation of the PI3K/AKT and MAPK pathways. Sci Rep. 2016;6(1):26859.
- Sun D, Huang J, Zhang Z, et al. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PloS One. 2012;7(3):e33491.
- Varga ZV, Giricz Z, Liaudet L, et al. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta Mol Basis Dis. 2015;1852(2):232–242.
- Yarahmadi A, Khademi F, Mostafavi-Pour Z, et al. In-vitro analysis of glucose and quercetin effects on m-TOR and Nrf-2 expression in HepG2 cell line (diabetes and cancer connection). Nutr Cancer. 2018;70(5):770–775.
- Xuan F, Jian J, Lin X, et al. 17-Methoxyl-7-hydroxy-benzene-furanchalcone ameliorates myocardial ischemia/reperfusion injury in rat by inhibiting apoptosis and autophagy via the PI3K-Akt signal pathway. Cardiovasc Toxicol. 2017;17(1):79–87.
- Dreyling M, Santoro A, Mollica L, et al. Phosphatidylinositol 3-kinase inhibition by copanlisib in relapsed or refractory indolent lymphoma. J Clin Oncol. 2017;35(35):3898–3905.
- Kimura H, Matsuyama Y, Araki S, et al. The effect and possible clinical efficacy of in vivo inhibition of neutrophil extracellular traps by blockade of PI3K-gamma on the pathogenesis of microscopic polyangiitis. Mod Rheumatol. 2018;28(3):530–541.
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293.
- B-j W, W-l Z, N-n F, et al. The effects of autophagy and PI3K/AKT/m-TOR signaling pathway on the cell-cycle arrest of rats primary Sertoli cells induced by zearalenone. Toxins (Basel). 2018;10(10):398.
- Yu FS, Yu CS, Chen JC, et al. Tetrandrine induces apoptosis via caspase‐8,‐9, and‐3 and poly (ADP ribose) polymerase dependent pathways and autophagy through beclin‐1/LC3‐I, II signaling pathways in human oral cancer HSC‐3 cells. Environ Toxicol. 2016;31(4):395–406.
