ABSTRACT
Oxidative stress and inflammation are implicated in the pathogenesis of cerebral ischemia-reperfusion (I/R) injury. SETD7 (SET Domain Containing 7) functions as a histone lysine methyltransferase, participates in cardiac lineage commitment, and silence of SETD7 exerts anti-inflammatory or antioxidant capacities. The effect of SETD7 in in vitro cell model of cerebral I/R injury was investigated in this study. Firstly, adrenal pheochromocytoma cell (PC12) was conducted with oxygen-glucose deprivation/reoxygenation (OGD/R) to establish cell model of cerebral I/R injury. OGD/R-enhanced SETD7 expression in PC12 cells. Cell viability of OGD/R-induced PC12 was reduced, while the apoptosis was promoted. Secondly, knockdown of SETD7 reversed the effect of OGD/R on cell viability and apoptosis of PC12. Moreover, OGD/R-induced inflammation in PC12 with decreased interleukin (IL)-10, increased IL-6, IL-1β, tumor necrosis factor-α (TNF-α), and cyclooxygenase 2 (COX-2) were restored by knockdown of SETD7. Thirdly, knockdown of SETD7 attenuated OGD/R-induced decrease of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and catalase (CAT), as well as increase of malondialdehyde (MDA) and reactive oxygen species (ROS) in PC12. Lastly, OGD/R-induced decrease of NF-κB inhibitor α (IκBα), increase of phosphorylated (p)-p65, p-IκBα, and Keap1 (Kelch-like ECH-associated protein 1) were reversed by silence of SETD7. Silence of SETD7 increased heme oxygenase-1 (HO-1) and nuclear factor erythroid 2-related factor 2 (Nrf2) expression in OGD/R-induced PC12. In conclusion, suppression of SETD7 ameliorated OGD/R-induced inflammation and oxidative stress in PC12 cell through inactivation of NF-κB and activation of Keap1/Nrf2/ARE pathway.
Graphical abstract
Introduction
Ischemic stroke is a common clinical cerebrovascular disease [Citation1]. Around 150,000 people suffer from the stroke injury every year, and nearly five million people died from stroke injury [Citation2]. Under the ischemic condition, brains could not get enough energy supply, including blood and oxygen, for biosynthesis, cell membrane structures maintenance, and proper function of enzymes, thus resulting in neurological damages [Citation3]. To date, restoration of blood and oxygen supply to the ischemic brain tissues has been considered to be the primary concern in clinical treatment of ischemic stroke [Citation2]. However, blood and oxygen supply might cause pathological damage in the ischemic tissue, leads to irreversible cerebral I/R injury [Citation4]. Therefore, protection of neural cells against I/R-induced damage might be beneficial for the treatment of stroke therapies [Citation5].
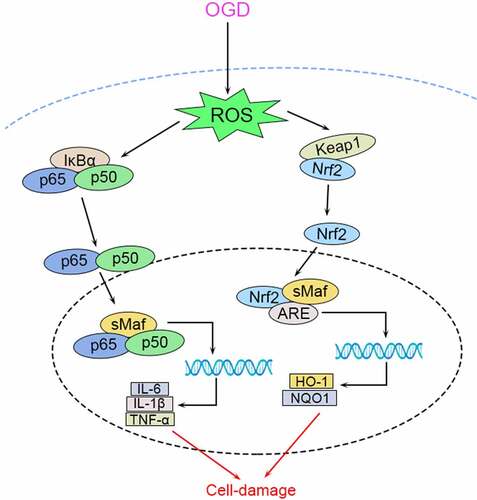
The basic mechanism of cerebral I/R injury has not been fully elucidated. Study has shown that oxidative stress caused by large amount of free radical production and reduced free radical scavenging ability after reperfusion is the critical regulator of cerebral I/R injury [Citation6]. Excessive oxidative stress could lead to lipid peroxidation, protein nitrification, nucleic acid damage, and severe inflammation of nerve cell membranes and organelles [Citation6]. Therefore, effective strategies to prevent oxidative stress and inflammatory response show beneficial effects on inhibiting cerebral I/R injury [Citation7].
SETD7 (SET Domain Containing 7) functions as a lysine methyltransferase specifically catalyze the demethylation of H3K4me1 [Citation8]. SETD7 has been reported to be related to various signaling or disease pathways [Citation9]. For example, SETD7 interacted with cofactors involved in the cardiomyocyte differentiation, thus potentiating cardiac lineage commitment [Citation10]. SETD7 also methylated Tau during the development of Alzheimer’s disease [Citation11]. SETD7 regulated expression of inflammatory cytokines to be involved in chronic constriction injury-induced neuropathic pain [Citation12]. Inhibition of SETD7 also promoted function of mitochondria antioxidant system, thus participating in reactive oxygen species-associated diseases [Citation13]. Moreover, SETD7 was overexpressed in rat cardiocytes induced by hypoxia and reoxygenation, and suppression of SETD7 reduced accumulation of reactive oxygen species suppressed cell apoptosis to prevent myocardial ischemia/reperfusion injury [Citation14]. However, the neuroprotective effect of SETD7 silence on I/R injury has not been fully understood.
In this study, we hypothesized that SETD7 might contribute to OGD/R-induced cytotoxicity in neurons. The effects of SETD7 on OGD/R-induced apoptosis, oxidative stress, and inflammation of neural cells were investigated. The underlying mechanism involved in SETD7-mediated OGD/R-induced neuronal cytotoxicity might provide potential therapeutic strategy for ischemic stroke.
Materials and methods
Cell culture, treatment and transfection
The PC12 cells were purchased from American Type Culture Collection (Manassas, VA, USA), and cultured in Dulbecco’ s modified eagle medium with 10% fetal bovine serum (Invitrogen, Grand Island, NY, USA). For establishment of ischemia-like condition, cells in glucose-free medium exposed to hypoxia condition: 1% O2, 5% CO2, and 94% N2 for 4 hours. Cells were then incubated with normal culture medium with 4.5 g/L glucose and reoxygenated for another 24 hours before functional assays according to previous study [Citation15]. The PC12 cells were seeded in a 96-well plate, and then transfected with 50 nM siRNA targeting SETD7 (si-SETD7) or the negative control (si-NC) by Lipofectamine 2000 (Invitrogen). Cells were then subjected to OGD/R 24 hours later.
Quantitative real-time polymerase chain reaction (qRT-PCR)
RNAs were isolated from PC12 cells using Trizol (Invitrogen), and then reverse-transcribed into cDNAs. The cDNAs were used as a template for qRT-PCR analysis of SETD7 by SYBR Green Master (Roche, Mannheim, Germany) under Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) according to previous study [Citation16]. The following primers: SETD7 (forward: 5’-GGGCCAGCCCAGGAGTACGA-3’; reve-rse: 5’-TTTGACCACAGGGGCAGGAA-3’), Keap1 (forward: 5’-CAACTTCGCTGAGCAGATTGGC-3’; reverse: 5’-TGATGAGGGTCACCAGTTGGCA-3’), Nrf2 (forward: 5’-GAGAGCCCAGTCTTCATTGC-3’; reverse: 5’-TGCTCAATGTCCTGTTGCAT-3’), HO-1 (forward: 5’-ATGGCCTCCCTGTACCACATC-3’; reverse: 5’-TGTTGCGCTCAATCTCCTCCT-3’), and GAPDH (forward: 5’-CTCTGCTCCTCCTGTTCGAC-3’; re-verse: 5’-GCGCCCAATACGACCAAATC-3’) were used in this study.
Cell viability and apoptosis assays
PC12 cells with indicated treatment and transfections were seeded in a 96-well plate for 48 hours. MTT solution (Dojindo, Tokyo, Japan) was added into each well and incubated for 4 hours. Absorbance at 570 nm was measured by Thermo Multiskan MK3 (Thermo Fisher Scientific Inc, Waltham, MA, USA) followed by incubation with dimethyl sulfoxide. For cell apoptosis, PC12 was harvested, and suspended in binding buffer of ApoDETECT Annexin V-FITC Kit (Thermo Fisher Scientific Inc). The apoptotic cells were analyzed by FACS flow cytometer (Attune, Life Technologies, Darmstadt, Germany) followed by staining with Annexin V-FITC and PI (Thermo Fisher Scientific Inc) according to previous study [Citation15].
ELISA and ROS measurement
PC12 was lysed in RIPA lysis buffer (Beyotime Institute of Biotechnology, Beijing, China), and then centrifuged at 12000 g for the collection of cell culture supernatants. Protein concentrations were determined by BCA Protein Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Levels of IL-10, IL-6, IL-1β, TNF-α, and COX-2, as well as MDA, SOD, GSH-Px, and CAT, were analyzed by commercial kits (Nanjing Jiancheng Bioengineering Institute) according to previous study [Citation16]. For the determination of ROS production, PC12 cells were incubated with dichlorofluoresceindiacetate (10 μM; Thermo Fisher Scientific Inc). Fluorescence spectrophotometer (BioTek, Winooski, VT, USA) was used to calculate the fluorescence intensity.
Western blot
Protein were separated by SDS-PAGE, and then transferred onto polyvinylidene difluoride membrane. The membrane was blocked, and probed with primary antibodies: anti-Bax and anti-Bcl-2 (1:2000; Abcam, Cambridge, MA, USA), anticleaved caspase-3 and anti-GAPDH (1:2500; Abcam), anti-p65 and anti-p-p65 (1:3000; Abcam), anti-IκBα and anti-p-IκBα (1:3500; Abcam), anti-Keap1 and anti-HO-1 (1:4000; Abcam), anti-Nuclear Nrf2, and anti-Lamin B1 (1:4500; Abcam). Followingly, the protein blots in the membrane were incubated with the corresponding secondary antibody (1:5000; Abcam) and visualized by ECL Western blotting Substrate Kit (AmyJet Scientific, Wuhan, China).
Statistical analysis
All the data were expressed as mean ± S.D., and analyzed by student’s t test or one-way analysis of variance under SPSS. A p value of < 0.05 was considered as statistically significant.
Results
Knockdown of SETD7 increased cell viability of OGD/R-induced PC12
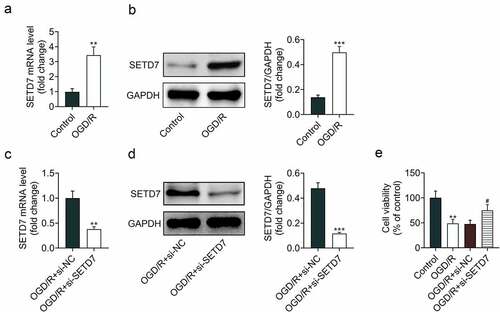
Expression level of SETD7 in response to OGD/R was detected to explore the relation between SETD7 and cerebral I/R injury. Data from qRT-PCR () and Western blot () showed that SETD7 was upregulated in OGD/R-induced PC12. Transfection with si-SETD7 reduced expression of SETD7 in OGD/R-induced PC12 (). Knockdown of SETD7 promoted neural survival in response to OGD/R (), suggesting the proliferative effect of SETD7 silence against OGD/R-induced PC12.
Figure 1. Knockdown of SETD7 increased cell viability of OGD/R-induced PC12 (a) mRNA expression of SETD7 was increase in OGD/R-induced PC12. (b) Protein expression of SETD7 was increase in OGD/R-induced PC12. (c) Transfection with si-SETD7 reduced expression of SETD7 in OGD/R-induced PC12. (d) Transfection with si-SETD7 reduced protein expression of SETD7 in OGD/R-induced PC12. (e) Knockdown of SETD7 promoted cell viability of OGD/R-induced PC12. # vs. OGD/R+ siNC, p < 0.05. ** vs. control, p < 0.01.
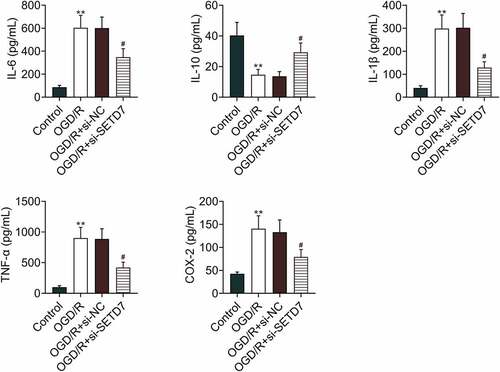
Knockdown of SETD7 suppressed inflammatory response in OGD/R-induced PC12
The effect of SETD7 on inflammation of OGD/R-induced PC12 was investigated. Expression level of IL-10 was reduced in OGD/R-induced PC12, while IL-6, IL-1β, TNF-α, and COX-2 were enhanced in PC12 post OGD/R treatment (). Moreover, knockdown of SETD7 attenuated OGD/R-induced decrease of IL-10, increase of IL-6, IL-1β, TNF-α, and COX-2 in PC12 (). However, over-expression of SETD7 enhanced levels of IL-6, IL-1β, TNF-α in OGD/R-induced PC12 (Supplemental Figure S1A), demonstrating anti-inflammatory effect of SETD7 silence against OGD/R-induced PC12.
Knockdown of SETD7 suppressed oxidative stress in OGD/R-induced PC12
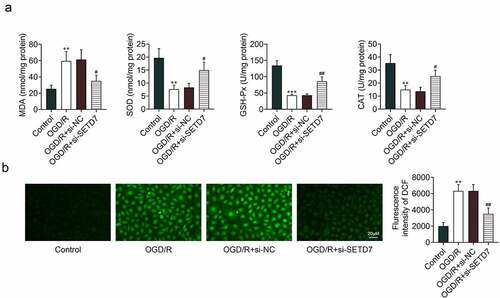
The effect of SETD7 on oxidative stress of OGD/R-induced PC12 was investigated. In addition to the proliferative and anti-inflammatory effects, enhanced MDA, as well as reduced SOD, GSH-Px, and CAT, in OGD/R-induced PC12 were restored by knockdown of SETD7 (). In addition, knockdown of SETD7 reversed the promotive effect of OGD/R on ROS level (), while over-expression of SETD7 enhanced levels of MDA (Supplemental Figure S1B) and ROS (Supplemental Figure S1C) in OGD/R-induced PC12, indicating antioxidant effect of SETD7 silence against OGD/R-induced PC12.
Figure 3. Knockdown of SETD7 suppressed oxidative stress in OGD/R-induced PC12. (a) Knockdown of SETD7 attenuated OGD/R-induced enhance of MDA, reduce of SOD, GSH-Px, and CAT in PC12. (b) Knockdown of SETD7 attenuated OGD/R-induced enhance of ROS in PC12. #, ## vs. OGD/R+ siNC, p < 0.05, p < 0.01. **, *** vs. control, p < 0.01, p < 0.001.
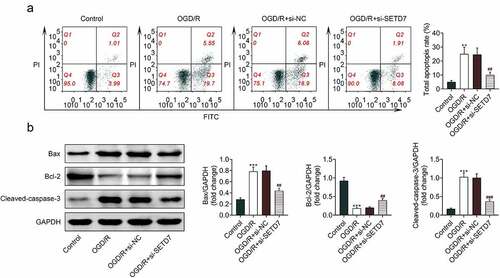
Knockdown of SETD7 suppressed cell apoptosis of OGD/R-induced PC12
The effect of SETD7 on cell apoptosis of OGD/R-induced PC12 was investigated. Cell apoptosis of PC12 was promoted by OGD/R condition (), while suppressed by silence of SETD7 (). Silence of SETD7 attenuated OGD/-induced decrease of Bcl-2, increase of Bax and cleaved caspase-3 in PC12 (). However, overexpression of SETD7 enhanced cell apoptosis of OGD/R-induced PC12 (Supplemental Figure S1D), revealing antiapoptotic effect of SETD7 silence against OGD/R-induced PC12.
Figure 4. Knockdown of SETD7 suppressed cell apoptosis of OGD/R-induced PC12. (a) Silence of SETD7 attenuated OGD/-induced increase of cell apoptosis in PC12. (b) Silence of SETD7 attenuated OGD/-induced decrease of Bcl-2, increase of Bax and cleaved caspase-3 in PC12. ##, ### vs. OGD/R+ siNC, p < 0.01, p < 0.001. **, *** vs. control, p < 0.01, p < 0.001.
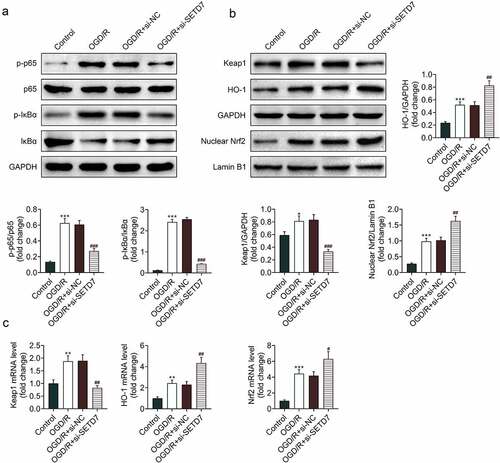
Knockdown of SETD7 inhibited NF-κB and activated Keap1/Nrf2/ARE pathways in OGD/R-induced PC12
The mechanism involved in SETD7-mediated OGD/R-induced PC12 was investigated. Protein expression of IκBα was down-regualted by OGD/R condition in PC12 (). Moreover, phosphorylated p65 (p-p65) and p-IκBα were up-regulated in OGD/R-induced PC12 (), showing that OGD/R induced activation of NF-κB in PC12. However, interference of SETD7 enhanced IκBα, reduced p-p65, and p-IκBα to suppress activation of NF-κB in OGD/R-induced PC12 (). Silence of SETD7 counteracted with the promotive effect of OGD/R on expression of Keap1 (). Expression of HO-1 and nuclear Nrf2 were up-regulated in OGD/R-induced PC12 (), showing that silence of SETD7 promoted activation of Keap1/Nrf2/ARE pathway in OGD/R-induced PC12.
Figure 5. Knockdown of SETD7 inhibited NF-κB and activated Keap1/Nrf2/ARE pathways in OGD/R-induced PC12. (a) Knockdown of SETD7 attenuated OGD/R-induced decrease of IκBα, increase of p-p65 and p-IκBα in PC12. (b) Silence of SETD7 reduced Keap1 protein, enhanced protein expression of HO-1 and nuclear Nrf2 in OGD/R-induced PC12. (c) Silence of SETD7 reduced Keap1 mRNA, enhanced mRNA expression of HO-1 and nuclear Nrf2 in OGD/R-induced PC12. #, ##, ### vs. OGD/R+ siNC, p < 0.05, p < 0.01, p < 0.001. *, **, *** vs. control, p < 0.05, p < 0.01, p < 0.001.
Discussion
Post-translational modifications, such as histone methylation, were implicated in the pathogenesis of I/R-induced injuries [Citation17]. For example, ischemic stroke-induced oxidative stress in neurons, and the oxidative stress-modulated epigenetic regulation, such as DNA methylation and histone modification, in neural networks influenced the redox system in neurons and induced cell damages [Citation18]. Inhibition of histone methyltransferases has been shown to protect neurons against cerebral ischemia injury [Citation19]. Since SETD7 was implicated in the pathogenesis of myocardial ischemia/reperfusion injury [Citation14], the effect of SETD7 on OGD/R-induced PC12 was investigated in this study.
Firstly, PC12 was conducted with OGD/R condition to mimic the in vitro I/R pathological process [Citation20]. In line with previous study that SETD7 was upregualted in hypoxia/reoxygenation-induced cardiomyocytes [Citation14], OGD/R condition also upregulated SETD7 in PC12. During the initiation of ischemia/reperfusion injury, cell apoptosis was promoted in the ischemic areas to induce cerebral ischemic damage [Citation21]. Alleviation of I/R-induced cell apoptosis attenuated the cerebral damages [Citation22]. Overexpression of SETD7 has been shown to promote hypoxia/reoxygenation-induced cell apoptosis of cardiomyocytes [Citation14]. Functional assays in this study showed that silence of SETD7 attenuated OGD/R-induced decrease of cell viability and increase of cell apoptosis in PC12, suggesting the antiapoptotic effect of SETD7 silence against OGD/R-induced PC12.
Previous study has shown that reperfusion induces cascade amplification of inflammatory responses in ischemic tissues, such as activation of inflammatory cells, secretion of proinflammatory cytokines, and sequestration of immunocytes, to exacerbate the injury [Citation21]. Therapeutic strategies to prevent inflammation were considered to be an effective treatment of ischemic stroke [Citation23]. TNF-α-induced inflammation in airway smooth muscle cells were promoted by SETD7 [Citation24]. Our results showed that knockdown of SETD7 attenuated OGD/R-induced decrease of IL-10, increase of IL-6, IL-1β, TNF-α, and COX-2 in PC12, demonstrating anti-inflammatory effect of SETD7 silence against OGD/R-induced PC12. Moreover, NF-κB, important for inflammatory response, was increased in the cortex post ischemia reperfusion injury [Citation25]. Suppression of NF-κB pathway prevented cerebral I/R injury [Citation25]. Activation of NF-κB was repressed by silence of SETD7, and block of NF-κB abrogated the effect of SETD7 on inflammation of TNF-α-induced airway smooth muscle cells [Citation24]. Our results showed that knockdown of SETD7 increased protein expression of IκBα, decreased p-p65, and p-IκBα to suppress activation of NF-κB in OGD/R-induced PC12.
Production of ROS induced by reperfusion-induced lipid peroxidation, inflammation, and cell apoptosis, thus aggravating the ischemic injury [Citation23]. Overexpression of SETD7 promoted the accumulation of ROS in hypoxia/reoxygenation-induced cardiomyocytes [Citation14]. Dichlorofluorescein diacetate is cell permeable and shows low fluorescence. Dichlorofluorescein diacetate is cleaved by intracellular esterases, and then oxidized into DCF with highly fluorescence by ROS. Therefore, the fluorescence of DCF was regarded as indicator of ROS level [Citation26]. The fluorescence of DCF in PC12 was upregulated by OGD/R, and knockdown of SETD7 reduced the fluorescence of DCF in OGD/R-induced PC12 to reduce ROS. Oxidative stress was also expected to be a target for the treatment of cerebral I/R injury [Citation23]. Levels of MDA in OGD/R-induced PC12 was upregulated, while SOD, GSH-Px and CAT were downregulated by knockdown of SETD7. Keap1 is a negative regulator of Nrf2 through ubiquitination and degradation, Nrf2 translocates into nucleus after dissociation with Keap1 to promote activation of antioxidant enzymes through binding to the antioxidant responsive elements [Citation27]. Activation of Keap1/Nrf2/HO-1 pathway protected against oxidative stress and exerted neuroprotection for stroke [Citation28]. Silence of SETD7 has been shown to decrease Keap1 expression, increase Nrf2 and HO-1 to prevent hypoxia/reoxygenation-induced injury [Citation14]. Our results also showed that knockdown of SETD7 reduced Keap1 expression, enhanced Nrf2 and HO-1 to ameliorate OGD/R-induced oxidative stress in PC12.
Conclusion
In summary, our results demonstrated that knockdown of SETD7 exerted antiapoptotic effect against OGD/R-induced PC12. Moreover, OGD/R-induced oxidative stress and inflammation were suppressed by silence of SETD7 through activation of Keap1/Nrf2/HO-1 pathway and inactivation of NF-κB pathway, respectively. Therefore, SETD7 might serve as a potential target for ischemic injury, that needs to further investigated in the in vivo animal model.
Highlights
Knockdown of SETD7 protected PC12 against OGD/R-induced cytotoxicity.
Knockdown of SETD7 suppressed inflammatory response and oxidative stress in OGD/R-induced PC12.
Knockdown of SETD7 mediated NF-κB and Keap1/Nrf2/ARE pathways in OGD/R-induced PC12.
Contribution of authors
Xianfang Pan and Jin Fan designed the study, supervised the data collection, Fang Peng Li Xiao and Zhiyi Yang analyzed the data, interpreted the data, Li Xiao prepare the manuscript for publication, and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Supplemental Material
Download JPEG Image (1.7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kang HG, Cheong JS, Lim IH, et al. Antioxidative activity of statins and HDL-PON1 association in lacunar ischemic stroke with and without white matter hyperintensity. Signa Vitae. 2021;17:104–110.
- Musuka TD, Wilton SB, Traboulsi M, et al. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ = Journal de l’Association Medicale Canadienne. 2015;187(12):887–893.
- Pan J, Konstas -A-A, Bateman B, et al. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49(2):93–102.
- Zarbock A, Eroglu A, Erturk E, et al. Ischemia-reperfusion injury and anesthesia. Biomed Res Int. 2014;2014:980318.
- D-s Q, J-h T, Zhang L-Q, et al. Neuroprotection of Cilostazol against ischemia/reperfusion-induced cognitive deficits through inhibiting JNK3/caspase-3 by enhancing Akt1. Brain Res. 2016;1653:67–74.
- Li S, Jiang D, Ehlerding EB, et al. Intrathecal Administration of Nanoclusters for Protecting Neurons against Oxidative Stress in Cerebral Ischemia/Reperfusion Injury. ACS Nano. 2019;13(11):13382–13389.
- Meng X, Xie W, Xu Q, et al. Neuroprotective Effects of Radix Scrophulariae on Cerebral Ischemia and Reperfusion Injury via MAPK Pathways. Molecules. 2018;23(9):2401.
- Tamura R, Doi S, Nakashima A, et al. Inhibition of the H3K4 methyltransferase SET7/9 ameliorates peritoneal fibrosis. PloS one. 2018;13(5):e0196844–e0196844.
- Soshnikova N, Liu H. Functions of SETD7 during development, homeostasis and cancer. Stem Cell Investig. 2019;6:6.
- Lee J, Shao N-Y, Paik DT, et al. SETD7 Drives Cardiac Lineage Commitment through Stage-Specific Transcriptional Activation. Cell Stem Cell. 2018;22(3):428–444.e425.
- Bichmann M, Prat Oriol N, Ercan-Herbst E, et al. SETD7-mediated monomethylation is enriched on soluble Tau in Alzheimer’s disease. Mol Neurodegener. 2021;16(1):46.
- Shen Y, Ding Z, Ma S, et al. SETD7 mediates spinal microgliosis and neuropathic pain in a rat model of peripheral nerve injury. Brain Behav Immun. 2019;82:382–395.
- He S, Owen DR, Jelinsky SA, et al. Lysine Methyltransferase SETD7 (SET7/9) Regulates ROS Signaling through mitochondria and NFE2L2/ARE pathway. Sci Rep. 2015;5(1):14368.
- Dang Y, Ma X, Li Y, et al. Inhibition of SETD7 protects cardiomyocytes against hypoxia/reoxygenation-induced injury through regulating Keap1/Nrf2 signaling. Biomed Pharmacother. 2018;106:842–849.
- Yang Z, Wang L, Hu Y, et al. Butorphanol protects PC12 cells against OGD/R-induced inflammation and apoptosis. Mol Med Rep. 2020;22(3):1969–1975.
- Shu K, Zhang Y. Protodioscin protects PC12 cells against oxygen and glucose deprivation-induced injury through miR-124/AKT/Nrf2 pathway. Cell Stress Chaperones. 2019;24(6):1091–1099.
- Tang J, Zhuang S. Histone acetylation and DNA methylation in ischemia/reperfusion injury. Clin sci. 2019;133(4):597–609.
- Zhao H, Han Z, Ji X, et al. Epigenetic Regulation of Oxidative Stress in Ischemic Stroke. Aging Dis. 2016;7(3):295–306.
- Schweizer S, Harms C, Lerch H, et al. Inhibition of Histone Methyltransferases SUV39H1 and G9a Leads to Neuroprotection in an in vitro Model of Cerebral Ischemia. J Cereb Blood Flow Metab. 2015;35(10):1640–1647.
- Zhang Z, Sun Y, Chen X. NLRC5 alleviated OGD/R-induced PC12-cell injury by inhibiting activation of the TLR4/MyD88/NF-κB pathway. J Int Med Res. 2020;48(8):300060520940455.
- Zhuang L, Kong Y, Yang S, et al. Dynamic changes of inflammation and apoptosis in cerebral ischemia‑reperfusion injury in mice investigated by ferumoxytol‑enhanced magnetic resonance imaging. Mol Med Rep. 2021;23(4):1–13.
- Chen W, Wang H, Feng J, et al. Overexpression of circRNA circUCK2 Attenuates Cell Apoptosis in Cerebral Ischemia-Reperfusion Injury via miR-125b-5p/GDF11 Signaling. Mol Ther Nucleic Acids. 2020;22:673–683.
- Wu L, Xiong X, Wu X, et al. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front Mol Neurosci. 2020;13:28.
- Wu Y, Zou F, Lu Y, et al. SETD7 promotes TNF-α-induced proliferation and migration of airway smooth muscle cells in vitro through enhancing NF-κB/CD38 signaling. Int Immunopharmacol. 2019;72:459–466.
- Zhao H, Chen Z, Xie L-J, et al. Suppression of TLR4/NF-κB Signaling Pathway Improves Cerebral Ischemia–Reperfusion Injury in Rats. Mol Neurobiol. 2018;55(5):4311–4319.
- Wang X, Roper MG. Measurement of DCF fluorescence as a measure of reactive oxygen species in murine islets of Langerhans. Anal Methods. 2014;6(9):3019–3024.
- Kobayashi M, Yamamoto M. Molecular Mechanisms Activating the Nrf2-Keap1 Pathway of Antioxidant Gene Regulation. Antioxid Redox Signal. 2005;7(3–4):385–394.
- Satoh T, Okamoto S-I, and Cui J, et al. Activation of the Keap1/Nrf2 pathway for neuroprotection by electrophilic phase II inducers. Proceedings of the National Academy of Sciences, USA. 2006; 103: 768–773.