ABSTRACT
Double homeobox A pseudogene 8 (DUXAP8) is a known tumor promoter in several malignancies. Nonetheless, its function in colon cancer (CC) is indefinite. Herein, we explored the significance of DUXAP8 and its underlying mechanism in CC. Our data indicated that DUXAP8 was upregulated in CC, and it was related to advanced stages and lymph node metastases. Based on our Kaplan-Meier survival analysis, elevated DUXAP8 expression resulted in shorter patient overall survival (OS). Conversely, DUXAP8 silencing strongly suppressed cellular proliferation, migration and invasion in vitro. Based on our western blot analysis, DUXAP8 deficiency strongly inhibited the epithelial–mesenchymal transition (EMT) in vitro. Alternately, DUXAP8 overexpression accelerated cellular proliferation migration and invasion in CC. Finally, silencing DUXAP8 prevented tumorigenesis in a mouse xenograft model in vivo. Collectively, our results demonstrated that DUXAP8 regulates the occurrence and advancement of CC, and may serve as a regulatory hub for this disease.
Graphical abstract
Introduction
Colon cancer (CC) is the third ranking carcinoma in the world, with 1,880,000 new cases, and 915,000 deaths in 2020 aloneCitation1. Lately, the overall survival (OS) rate of CC has improved tremendously, due to new treatment regimens, including, preoperative chemotherapy and targeted treatment. However, 50% of CC patients still experience recurrence and metastasis following treatmentCitation2. Therefore, identifying the pivotal molecular mechanism regulating tumor occurrence and progression in CC is crucial for the screening of novel targeted therapeutics that enhance prognosis of these patients.
Long non-coding RNAs (lncRNAs) have extensive functions, and participate in multiple cellular processes, such as, tumorigenesis and progressionCitation3,Citation4. LncRNA FAM225A is upregulated in nasopharyngeal carcinoma (NPC), and its overexpression promotes NPC tumorigenesis and metastasisCitation5. Another important lncRNA is LncRNA GLCC1, which is reported to promote colorectal carcinogenesis and glucose metabolism via stabilization of c-MycCitation6. Being a special class of lncRNAs, pseudogenes were initially regarded as nonfunctional genomic relics of functional genes. Current research demonstrated that pseudogene-derived RNAs in fact modulate human cancer progression via other mechanisms, such as, serving as competing endogenous RNAs (ceRNAs), and as competitors for RBP or translational machineryCitation7,Citation8. Recent investigations suggested that DUXAP10 functions as an oncogene to promote cell proliferation via the PI3K/AKT pathway in hepatocellular carcinoma (HCC)Citation9. HMGA1P6 is activated by MYC, and promotes cell malignancy by serving as a ceRNA in ovarian cancerCitation10. Double homeobox A pseudogene 8 (DUXAP8) is a retrotransposed pseudogene, encoded by the human double homeobox A (DUXA), residing in 22q11, and carrying a length of 2107 bpCitation11. DUXAP8 serves a critical function in numerous human malignant tumors, such as, HCCCitation12, gliomaCitation13, and gastric cancerCitation14. A recent study suggested that DUXAP8 accelerates cellular proliferation of papillary thyroid carcinoma via the ceRNAs mechanismCitation15. In triple negative breast cancer, DUXAP8 is activated by the YY1 transcription factor, and it promotes cellular proliferation of cancer cells via upregulation of SAPCD216. However, the function of DUXAP8 in CC is still undetermined, and requires additional investigation.
Our goal here was to assess the DUXAP8 expression in CC, elucidate its potential regulation of cellular proliferation, migration and invasion, and clarify the underlying mechanism. To this end, we examined DUXAP8 expression in CC tissues and cells. In addition, we investigated the relation between DUXAP8 and pathologic characteristics. Next, we examined the DUXAP8-mediated modulation of cellular proliferation, migration and invasion. Mechanistically, we evaluated vimentin and e-cadherin levels under DUXAP8 overexpression and deficiency, to better understand its influence on the epithelial–mesenchymal transition (EMT) in vitro. Our analysis identified a new function for DUXAP8, which may provide a novel insight into the treatment of CC patients.
Materials and methods
Tissue samples
Between March 2014 and September 2015, we obtained the surgical samples of 60 CC patients from the Second Affiliated Hospital of Nanchang University (Nanchang, China). This included CC tissues and corresponding adjoining non-tumor colon mucosa tissues (NTT). CC disease was diagnosed via pathology, and none of the subjects received chemo- or radiotherapy prior to surgery. Our work received ethical approval from our hospital (2019 No. 100). The resected samples were immediately stored at –80°C. The patient clinicopathologic profiles are summarized in Supplemental Table 1.
Cell culture
The cell lines HCT116, SW480, LoVo, SW620, HT29, and NCM460 were obtained from the Chinese Academy of Science. All cells were maintained in DMEM (Gibco, USA) with 10% of fetal bovine serum (FBS; Invitrogen, Carlsbad, USA), 100 U/ml penicillin, and 100 mg/ml streptomycin (Beyotime Biotechnology, Shanghai, China) in a humid chamber with 5% CO2, and at 37°C.
Quantitative real-time PCR (qRT-PCR)
Total RNA was retrieved with Trizol® (Invitrogen, Carlsbad, USA), and cDNA synthesis was done with the PrimeScript™ RT Master Mix (Takara Biomedical Technology Co., Ltd. Beijing). Then, qRT-PCR analysis was carried out with TB Green™ Premix Ex Taq™ II in an ABI PRISM® 7500 FAST System (Applied Biosystems, Foster City, CA, USA), as per kit directions. Briefly, the reaction conditions were as follows: denaturation at 95°C for 5 min; then 35 cycles of 95°C for 10s, 60°C for 30s, and 72°C for 30s. GAPDH was employed as the housekeeping gene. The relative DUXAP8 expressions were obtained with the 2-ΔΔCt methodCitation16. The DUXAP8 primers were as follow: 5’-AGGATGGAGTCTCGCTGTATTGC-3’ (forward) and 5’-GGAGGTTTGTTTTCTTCTTTTTT-3’ (reverse). The GAPDH primers were as follow: 5’-GGGAGCCAAAAGGGTCAT-3’ (forward) and 5’-GAGTCCTTCCACGATACCAA-3’ (reverse).
Cell transfection
The overexpression vectors, shRNA vectors, and relevant negative control vector were manufactured by TSINHKE Biological Technology (Guangzhou, China). Transfection was performed using Lipofectamine 3000 (Invitrogen, Carlsbad, USA). Briefly, 1 × 105 cells were placed on a 6-well plate, and incubated until 70% confluency. The transfection reagent and vector were mixed after dilution with serum‐free medium (SFM). Next, the cells were cultured with the mixture at 37°C for 12 hours, after which the medium was refreshed. After 24 hours, the cells were harvested for RNA extraction. The sequences of the sh-DUXAP8 were as follows: sh-DUXAP8-1:5’-CACCAAGATAAAGGTG GTTTCCACAAGAACGAATTCTTGTGGAAACCACCTTTATC-3’; shDUXAP8-2: 5’-CACCGCA GCATACTTCAAATTCACAGCAAACGAATTTGCTGTGAATTTGAAGTATGCTG-3’; sh-NC: 5’- CCGGGTTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAACCTTTTTG-3’.
Cell proliferation
The Cell Counting kit-8 (CCK-8) was employed for the CC cellular proliferation capacity assessment. Briefly, 1 × 103 cells were placed per well of a 96-well plate. Subsequently, we introduced the CCK‐8 solution, and evaluated absorbance at 450 nm following a 2 h incubation at 37°C.
Migration and invasion assays
The migration and invasion chamber systems were purchased from Corning (NY 14831 USA), and the experiments were performed as described previouslyCitation17. In short, 2 × 105 cells were placed into the top chamber of inserts, with or without pre-coated Matrigel, and incubated with SFM. In the bottom chamber, we introduced 10% FBS containing culture medium to induce chemotaxis. Following a 24 h incubation, the cells that traveled to the bottom chamber underwent fixation in 4% paraformaldehyde, and staining with 0.1% crystal violet. Lastly, the invading cells were counted under a fluorescent microscope (Carl Zeiss AG).
Wound healing assay
Overall, 1 × 105 cells were placed per well in a 6-well plate, and incubated until 80–90% confluency. Then, a 10 μl pipette tip was employed to generate a scratch, and the migration distance was measured under a phase-contrast microscope (Carl Zeiss AG), after 48 h of culture.
Western blot analysis
Total protein was retrieved with RIPA buffer and protease inhibitors, and protein quantification was done with the BCA protein Assay Kit (Millipore, Darmstadt, Germany). Next, 10ug proteins underwent separation via SDS-PAGE, then transfer to a polyvinylidene fluoride membrane (Millipore, Darmstadt, Germany), and subsequent treatments as follows: sealing with 5% nonfat milk, incubation with specific primary and secondary antibodies, and lastly, quantification via chemiluminescence. All primary antibodies were diluted 1:1000 and were acquired from Cell Signaling Technology (Danvers, MA, USA). The employed antibodies were as follows: anti-GAPDH (#5174), anti-E-cadherin(#3195), and anti-vimentin (#5741). The protein band intensity was visualized and quantifed via the ImageJ software. GAPDH expression was employed as internal reference.
In vivo tumor growth experiments
Five to six week old BALB/c nude mice were selected (n = 4 per group). Our animal protocols received ethical approval from our institution. HCT116 and HT29 cells (1x107 cells/ml) were administered into the posterior sides of mice in various groups (shRNA-DUXAP8 and control). Following two weeks of icubation, we euthanized the mice, and evaluated their tumor volumes.
Statistical analysis
All data analyses were carried out with the SPSS 22.0 software (SPSS, Inc, IL, USA). All experiments were conducted in triplicates. Inter-group comparisons were done with the Student’s t test. We plotted OS curves via the Kaplan–Meier curve analysis. P < 0.05 was set as the significance threshold.
Results
In the last decade, growing evidences suggested that the pseudogene-derived RNAs serve a crucial role in CC progression. In particular, DUXAP8 is upregulated and promotes cellular proliferation in papillary thyroid carcinoma and triple negative breast cancerCitation15,Citation18. Nevertheless, the DUXAP8 significance in CC remains unclear, and requires additional investigation. Herein, we elucidated the DUXAP8-mediated regulation of cellular proliferation, migration, and invasion. We also clarified potential mechanisms, in order to provide a novel insight into the treatment of CC patients.
DUXAP8 levels are elevated in CC, and it is correlated with poor outcome
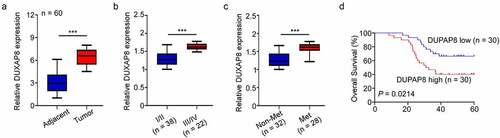
To delineate the profile and significance of DUXAP8 in CC, 60 pairs of CC tissues and corresponding adjoining NTT were included. Based on our qRT-PCR results, the DUXAP8 levels were markedly elevated in CC tissues, relative to NTT (). We, next, classified CC patients into two groups, based on their stage and lymph node metastatic statuses. As shown in , DUXAP8 upregulation was strongly association with advanced stages and lymph node metastases. Moreover, our Kaplan-Meier OS analysis revealed shorter OS rates in the CC patients with elevated DUXAP8 expression, relative to controls ().
Figure 1. Elevation in DUXAP8 levels are connected to poor cancer prognosis in colon cancer (CC) patients, as evidenced by qRT-PCR. (a) enhanced DUXAP8 levels in CC tissues versus corresponding non-tumor samples. (b) elevated DUXAP8 expression in patients at III/IV stages versus I/II stages. (c) elevated DUXAP8 levels in patients with lymphatic metastasis than without lymphatic metastasis. (d) CC patients expressing higher DUXAP8 levels experience shorter overall survival (OS) than patients with low DUXAP8 levels. ***P < 0.001.
DUXAP8 knockdown suppressed CC cell proliferation
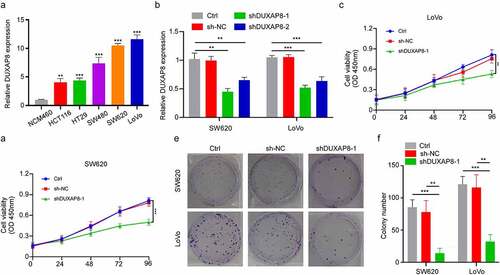
Given that DUXAP8 was highly expressed in CC patients, we further examined its influence in CC cellular proliferation. Using qRT-PCR, we demonstrated that the DUXAP8 levels were significantly increased in CC cell lines versus colon epithelial cells (). Next, we incorporated shRNA to knockdown DUXAP8 expression in the SW620 and LoVo cells, and assessed its subsequent biological effects (). Our CCK8-assay indicated that the DUXAP8 knockdown markedly suppressed cellular proliferation (). Moreover, as depicted in , DUXAP8 knockdown also prevented colony formation, relative to controls.
Figure 2. DUXAP8 knockdown inhibits cell proliferation of colon cancer (CC) cells, as evidenced by qRT-PCR. (a) markedly elevated DUXAP8 levels in five CC cell lines versus healthy colonic epithelial cells. (b) descended DUXAP8 levels following transfection with the sh-DUXAP8. (c-d) DUXAP8 deficiency in SW620 and LoVo cells significantly inhibits cellular proliferation. (e) DUXAP8 deficiency in SW620 and LoVo cells suppresses colony formation, relative to controls. (f) colonies were counted in (E). **P < 0.01, ***P < 0.001.
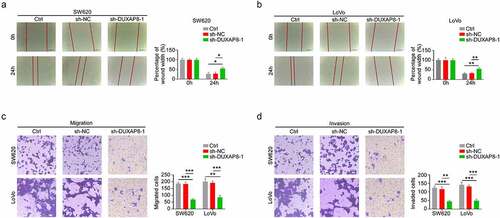
DUXAP8 knockdown inhibited CC migration and invasion
We, next, elucidated whether DUXAP8 affects migration and invasion of CC cells. Based on our wound healing assay results, the relative wound width was significantly enhanced in the sh-DUXAP8 group, thereby suggesting that the migration of CC was inhibited after DUXAP8 knockdown (). Similarly, using transwell assays, we demonstrated that, compared to the control group, the migration and invasion were drastically suppressed in the sh-DUXAP8 group, relative to controls (). Collectively, these results suggested that DUXAP8 knockdown suppresses both CC migration and invasion.
DUXAP8 overexpression promoted CC cellular proliferation
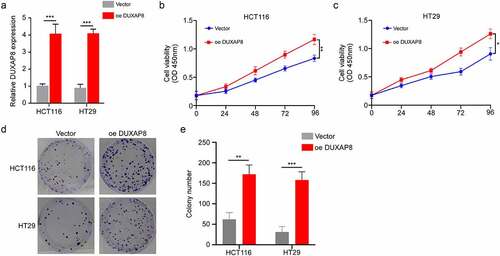
In previous experiments, we observed that DUXAP8 expression was reduced in HCT116 and HT29 CC cells. Hence, we overexpressed DUXAP8 in those cells, and evaluated its influence on cellular proliferation. We incorporated cells with the structured pcDNA 3.1-DUXAP8 to enhance DUXAP8 levels. Using qRT-PCR, we revealed that the pcDNA 3.1-DUXAP8 incorporation significantly elevated DUXAP8 levels within cells (). Based on our CCK8-assay, the cellular proliferation was markedly enhanced following DUXAP8 overexpression, relative to controls (). Furthermore, increased DUXAP8 levels accelerated colony formation, relative to controls ().
Figure 4. DUXAP8 incorporation accelerates colon cancer (CC) cell proliferation. (a) structured pcDNA 3.1-DUXAP8 incorporation significantly enhanced DUXAP8 levels. (b-c) DUXAP8 overexpression markedly increased cellular proliferation. (d) following pcDNA 3.1-DUXAP8 transfection, colonies were more and larger than the control groups. (e) colonies were counted in (D). **P < 0.01, ***P < 0.001.
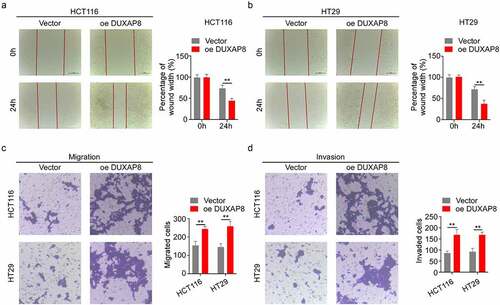
DUXAP8 overexpression accelerated CC migration and invasion
We, next, assessed the impact of DUXAP8 overexpression on CC migration and invasion. The relative wound width was drastically diminished in the oe-DUXAP8 group, which suggested that the CC migration was markedly increased after DUXAP8 overexpression (). Similarly, the transwell assays revealed that, compared to the control group, the oe-DUXAP8 group exhibited accelerated migration and invasion ().
DUXAP8 modulated CC tumorigenesis in vivo
Given that we already established that DUXAP8 promotes CC cellular proliferation in vitro. We, next, examined whether DUXAP8 also accelerates CC growth in a mouse xenograft model in vivo. Two weeks after CC cell administration, the tumors volume was measured. Based on our analysis, DUXAP8 knockdown remarkably decreased tumor volume, relative to controls (). These data indicated that DUXAP8, indeed, modulated CC tumorigenesis in vivo.
DUXAP8 was essential for EMT
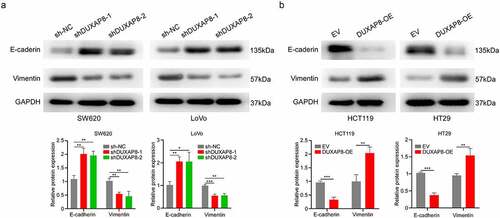
In order to elucidate the underlying mechanism behind the DUXAP8-mediated regulation of CC migration and invasion, we investigated the EMT-related protein expressions. The e‐cadherin levels were markedly increased, whereas, vimentin levels were strongly suppressed after DUXAP8 knockdown, as evidenced by western blot (). In contrast, the e‐cadherin levels were strongly suppressed, whereas, the vimentin levels were enhanced in the oe-DUXAP8 group versus controls (). Collectively, these results indicated that DUXAP8 induces EMT in CC.
Figure 7. DUXAP8 is critical for epithelial–mesenchymal transition (EMT). (a) DUXAP8 knockdown results in an upregulation of e‐cadherin expression, and suppression of vimentin levels. (b) DUXAP8 overexpression leads to the downregulation of e‐cadherin expression, and elevation of vimentin levels. **P < 0.01, ***P < 0.001.
Discussion
Prior studies suggested that pseudogene serves an essential function in promoting tumor occurrence in breast cancerCitation19, non-small cell lung cancerCitation11, CCCitation20 and pancreatic carcinomaCitation21. In this study, we demonstrated that DUXAP8 is a principal modulator of tumor occurrence and advancement in CC. Based on our analysis, DUXAP8 levels were markedly elevated in CC tissues, and they were strongly associated with the pathological stage and lymph node metastatic status of CC. Furthermore, the OS curves indicated that the higher expression of DUXAP8 represented worse OS in CC patients. Collectively, these results suggested that DUXAP8 serves as an oncogene, and is linked to poor prognosis in CC patients.
Pseudogene-derived RNAs are critical for various aspects of tumor biology in multiple tumors, including, tumorigenesis, apoptosis, and invasionCitation22,Citation23. Decreased pseudogene-derived RNASFTA1P suppresses cellular proliferation in gastric cancerCitation24. Lian et al. reported that the pseudogene-derived RNADUXAP10 is highly expressed in colorectal cancer tissues. Furthermore, several functional and mechanistic investigations revealed that DUXAP10 promotes cellular proliferation and tumorigenesis via silencing of the p21 and PTEN gene expressionsCitation25. Recently, increasing studies are investigating the DUXAP8 role in tumor. Wei et al. demonstrated marked upregulation in DUXAP8 levels in HCC, which, in turn, triggers cellular proliferation, migration, and invasion of HCC cells via regulation of the miR-422a/PDK2 axisCitation26. Another study reported that DUXAP8 downregulates miR-126 expression, which, in turn, promotes the renal cell carcinoma progressionCitation27. In our investigation, DUXAP8 knockdown strongly suppressed CC cellular proliferation, migration, and invasion. In contrast, DUXAP8 overexpression markedly enhanced cellular proliferation, migration, and invasion, similar to prior studies involving HCCCitation26. Further analysis indicated that the transplanted CC growth was significantly abrogated after DUXAP8 knockdown in the xenograft experiment in vivo.
EMT is strongly associated with tumor invasion and metastasis in numerous cancers, including, colonCitation28, gastricCitation29, and prostate cancersCitation30. It was previously demonstrated that several lncRNAs modulate EMT to regulate tumor metastasisCitation31,Citation32. Following EMT development, a polarized epithelial cell assumes the phenotype of a mesenchymal cell via multiple biochemical alterationsCitation33. In this study, DUXAP8 knockdown inhibited vimentin, and raised e-cadherin expression in CC cells. Alternately, DUXAP8 overexpression enhanced vimentin levels, while downregulating e-cadherin expression in CC cells. Our study demonstrated that DUXAP8 accelerated CC metastasis by facilitating EMT. We also demonstrated that DUXAP8 levels were markedly elevated in CC, and induced CC cellular proliferation, migration, and invasion. Nevertheless, it is unclear whether other downstream genes or regulatory mechanisms are involved in this process. Therefore, further investigations are warranted to clarify the functions of DUXAP8, and identify underlying mechanisms that promote the development of CC.
Conclusion
In summary, we demonstrated that DUXAP8 was significantly upregulated in CC. Additional analyses indicated that DUXAP8 accelerated CC cellular proliferation, migration, and EMT. Our work also indicated that CC patients with elevated DUXAP8 expression experience worse prognosis. Based on these evidences, DUXAP8 influences the occurrence and progression of CC, and may, therefore, serve as a regulatory hub for this disease.
Highlights
DUXAP8 is highly expressed in CC.
DUXAP8 may promote the proliferation, migration and invasion of CC cells.
DUXAP8 was required for EMT.
Supplemental Material
Download MS Word (20 KB)Acknowledgements
The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249.
- Xu F, Tang B, Jin TQ, et al. Current status of surgical treatment of colorectal liver metastases. World J Clin Cases. 2018;6(14):716–734.
- Chen S, Shen X. Long noncoding RNAs: functions and mechanisms in colon cancer. Mol Cancer. 2020;19(1):167.
- Yim GW, Lee DW, Kim JI, et al. Long non-coding rna loc285194 promotes epithelial ovarian cancer progression via the apoptosis signaling pathway. In Vivo (Athens, Greece). 2022;36(1):121–131.
- Zheng ZQ, Li ZX, Zhou GQ, et al. Long noncoding RNA FAM225A promotes nasopharyngeal carcinoma tumorigenesis and metastasis by acting as ceRNA to sponge mir-590-3p/mir-1275 and upregulate ITGB3. Cancer Research. 2019;79(18):4612–4626.
- Tang J, Yan T, Bao Y, et al. LncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-Myc. Nature Communications. 2019;10(1):3499.
- Xiao-Jie L, Ai-Mei G, Li-Juan J, et al. Pseudogene in cancer: real functions and promising signature. J Med Genet. 2015;52(1):17–24.
- Lou W, Ding B, Fu P. Pseudogene-Derived lncRNAs and their mirna sponging mechanism in human cancer. Front Cell Dev Biol. 2020;8:85.
- Yue C, Liang C, Ge H, et al. Pseudogene DUXAP10 acts as a diagnostic and prognostic marker and promotes cell proliferation by activating PI3K/AKT pathway in hepatocellular carcinoma. Onco Targets Ther. 2019;12:4555–4566.
- Tian X, Song J, Zhang X, et al. MYC-regulated pseudogene HMGA1P6 promotes ovarian cancer malignancy via augmenting the oncogenic HMGA1/2. Cell Death & Disease. 2020;11(3):167.
- Sun M, Nie FQ, Zang C, et al. The pseudogene DUXAP8 promotes non-small-cell lung cancer cell proliferation and invasion by epigenetically silencing EGR1 and RHOB. Mol ther. 2017;25(3):739–751.
- Zhang H, Chu K, Zheng C, et al. Pseudogene DUXAP8 promotes cell proliferation and migration of hepatocellular carcinoma by sponging MiR-490-5p to induce BUB1 expression. Front Genet. 2020;11:666.
- Zhao X, Hao S, Wang M, et al. Knockdown of pseudogene DUXAP8 expression in glioma suppresses tumor cell proliferation. Oncol Lett. 2019;17(3):3511–3516.
- Ma HW, Xie M, Sun M, et al. The pseudogene derived long noncoding RNA DUXAP8 promotes gastric cancer cell proliferation and migration via epigenetically silencing PLEKHO1 expression. Oncotarget. 2017;8(32):52211–52224.
- Liu Y, Zhang H, Wang H, et al. Long non-coding RNA DUXAP8 promotes the cell proliferation, migration, and invasion of papillary thyroid carcinoma via miR-223-3p mediated regulation of CXCR4. Bioengineered. 2021;121:496–506
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25(4):402–408.
- Song Z, Wang J. LncRNA ASMTL-AS1/microRNA-1270 differentiate prognostic groups in gastric cancer and influence cell proliferation, migration and invasion. Bioengineered. 2022;13(1):1507–1517.
- Yang Z, Ding H, Pan Z, et al. YY1-inudced activation of lncRNA DUXAP8 promotes proliferation and suppresses apoptosis of triple negative breast cancer cells through upregulating SAPCD2. Cancer Biology & Therapy. 2021;22(3):216–224.
- Yndestad S, Austreid E, Skaftnesmo KO, et al. Divergent activity of the pseudogene PTENP1 in ER-positive and negative breast cancer. Mol Cancer Res. 2018;16(1):78–89.
- Johnson GS, Li J, Beaver LM, et al. A functional pseudogene, NMRAL2P, is regulated by Nrf2 and serves as a coactivator of NQO1 in sulforaphane-treated colon cancer cells. Mol Nutr Food Res. 2017;61:4.
- Lian Y, Yang J, Lian Y, et al. DUXAP8, a pseudogene derived lncRNA, promotes growth of pancreatic carcinoma cells by epigenetically silencing CDKN1A and KLF2. Cancer Commun (Lond). 2018;38(1):64.
- Li J, Jiang L, Liu Z, et al. Oncogenic pseudogene DUXAP10 knockdown suppresses proliferation and invasion and induces apoptosis of papillary thyroid carcinoma cells by inhibition of Akt/mTOR pathway. Clinical and Experimental Pharmacology & Physiology. 2020;47(8):1473–1483.
- Yu L, Qiao R, Xu J, et al. FAM207BP, a pseudogene-derived lncRNA, facilitates proliferation, migration and invasion of lung adenocarcinoma cells and acts as an immune-related prognostic factor. Life Sci. 2021;268:119022.
- Ma H, Ma T, Chen M, et al. The pseudogene-derived long non-coding RNA SFTA1P suppresses cell proliferation, migration, and invasion in gastric cancer. Biosci Rep. 2018;38(2):2.
- Lian Y, Xu Y, Xiao C, et al. The pseudogene derived from long non-coding RNA DUXAP10 promotes colorectal cancer cell growth through epigenetically silencing of p21 and PTEN. Sci Rep. 2017;7(1):7312.
- Wei F, Yang L, Jiang D. Long noncoding RNA DUXAP8 contributes to the progression of hepatocellular carcinoma via regulating miR-422a/PDK2 axis. Cancer Medicine. 2020;9(7):2480–2490.
- Huang T, Wang X, Yang X, et al. Long non-coding rna duxap8 enhances renal cell carcinoma progression via downregulating miR-126. Med Sci Monit. 2018;24:7340–7347.
- Chae U, Kim B, Kim H, et al. Peroxiredoxin-6 regulates p38-mediated epithelial-mesenchymal transition in HCT116 colon cancer cells. Journal of Biological Research (Thessalonike, Greece). 2021;28(1):22.
- Bossaghzadeh F, Hajjari M, Sheikhi A, et al. HOTAIR induces the downregulation of miR-200 family members in gastric cancer cell lines. Iran Biomed J. 2022;26(1):77–84.
- El-Benhawy SA, Morsi MI, Fahmy EI, et al. Role of resveratrol as radiosensitizer by targeting cancer stem cells in radioresistant prostate cancer cells (PC-3). Asian Pac J Cancer Prev. 2021;22(12):3823–3837.
- Lu W, Zhang H, Niu Y, et al. Long non-coding RNA linc00673 regulated non-small cell lung cancer proliferation, migration, invasion and epithelial mesenchymal transition by sponging miR-150-5p. Mol Cancer. 2017;16(1):118.
- Li J, Zhang S, Wu L, et al. Interaction between LncRNA-ROR and miR-145 contributes to epithelial-mesenchymal transition of ovarian cancer cells. Gen Physiol Biophys. 2019;38(6):461–471.
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196.