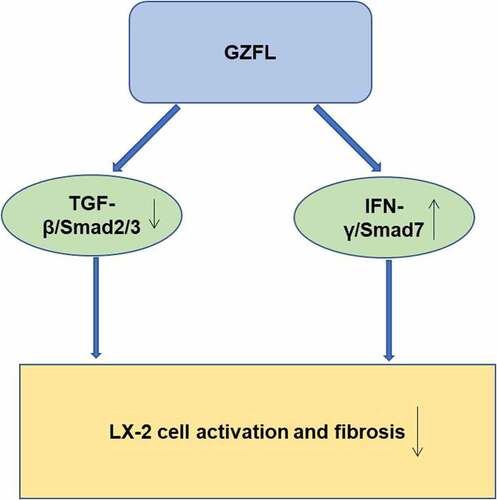
ABSTRACT
Liver fibrosis resulting from chronic liver injuries (CLI) is a common health problem globally. Guizhi Fuling pill (GZFL), a modern preparation from traditional Chinese medicine, exhibited anti-dysmenorrhea, anti-inflammatory, and immune-regulative effects. However, the effect of GZFL on liver fibrosis remains unknown. In this research, LX-2 cells were stimulated with acetaldehyde for mimicking liver fibrosis progression in vitro. In addition, carbon tetrachloride (CCl4)-induced mouse model of liver fibrosis was established as well. The data revealed GZFL obviously suppressed the proliferation and triggered the apoptosis of acetaldehyde-stimulated LX-2 cells. In addition, GZFL prevented acetaldehyde-induced activation of LX-2 cells via downregulation of TGF-β1, p-Smad2, p-Smad3, CUGBP1, and upregulation of p-STAT1 and Smad7. Meanwhile, GZFL significantly alleviated CCl4‑induced liver fibrosis, as evidenced by the decrease of ALT and AST levels. Moreover, GZFL downregulated the expressions of TGF-β1, p-Smad2, p-Smad3, and CUGBP1 in CCl4-treated mice. Furthermore, GZFL remarkably elevated the levels of IFN-γ, p-STAT1, and Smad7 in CCl4-treated mice. To sum up, GZFL was able to inhibit liver fibrosis in vitro and in vivo through suppressing TGF-β1/Smad2/3-CUGBP1 signaling and activating IFN-γ/STAT1/Smad7 signaling. Thus, GZFL might have a potential to act as a therapeutic agent for anti-fibrotic therapy.
Graphical Abstract
Introduction
Chronic liver diseases (CLD) have become a major disorder worldwide, which is characterized by sustained inflammatory response and fibrogenesis[Citation1]. Liver fibrosis is a wound-healing response to chronic liver injuries (CLI) that occurs in various types of CLD [Citation2]. CLD including hepatitis, nonalcoholic steatohepatitis, alcoholic fatty liver, and cholestasis could lead to liver fibrosis, and liver fibrosis may ultimately evolve into liver cirrhosis even hepatocellular carcinoma [Citation3–6]. Meanwhile, there are about 18 million new cases of liver fibrosis per year in China [Citation7]. At present, obethicolic acid has been approved for the treatment of liver fibrosis, while the outcomes remain limited. Thus, exploration of novel effective anti-fibrotic drugs for patients with liver fibrosis is extremely necessary.
Liver fibrosis is characterized by increased accumulation of extracellular matrix (ECM) proteins including type I collagen (collagen I) [Citation8,Citation9]. In addition, hepatic stellate cells (HSCs) play a major role in hepatic fibrogenesis[Citation10]. HSCs activated by inflammation was able to convert to myofibroblast-like cells and then generate excessive ECM, eventually leading to hepatic fibrosis [Citation11,Citation12]. Evidence has shown that acetaldehyde, a main toxic metabolite of alcohol, could trigger liver fibrosis via HSCs[Citation13]. Acetaldehyde is able to stimulate the activation of HSCs by promoting transforming growth factor‐β1 (TGF‐β1) synthesis in cells[Citation14]. In addition, acetaldehyde could increase the expressions of collagens and smooth muscle α‐action (α‐SMA) in HSCs[Citation14].
It has been reported that Chinese herbal compounds exerted anti-fibrotic effects, including Guizhi Fuling pill (GZFL)[Citation15]. GZFL is a Chinese herbal compound composed of five medicinal herbs, including Cinnamomi Ramulus, Poria, Moutan Cortex, Persicae Semen, and Paeoniae Radix Alba[Citation16]. GZFL exhibits various pharmacological properties including anti-dysmenorrhea, anti-inflammatory, and immunoloregulation effects [Citation16–18]. In addition, Lian et al. found that a heteropolysaccharide from Moutan Cortex could alleviate tubulointerstitial fibrosis in a rat with diabetic nephropathy[Citation19]. Zhang et al. found that amygdalin, a component from Semen Persicae, was able to attenuate pancreatic fibrosis in a rat with chronic pancreatitis[Citation20]. Moreover, Bushen Yijing Decoction (BSYJ) exerts an anti-fibrotic effect via regulating MicroRNA-26a/FLI1 axis[Citation21]. However, the detailed function of GZFL in liver fibrosis remains unexplored. Based on the above backgrounds, we hypothesized that GZFL might have the potential in the treatment of liver fibrosis. Hence, we explored the effects of GZFL on liver fibrosis in the current study.
Since HSC activation could lead to the progression of liver fibrosis[Citation22], the effect of GZFL on the viability of HSCs should be assessed. Additionally, the inflammatory responses and the activation of TGF-β signaling could cause the occurrence of liver fibrosis[Citation23]. Moreover, the data of liver function was important for the diagnosis of liver fibrosis[Citation24]. Thus, the related bioassays were required in this study. As expected, the results confirmed that GZFL could inhibit the progression of liver fibrosis, and this research might shed new lights on exploring new strategies against liver fibrosis.
Materials and methods
Cell culture and Materials
LX-2 cell line was obtained from the Xiangya Central Experiment Laboratory, Central South University (Changsha, China). Cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (FBS, Gibco, Waltham, MA, USA), 100 mg/ml streptomycin, and 100 U/ml penicillin at 37°C in a humidified atmosphere with 5% CO2. LX-2 cells were treated with acetaldehyde (400 μM; Macklin Inc., Shanghai, China) for 24 h, and then treated with colchicine (Sigma Aldrich, St. Louis, MO, USA) or GZFL (Chengdu Jiuzhitang Jinding Pharmaceutical Co., Ltd., Chengdu, China) for another 24 h. The procedure was performed in accordance with the previous reference[Citation15].
Cell viability assay
LX-2 cells (5 × 103 cells) were plated onto 96-well plates overnight. Then, cells were treated with GZFL (0, 1, 2, 4, 6, 8, 10, 25, or 50 mg/ml) or colchicine (0, 1, 2, 4, 8, 16, 32, or 64 μg/ml) for 24 h. After that, cell-counting kit 8 (CCK-8) reagent (10 μL; Dojindo, Shanghai, China) was added into each well for 2 h. Subsequently, the absorbance was recorded using a SpectraMax M2 microplate reader (Molecular Devices, San Jose, CA, USA) at 450 nm. The procedure was in accordance with the previous reference[Citation25].
AO/EB staining assay
LX-2 cells were stained with the dye mixture (1:1) of acridine orange (AO; 100 μg/ml) and ethidium bromide (EB; 100 μg/ml) for 5 min in darkness. Later on, cells were photographed with a fluorescent microscope (Nikon, Tokyo, Japan). The procedure was conducted in accordance with the previous reference[Citation26].
Hochest33342/PI staining assay
LX-2 cells were fixed for 15 min in 4% paraformaldehyde. After that, cells were stained with Hochest33342 (10 μl, 100 mg/ml) for 15 min and then stained with PI (5 μl, 1 g/l) for 30 min. Later on, cells were observed using a fluorescent microscope[Citation27].
Western blot assay
Each group of proteins (30 μg/lane) were separated by 10% SDS-PAGE and transferred to a PVDF membrane. Then, the membrane was incubated with primary antibodies against α-SMA (1:5000; Proteintech, Wuhan, China), Collagen I (1:8000; Proteintech), p-STAT1 (1:1000; Affinity biosciences, Cambridge, UK), STAT1 (1:5000; Proteintech), CUG-binding protein 1 (CUGBP1; 1:2000; Proteintech), TGF-β1 (1:1000; Proteintech), TGFBR2 (1:1000; Affinity biosciences), Smad1 (1:5000; Proteintech), p-Smad2 (1:1000; Affinity biosciences), Smad2 (1:5000; Proteintech), p-Smad3 (1:1000; Affinity biosciences), Smad3 (1:3000; Proteintech), Smad7 (1:1000; Proteintech), Bax (1:1000; Affinity biosciences), active caspase 3 (1:1000; Affinity biosciences), BCL-2 (1:1000; Proteintech), active caspase 9 (1:1000; Affinity biosciences), and GAPDH (1:10,000; Proteintech) overnight at 4°C. Later on, the membrane was probed with the secondary antibody for 1 h. Subsequently, the blots were developed using an electrochemiluminescence reagent (Thermo Fisher Scientific). The procedure was conducted in accordance with the previous reference[Citation28].
Animal study
Male ICR mice (4–6‐week age) were obtained from Zhejiang Provincial Experimental Animal Center. All animal experiments were approved by the Ethics Committee of Experimental Animal Ethics Committee of Zhejiang Ocean University (Zhoushan, China) (Permission No. 2020–0026) and performed according to the procedures of National Institutes of Health guide for the care and use of laboratory animals. Animals were allowed to adapt to their environment for 7 days. After that, animals were divided into five groups randomly: control, CCl4, CCl4 + colchicine, CCl4 + low dose GZFL (L-GZFL), CCl4 + high dose GZFL (H-GZFL) groups (n = 5). In the control group, mice were intraperitoneally treated with saline (0.9% NaCl) for 4 weeks (twice per week). Mice in the CCL4, CCl4 + colchicine, CCl4 + L-GZFL or CCl4 + H-GZFL groups were intraperitoneally administrated with CCl4 solution (3 ml/kg, dissolved in soybean oil) for 4 weeks (twice per week). After 4 weeks of treatment, mice in the control and CCl4 groups were given 0.9% saline by gastric gavage per day for another 4 weeks. Meanwhile, mice in the CCl4 + colchicine, CCl4 + L-GZFL or CCl4 + H-GZFL groups were given colchicine (0.1 mg/kg), L-GZFL (125 mg/kg) or H-GZFL (250 mg/kg) three times a day by gastric gavage for another 4 weeks, respectively. The drug administration was in line with previous described[Citation29]. The body weights of mice were recorded weekly. All mice were sacrificed at 9 weeks, and the liver and spleen weights of mice were recorded. Liver or spleen coefficient = liver or spleen weight/body weight.
Histological evaluation
Liver samples were sectioned to a thickness of 5 μm. Subsequently, slides were subjected to H&E staining and Masson’s trichrome staining as previously described[Citation30]. Next, the injury of liver tissue was observed using an Olympus BH2 microscope.
ELISA assay
The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (T-BIL), albumin (ALB), alkaline phosphatase (AKP), glutathione sulfotransferase (GSH-ST) in serum of mice were detected using ELISA kits. In addition, the concentrations of hydroxyproline (Hyp), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), total antioxidant capacity (T-AOC), catalase (CAT), hyaluronic acid (HA) and laminin (LN), type III procollagen (PC III) and type IV collagen (Col-IV) in the liver tissues of mice were detected using ELISA kits. These ELISA kits were obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Meanwhile, the concentrations of IL-6, IFN-γ, IL-1β, TNF-α in the liver tissues of mice were detected by ELISA kits (Elabscience Biotechnology Co., Ltd., Wuhan, China).
RT-qPCR assay
Total RNA was isolated from liver tissues using the TRIzol reagent and transcribed using a HiScript 1st Strand cDNA Synthesis Kit (Vazyme, Piscataway, NJ, USA). After that, qPCR was carried out using AceQ qPCR SYBR Green Master Mix (without ROX) (Vazyme). Gene expression was normalized against GAPDH and calculated using 2−ΔΔct method[Citation31]. The sequences of primers were as follows: TGF-βR2 forward, 5’-AAACUACUAGGUAAAGGCACUUUU-3’ and reverse, 5’-GATAAAUUUAAAGCUCUGUGCC-3’; Smad2 forward, 5’-GGAGGACTGAGAAGGTGAGGC-3’ and reverse, 5’-GGCAAGGGGACATCCTCTG-3’; Smad3 forward, 5’-CTCTTCTCATTCCTGCTTG-3’ and reverse, 5’-CTCCACTTGGTGGTTTGT-3’; α-SMA forward, 5’-CACAGAAGGAGTGGCTAA-3’ and reverse, 5’-CCATAACGCACTAGGTTT-3’; GAPDH forward, 5’-GTCCACCGCAAATGCTTCTA-3’ and reverse, 5’-TGCTGTCACCTTCACCGTTC-3’.
Statistical analysis
Each experiment was repeated independently at least three times. Data are presented as the mean ± standard deviation (S.D.) The statistical significance of differences was calculated by One-way analysis of variance (ANOVA) and Tukey’s tests. P values of <0.05 were considered as statistically significant.
Results
GZFL inhibits acetaldehyde-induced LX-2 cells activation by regulating cell proliferation and apoptosis
In order to explore the effect of GZFL on liver fibrosis in vitro, LX-2 cells were activated with acetaldehyde firstly. Then, the effect of acetaldehyde on LX-2 cell viability was evaluated. The result showed that 400 μM acetaldehyde remarkably increased the viability of LX-2 cells (). In addition, colchicine (4 μg/ml), used as a positive control, induced about 50% growth inhibition in LX-2 cells (). Moreover, 8 and 10 mg/ml GZFL markedly decreased the proliferation of LX-2 cells (). Thus, GZFL at 8 or 10 mg/ml dose was used for the subsequent in vitro experiments.
Figure 1. GZFL inhibits acetaldehyde-induced LX-2 cells activation through regulating cell viability and apoptosis. (a) LX-2 cells were treated with different concentrations of acetaldehyde and cell viability was detected with CCK-8 assay (b) LX-2 cells were treated with different concentrations of colchicine and cell viability was detected with CCK-8 assay. (c) LX-2 cells were treated with different concentrations of GZFL and cell viability was detected with CCK-8 assay. (d, e) LX-2 cells were stimulated with 400 μM for 24 h, and then treated with colchicine (4 μg/ml) or GZFL (8 or 10 mg/ml) for another 24 h. AO/EB and Hochest3342/PI staining assays were performed to determine cell apoptosis. (f) Western blot was used to determine Bax, BCL-2, active caspase 3 and active caspase 9 expressions in LX-2 cells. **P < 0.01 vs. control group; ##P < 0.01 vs. AA group; n = 3.
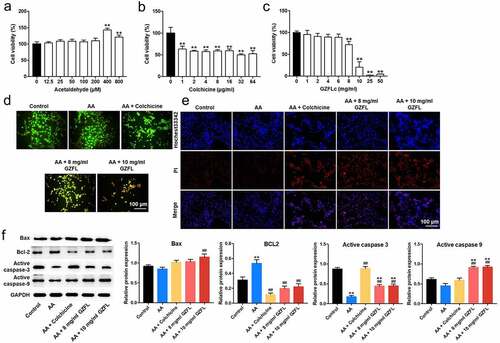
Next, AO/EB staining and Hochest3342 staining indicated that 8 or 10 mg/ml GZFL obviously induced the apoptosis in acetaldehyde-stimulated LX-2 cells ( and 1E). Meanwhile, acetaldehyde significantly reduced the expression of active caspase 3 and upregulated BCL2 expression in LX-2 cells, whereas the effects of acetaldehyde were partially reversed by GZFL treatments (). To sum up, GZFL could suppress acetaldehyde-induced LX-2 cells activation by regulating cell proliferation and apoptosis.
GZFL inhibits acetaldehyde-induced LX-2 cells activation through suppressing TGF-β1/Smad2/3 signaling and activating IFN-γ/STAT1/Smad7 pathway
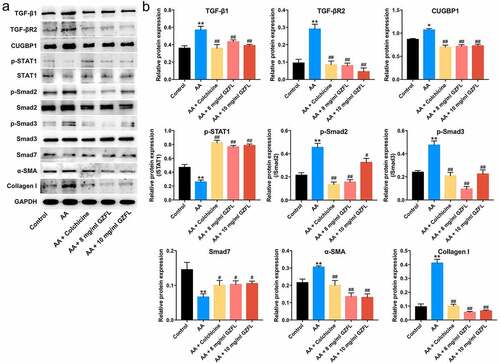
Evidences have shown that TGF-β1/Smad2/3 signaling play a pro-fibrotic role, while IFN-γ/STAT1/Smad7 signaling pathway exhibit an anti-fibrotic role in liver fibrosis progression [Citation32,Citation33]. Importantly, CUGBP1 has been emerged as a key molecule in liver fibrosis, which may affect the pro- and anti-fibrotic signaling pathways[Citation34]. Therefore, we investigated the effects of GZFL on TGF-β1/Smad2/3, IFN-γ/STAT1 or CUGBP1 signaling in acetaldehyde-treated LX-2 cells. The data indicated that GZFL notably decreased the expressions of TGF-β1, TGF-βR2, CUGBP1, p-Smad2, p-Smad3, α-SMA and Collagen I and upregulated the expressions of p-STAT1 and Smad7 in acetaldehyde-treated LX-2 cells ( and 2B). Furthermore, no significant differences in IFN-γ levels were detected between acetaldehyde and GZFL treatment groups (Supplementary Fig. 1). These data suggested that GZFL could inhibit acetaldehyde-induced LX-2 cells activation through suppressing TGF-β1/Smad2/3, CUGBP1 signaling and activating IFN-γ/STAT1/Smad7 pathway.
Figure 2. GZFL inhibits acetaldehyde-induced LX-2 cells activation through suppressing TGF-β1/Smad2/3 signaling and activating IFN-γ/STAT1/Smad7 signaling. LX-2 cells were exposed to acetaldehyde (AA; 400 μM) for 24 h, followed by exposure to colchicine (4 μg/ml) or GZFL (8 or 10 mg/ml) for another 24 h. (a, b) TGF-β1, TGF-βR2, CUGBP1, p-STAT1, p-Smad2, p-Smad3, Smad7, α-SMA and Collagen I expressions in LX-2 cells were detected by western blot assay. *P < 0.05, **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. AA group; n = 3.
GZFL ameliorates CCl4-induced liver fibrosis in mouse in vivo
To further explore the role of GZFL in liver fibrosis in vivo, CCl4-induced mouse model of liver fibrosis was constructed. As shown in , mice subjected to intraperitoneal injection with CCl4 displayed an increase of liver or spleen index, while these phenomena were reversed by GZFL treatment. Furthermore, HE staining assay revealed that mice subjected to CCl4 displayed obvious liver injury (unordered liver structure and localized injury and necrosis); however, CCl4-induced liver injury were completely reversed by GZFL treatment (). Moreover, masson’s trichrome staining revealed a higher accumulation of collagen in CCl4-treated mice compared to control group; whereas GZFL treatment greatly attenuated these pathologic changes ().
Figure 3. GZFL ameliorates CCl4-induced liver fibrosis in vivo. (a) The liver and spleen index in mice with CCl4-induced liver fibrosis. (b) Analysis of liver injury in liver tissues with HE staining. Analysis of collagen deposition in liver tissues with Masson’s trichrome staining assay. (c) ALT, AST, T-BIL, ALB and AKP levels in serum samples in mice with CCl4-induced liver fibrosis were measured with ELISA assay. (d) The levels of liver fibrotic markers Hyp, HA, LN, PC III and Col-IV in liver tissues in mice with CCl4-induced liver fibrosis were measured with ELISA assay. *P < 0.05, **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. CCL4 group; n = 5.
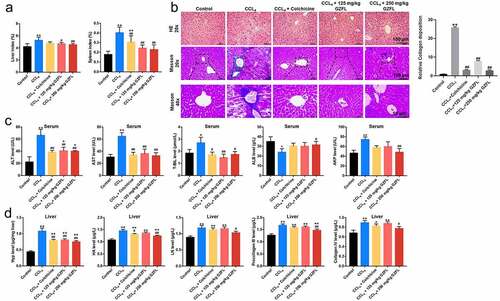
Next, the mouse liver function was determined by analyzing serum ALT, AST, and T-BIL, ALB and AKP levels. As indicated in , CCl4 significantly elevated serum ALT, AST, T-BIL and AKP levels and reduced ALB levels in mouse; however, these changes were all reversed by GZFL treatments. Meanwhile, GZFL treatment notably downregulated the levels of liver fibrotic markers Hyp, HA, LN, PC III and Col-IV in CCl4-treated mice (). Collectively, GZFL was able to ameliorate CCl4-induced liver fibrosis in mouse in vivo.
GZFL ameliorates CCl4-induced liver fibrosis in mouse in vivo via inhibiting inflammation and oxidative stress
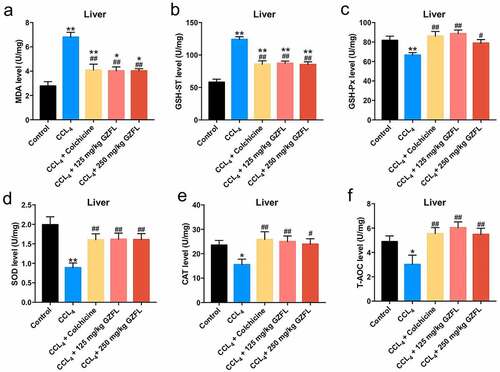
With the purpose of exploring whether GZFL could exhibit anti-inflammatory and anti-oxidative effects in CCl4-treated mice, ELISA assay was applied. We found that GZFL treatment markedly decreased the levels of IL-1β, IL-6, TNF-α and elevated the expression of IFN-γ in the liver tissues of CCl4-treated mice (). Meanwhile, GZFL treatment notably reduced MDA and GSH-ST and upregulated GSH-Px, SOD, CAT, and T-AOC levels in the liver tissues of CCl4-treated mice (, 5B, 5C, 5D, 5E, and 5 F). To sum up, GZFL could ameliorate CCl4-induced liver fibrosis in mouse in vivo via exerting anti-inflammatory and anti-oxidative activities.
Figure 4. GZFL ameliorates CCl4-induced liver fibrosis in vivo via exerting anti-inflammation effect. The levels of IL-1β, IL-6, TNF-α, IFN-γ in liver tissues of CCl4-treated mice were measured with ELISA assay. **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. CCL4 group; n = 5.
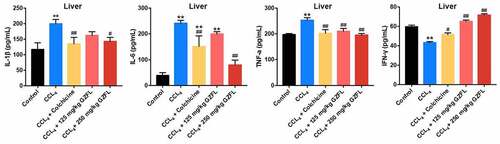
Figure 5. GZFL ameliorates CCl4-induced liver fibrosis in vivo via exerting anti-oxidation effect. (A, B, C, D, E, F) The levels of MDA, GSH-ST, GSH-Px, SOD, CAT, T-AOC in liver tissues of CCl4-treated mice were measured with ELISA assay. *P < 0.05, **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. CCL4 group; n = 5.
GZFL ameliorates CCl4-induced liver fibrosis in mouse in vivo by inhibiting TGF-β1/Smad2/3 signaling and activating IFN-γ/STAT1 signaling
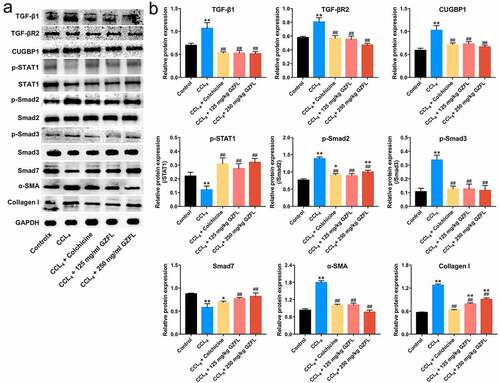
We finally explored the effects of GZFL on TGF-β1/Smad2/3 signaling and IFN-γ/STAT1 signaling in CCl4-induced mouse model of liver fibrosis by using western blot. As shown in and 6B and Supplementary Fig. 2A-2D, TGF-β1, TGF-βR2, CUGBP1, p-Smad2, p-Smad3, Collagen I and α-SMA levels were remarkably elevated and p-STAT1 and Smad7 levels were reduced in liver tissues of CCl4-treated mice; whereas these changes were reversed by GZFL treatment. Collectively, GZFL could reduce CCl4-induced liver fibrosis in vivo by inhibiting TGF-β1/Smad2/3, CUGBP1 signaling and activating IFN-γ/STAT1/Smad7 signaling.
Figure 6. GZFL ameliorates CCl4-induced liver fibrosis in vivo by inhibiting TGF-β1/Smad2/3 signaling and activating IFN-γ/STAT1/Smad7 signaling. (a, b) TGF-β1, TGF-βR2, CUGBP1, p-STAT1, p-Smad2, p-Smad3, Smad7, α-SMA and Collagen I expressions in liver tissues of CCl4-treated mice were detected using western blot assay. *P < 0.05, **P < 0.01 vs. control group; ##P < 0.01 vs. CCL4 group; n = 5.
Discussion
Liver fibrosis, a serious health problem worldwide, is the common pathological basis of chronic liver diseases [Citation35,Citation36]. Currently, there are no effective therapies for liver fibrosis. Fortunately, Traditional Chinese medicine has attracted more attention for their anti-fibrosis effects [Citation37,Citation38]. Hsu et al. found that Graptopetalum paraguayense could inhibit liver fibrosis in diethylnitrosamine-induced rat liver injury model via suppressing TGF-β signaling[Citation39]. Chinese herbal formula Fuzheng Huayu was able to alleviate liver fibrosis in CCl4-treated rats[Citation40]. However, the role of GZFL in liver fibrosis remains unclear.
The activated HSCs, main producers of ECM, contribute to the formation of liver fibrosis [Citation23,Citation41]. Acetaldehyde was able to trigger the activation of HSCs via inducing cell proliferation, which is accompanied by the increase of Collagen I and α-SMA[Citation14]. In this study, we found GZFL treatments significantly reversed acetaldehyde-induced LX-2 cells activation. Furthermore, GZFL obviously induced the apoptosis of acetaldehyde-stimulated LX-2 cells. Meng et al. reported that carvedilol could inhibit HSCs activation via promoting cell apoptosis[Citation42]. Consistent with previous reports, GZFL was able to inhibit acetaldehyde-induced LX-2 cells activation through suppressing cell proliferation and promoting apoptosis.
Furthermore, we found that CCl4 notably induced liver injury and fibrosis in mice, as determined by the elevated levels of ALT and AST. Meanwhile, CCl4 upregulate liver fibrotic markers HA, LN, PC III, and Col-IV in mice. However, GZFL treatments significantly alleviated liver injury and fibrosis in CCl4-treated mice. In addition, we found that GZFL exhibited anti-inflammation and anti-oxidation effects in CCl4-treated mice. Consistently, Feriani et al. showed that zygophyllum album leaves extract alleviated liver fibrosis in deltamethrin-treated rats via improving liver function and inhibiting inflammation and oxidative stress[Citation43]. These data showed that GZFL could ameliorate liver fibrosis in vivo via exerting anti-inflammatory and anti-oxidative activities.
Evidences have shown that TGF-β could trigger HSCs activation and fibrotic injury; suppressing TGF-β signaling could alleviate the liver fibrosis process [Citation41,Citation44]. As we know, TGF-β promotes fibrogenesis via activating Smad2 and Smad3, which is negatively regulated by Smad7[Citation23]. Chen et al indicated that Baihe Wuyao decoction could reduce liver fibrosis in CCl4-treated mice through inhibiting TGF-β1/Smads signaling[Citation45]. Our data indicated that GZFL remarkably decreased the levels of TGF-β1, TGF-βR2, p-Smad2, p-Smad3 and increased the level of Smad7 in vitro and in vivo. All these data indicated that GZFL was able to ameliorate liver fibrosis in vitro and in vivo though suppressing TGF-β1/Smad2/3 signaling pathway.
It has been shown that IFN-γ/STAT1 signaling exhibits anti-fibrotic activity in liver cells [Citation33,Citation46]. IFN-γ could elevate Smad7 expression through activation of STAT-1 signaling, thereby inhibiting TGF-β signaling[Citation33]. Jeong et al. showed that STAT1 could alleviate liver fibrosis by inhibition of TGF-β signaling[Citation47]. In this study, GZFL elevated IFN-γ, p-STAT1, and Smad7 expressions in liver tissues of CCl4-treated mice. These data showed that GZFL was able to ameliorate liver fibrosis by activating IFN-γ/STAT1/Smad7 signaling.
RNA‐binding protein CUGBP1 is a key molecule in regulating liver injury [Citation48,Citation49]. Recently, Wu et al. revealed that TGF-β could elevate the CUGBP1 expression in HSCs[Citation34]. In addition, CUGBP1 promoted HSCs activation via downregulating IFN-γ levels[Citation50]. These data suggested that CUGBP1 is a key molecule in liver fibrosis, which mediated the crosstalk between TGF-β1/Smad2/3 and IFN-γ/STAT1 signaling pathway. The present results showed that GZFL notably decreased the levels of TGF-β1 and CUGBP1 and upregulated the level of IFN-γ in liver tissues of CCl4-treated mice. Thus, we deduced GZFL decreased the expression of CUGBP1, and then triggered IFN-γ/Smad7 signaling in activated HSCs. In another word, the pro-fibrogenic activity of TGF-β was limited by GZFL. All these data suggested that GZFL was able to ameliorate liver fibrosis in vitro and in vivo by modulating the crosstalk between TGF-β1/Smad2/3 and IFN-γ/STAT1/Smad7 signaling pathway via CUGBP1.
Conclusion
GZFL could inhibit acetaldehyde‑induced cellular fibrosis and alleviate CCL4‑induced liver fibrosis by inhibiting TGF-β1/Smad2/3, CUGBP1 signaling and activating IFN-γ/STAT1/Smad7 signaling. Therefore, GZFL might have a potential to act as a therapeutic agent for anti-fibrotic therapy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All animal procedures were approved by the Ethics Committee of Zhejiang Ocean University (Zhoushan, China).
Supplemental Material
Download Zip (5.7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here
Additional information
Funding
References
- Parola M, Pinzani M. Liver fibrosis: pathophysiology, pathogenetic targets and clinical issues. Mol Aspects Med. 2019;65:37–55.
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115(2):209–218.
- Ye J, Lin Y, Yu Y, et al. LncRNA NEAT1/microRNA-129-5p/SOCS2 axis regulates liver fibrosis in alcoholic steatohepatitis. J Transl Med. 2020;18(1):445.
- Blonsky JJ, Harrison SA. Review article: nonalcoholic fatty liver disease and hepatitis C virus–partners in crime. Aliment Pharmacol Ther. 2008;27(10):855–865.
- Dong Z, Li S, Wang X, et al. lncRNA GAS5 restrains CCl 4-induced hepatic fibrosis by targeting miR-23a through the PTEN/PI3K/Akt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2019;316(4):G539–g50.
- Kwon HJ, Won YS, Park O, et al. Aldehyde dehydrogenase 2 deficiency ameliorates alcoholic fatty liver but worsens liver inflammation and fibrosis in mice. Hepatology. 2014;60(1):146–157.
- Wang S, Fan X, Gao Y, et al. The Relationship Between Zinc Deficiency and Hepatocellular Carcinoma Associated with Hepatitis B Liver Cirrhosis: a 10-year Follow-up Study. Biol Trace Elem Res. 2022. DOI:10.1007/s12011-022-03156-z
- Petitclerc L, Sebastiani G, Gilbert G, et al. Liver fibrosis: review of current imaging and MRI quantification techniques. J Magn Reson Imaging. 2017;45(5):1276–1295.
- Urtasun R, Conde de la Rosa L, Nieto N. Oxidative and nitrosative stress and fibrogenic response. Clin Liver Dis. 2008;12(4):769–90, viii.
- Nakano Y, Kamiya A, Sumiyoshi H, et al. A Deactivation Factor of Fibrogenic Hepatic Stellate Cells Induces Regression of Liver Fibrosis in Mice. Hepatology. 2020;71(4):1437–1452.
- Shajari S, Laliena A, Heegsma J, et al. Melatonin suppresses activation of hepatic stellate cells through ROR α-mediated inhibition of 5-lipoxygenase. J Pineal Res. 2015;59(3):391–401.
- Fagone P, Mangano K, Pesce A, et al. Emerging therapeutic targets for the treatment of hepatic fibrosis. Drug Discov Today. 2016;21(2):369–375.
- Takase T, Toyoda T, Kobayashi N, et al. Dietary iso-α-acids prevent acetaldehyde-induced liver injury through Nrf2-mediated gene expression. PloS one. 2021;16(2):e0246327.
- Lestari N, Louisa M, Soetikno V, et al. Alpha Mangostin Inhibits the Proliferation and Activation of Acetaldehyde Induced Hepatic Stellate Cells through TGF-β and ERK 1/2 Pathways. J Toxicol. 2018;2018:5360496.
- Chen L, Chen H, Yang Q, et al. Guizhi Fuling Capsule inhibits uterine fibroids growth by modulating Med12-mediated Wnt/beta-Catenin signaling pathway. J Ethnopharmacol. 2022;290:115115.
- Zheng Y, Xin G, Gong G, et al. Evaluation of Anti-Inflammatory Components of Guizhi Fuling Capsule, an Ancient Chinese Herbal Formula, in Human Umbilical Vein Endothelial Cells. Evidence-based complementary and alternative medicine: eCAM 2020. 2020;10(2):2029134.
- Zheng W, Li M, Wang Y, et al. Guizhi Fuling Capsule Exhibits Antidysmenorrhea Activity by Inhibition of Cyclooxygenase Activity. Evidence-based complementary and alternative medicine: eCAM 2020. 2020;23(4):8607931.
- Wang YR, Li N, Cao L, et al. Study on anti-inflammation and immunoloregulation effect of Guizhi Fuling capsule ingredients using high content screening. Zhongguo Zhong Yao Za Zhi. 2015;40(6):1005–1011.
- Lian Y, Zhu M, Chen J, et al. Characterization of a novel polysaccharide from Moutan Cortex and its ameliorative effect on AGEs-induced diabetic nephropathy. Int J Biol Macromol. 2021;176:589–600.
- Zhang X, Hu J, Zhuo Y, et al. Amygdalin improves microcirculatory disturbance and attenuates pancreatic fibrosis by regulating the expression of endothelin-1 and calcitonin gene-related peptide in rats. J Chin Med Assoc. 2018;81(5):437–443.
- Cheng Z, Zhang J, Deng W, et al. Bushen Yijing Decoction (BSYJ) exerts an anti-systemic sclerosis effect via regulating MicroRNA-26a /FLI1 axis. Bioengineered. 2021;12(1):1212–1225.
- Ye Y, Wu W, Zheng J, et al. Role of long non-coding RNA-adducin 3 antisense RNA1 in liver fibrosis of biliary atresia. Bioengineered. 2022;13(3):6222–6230.
- Xu XY, Geng Y, Xu HX, et al. Antrodia camphorata-Derived Antrodin C Inhibits Liver Fibrosis by Blocking TGF-Beta and PDGF Signaling Pathways. Front Mol Biosci. 2022;9:835508.
- Zhu T, Zhang L, Li C, et al. The S100 calcium binding protein A11 promotes liver fibrogenesis by targeting TGF-beta signaling. J Genet Genomics. 2022. DOI:10.1016/j.jgg.2022.02.013.
- Chen CC, Huang CY, Shiu LY, et al. Combinatory effects of current regimens and Guizhi Fuling Wan on the development of endometriosis. Taiwan J Obstet Gynecol. 2022;61(1):70–74.
- Zhou Z, Chen H, Li Y, et al. Transcriptome and biochemical analyses of rainbow trout (Oncorhynchus mykiss) RTG-2 gonadal cells in response to BDE-47 stress indicates effects on cell proliferation. Aquat Toxicol. 2022;245:106108.
- Zhu YF, Wang R, Chen W, et al. miR-133a-3p attenuates cardiomyocyte hypertrophy through inhibiting pyroptosis activation by targeting IKKepsilon. Acta Histochem. 2021;123(1):151653.
- Bai Z, Liu W, He D, et al. Protective effects of autophagy and NFE2L2 on reactive oxygen species-induced pyroptosis of human nucleus pulposus cells. Aging (Albany NY). 2020;12(8):7534–7548.
- Zhang Y, Su N, Liu W, et al. Metabolomics Study of Guizhi Fuling Capsules in Rats With Cold Coagulation Dysmenorrhea. Front Pharmacol. 2021;12:764904.
- Zhong X, Song Z, Ning Z, et al. Inhibition of Src improves cardiac fibrosis in AngII-induced hypertrophy by regulating the expression of galectin-3. Microvasc Res. 2022;104347. DOI:10.1016/j.mvr.2022.104347
- Liu N, Guan Y, Zhou C, et al. Pulmonary and Systemic Toxicity in a Rat Model of Pulmonary Alveolar Proteinosis Induced by Indium-Tin Oxide Nanoparticles. Int J Nanomedicine. 2022;17:713–731.
- Song L, Chen TY, Zhao XJ, et al. Pterostilbene prevents hepatocyte epithelial-mesenchymal transition in fructose-induced liver fibrosis through suppressing miR-34a/Sirt1/p53 and TGF-β1/Smads signalling. Br J Pharmacol. 2019;176(11):1619–1634.
- Weng H, Mertens PR, Gressner AM, et al. IFN-gamma abrogates profibrogenic TGF-beta signaling in liver by targeting expression of inhibitory and receptor Smads. J Hepatol. 2007;46(2):295–303.
- Wu X, Wu X, Ma Y, et al. CUG-binding protein 1 regulates HSC activation and liver fibrogenesis. Nat Commun. 2016;7(1):13498.
- Wang P, Cui Y, Wang J, et al. Mesenchymal stem cells protect against Acetaminophen hepatotoxicity by secreting regenerative cytokine hepatocyte growth factor. Stem Cell Res Ther. 2022;13(1):94.
- Abassa KK, Xiao XP, Zhou HX, et al. FcGBP and VCAM-1 are ponderable biomarkers for differential diagnosis of alcoholic liver cirrhosis. Drug Alcohol Depend. 2022;233:109377.
- Wang R, Song F, Li S, et al. Salvianolic acid A attenuates CCl(4)-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des Devel Ther. 2019;13:1889–1900.
- Cai FF, Bian YQ, Wu R, et al. Yinchenhao decoction suppresses rat liver fibrosis involved in an apoptosis regulation mechanism based on network pharmacology and transcriptomic analysis. Biomed Pharmacothe. 2019;114:108863.
- Hsu WH, Liao SC, Chyan YJ, et al. Graptopetalum paraguayense Inhibits Liver Fibrosis by Blocking TGF-β Signaling In Vivo and In Vitro. Int J Mol Sci. 2019;20(10) :2592.
- Dong S, Cai FF, Chen QL, et al. Chinese herbal formula Fuzheng Huayu alleviates CCl(4)-induced liver fibrosis in rats: a transcriptomic and proteomic analysis. Acta Pharmacol Sin. 2018;39(6):930–941.
- Yu X, Elfimova N, Muller M, et al. Autophagy-related activation of hepatic stellate cells reduces cellular miR-29a by promoting its vesicular secretion. Cell Mol Gastroenterol Hepatol. 2022. DOI:10.1016/j.jcmgh.2022.02.013.
- Meng D, Li Z, Wang G, et al. Carvedilol attenuates liver fibrosis by suppressing autophagy and promoting apoptosis in hepatic stellate cells. Biomed Pharmacothe. 2018;108:1617–1627.
- Feriani A, Tir M, Gómez-Caravaca AM, et al. Zygophyllum album leaves extract prevented hepatic fibrosis in rats, by reducing liver injury and suppressing oxidative stress, inflammation, apoptosis and the TGF-β1/Smads signaling pathways. Exploring of bioactive compounds using HPLC-DAD-ESI-QTOF-MS/MS. Inflammopharmacology. 2020;28(6):1735–1750.
- Wang T, Zhang C, Meng X, et al. Long Noncoding RNA Metastasis-Associated Lung Adenocarcinoma Transcript 1 in Extracellular Vesicles Promotes Hepatic Stellate Cell Activation, Liver Fibrosis and beta-Catenin Signaling Pathway. Front Physiol. 2022;13:792182.
- Chen Y, Li R, Hu N, et al. Baihe Wuyao decoction ameliorates CCl(4)-induced chronic liver injury and liver fibrosis in mice through blocking TGF-β1/Smad2/3 signaling, anti-inflammation and anti-oxidation effects. J Ethnopharmacol. 2020;263:113227.
- Ramalingam TR, Gieseck RL, Acciani TH, et al. Enhanced protection from fibrosis and inflammation in the combined absence of IL-13 and IFN-γ. J Pathol. 2016;239(3):344–354.
- Jeong WI, Park O, Radaeva S, et al. STAT1 inhibits liver fibrosis in mice by inhibiting stellate cell proliferation and stimulating NK cell cytotoxicity. Hepatology. 2006;44(6):1441–1451.
- Cast A, Kumbaji M, D’Souza A, et al. Liver Proliferation Is an Essential Driver of Fibrosis in Mouse Models of Nonalcoholic Fatty Liver Disease. Hepatol Commun. 2019;3(8):1036–1049.
- Liu Y, Huang H, Yuan B, et al. Suppression of CUGBP1 inhibits growth of hepatocellular carcinoma cells. Clin Invest Med. 2014;37:E10–8.
- Huang Q, Zhang X, Bai F, et al. Methyl helicterte ameliorates liver fibrosis by regulating miR-21-mediated ERK and TGF-β1/Smads pathways. Int Immunopharmacol. 2019;66:41–51.
