?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The mRNA turnover and ribosome assembly are facilitated by Mrt4 protein from Saccharomyces cerevisiae. In present study, we are reporting the cloning, expression and homogeneous purification of recombinant Mrt4. Mrt4 is a 236-amino-acid-long nuclear protein that plays a very crucial role in mRNA turnover and ribosome assembly during the translation process. mrt4 gene was amplified by polymerase chain reaction and cloned in expression vector pET23a (+) under the bacteriophage T7-inducible promoter and lac operator. Furthermore, protein was purified to homogeneity using immobilized metal affinity chromatography (IMAC) and its homogeneous purification was further validated by immunoblotting with anti-His antibody. The far-UV CD spectra represent that Mrt4 has a typical α helix with characteristic negative minima at 222 and 208 nm. At physiological pH, the fluorescence spectra and CD spectra showed properly folded tertiary and secondary structures of Mrt4, respectively. Saccharomyces Mrt4 protein possesses putative bipartite NLS (nuclear localization signal) at the N-terminal part followed by two well-conserved domains, rRNA-binding domains and translation factor (TF) binding domain. PIPSA analysis evaluates electrostatic interaction properties of proteins and concluded that Mrt4 protein can be used as a fingerprint for classifying Mrt4-like mRNA turnover protein from various species. The availability of an ample amount of protein may help in its biochemical and biophysical characterization, crystallization and identification of new interacting partners of Mrt4.
Highlights
Mrt4 is a key player of mRNA turnover and ribosome assembly.
Recombinant expression and homogeneous purification were established.
Structure of yeast Mrt4 is largely unknown.
Biophysical characterization of Mrt4 was performed.
Introduction
Eukaryotic ribosome maturation is dependent on a wide range of trans-acting factors. To date, more than 150 factors have been identified in the ribosome assembly that associate and dissociate from preribosomal particles during the maturation pathway [Citation1,Citation2]. Mrt4 (mRNA turnover protein) is a major subunit of the ribosome assembly machinery. It also works as a nuclear paralog of the ribosomal P0 protein, interacts with pre-60S subunits at an initial stage of assembly into the nucleolus. Finally, replaced by ribosomal P0 protein in cytoplasmic mature 80S ribosomes and pre-60S subunits. Mrt4 is a kind of ribosomal stalk protein well known to be involved in mRNA turnover and ribosome biogenesis [Citation1]. Initially, Mrt4 was thought to be involved in turnover of mRNA only, but later on reported in preribosomes and now well characterized as part of the ribosome assembly machinery [Citation2,Citation3]. Mrt4 and P0 protein contain a wide range of amino acid sequence homology. Both proteins can interact and compete for the same site in the rRNA. They play mutually exclusive role in ribosome [Citation4–6]. Dual-specific phosphatase Yvh1 played a very crucial role in release of Mrt4 from pre-60S subunits. Yvh1 deletion resulted in persistence of Mrt4 on pre-60S subunits in the cytoplasm [Citation7–10]. Yeast and human Mrt4 proteins share very fine sequence conservation and homology. Recent reports showed that mrt4 deletion in yeast exhibits a very slow growth phenotype in cells that could be easily complemented with hMrt4 (human Mrt4) protein. This clearly represents that the Mrt4 protein structure and function are fairly conserved throughout the evolution [Citation11]. In recent studies, temperature-sensitive yeast strain ts1189 defects lead to decay for a number of mRNAs. The human counterpart was fairly able to complement the growth of the mrt4 temperature-sensitive mutant strain, but not completely [Citation12]. The human and yeast Mrt4 proteins show strong conservation of the sequence with the P0 protein, not only in the case of the rRNA-binding domain but also in the translation factor (TF) binding domain. Mrt4 protein coding gene also play a crucial role in some human genetic diseases. Major diseases associated with mrt4 gene include Robinow Syndrome, Autosomal Dominant 1(RSAD1) and Shwachman-Diamond Syndrome 1(SDS1) [Citation13]. RSAD1 is characterized by acral dysostosis with genital and facial abnormalities, while SDS1 by bone marrow and dysfunction pancreatic insufficiency [Citation14]. Recombinant protein expression in Escherichia coli provides the opportunity to get ample amount of recombinant proteins swiftly [Citation15]. Nowadays, pET23a (+) is a widely used vector for the expression and purification of recombinant proteins [Citation16]. Although the expression and production of recombinant proteins are a well-established phenomenon, certain obstacles can affect the process [Citation17,Citation18]. Limiting factors in the recombinant protein production include insoluble protein production, no expression, improper purification, etc. [Citation19,Citation20]. In the present work, we are reporting cloning, successful overexpression and purification of Mrt4 protein that may lead to revelation of its novel interacting partners and its crystallization.
Materials and methods
Restriction enzymes and deoxynucleotide triphosphates (dNTPs) were indented from New England Biolabs (NEB), USA. All other chemicals and reagents were purchased from the Sisco Research Laboratories (SRL), Sigma-Aldrich Chemical Company, Mumbai, India, and USA, and were of the highest purity available. The Nickel-Nitrilotriacetic Acid (Ni–NTA) Agarose matrix and molecular biology kits were purchased from Qiagen, CA, USA. Bacterial culture media were indented from Himedia Laboratories, Mumbai, India.
Isolation of S. cerevisiae genomic DNA
S. cerevisiae BY4741 strain (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) genomic DNA was isolated manually by the method mentioned elsewhere [Citation21].
Amplification of mrt4 gene
Gene-specific primers, 5’- CGC GGA TCC ATG CCA AGG TCA AAA CGT TCC AAG-3’ (forward) and 5’-ACG CGT CGA CTT CCA TGT TGA TGT TAG TGC TTT C-3’ (reverse), were constructed for PCR amplification of the S. cerevisiae Mrt4 gene. Primers were constructed with BamHI (forward) and Sal1 (reverse) restriction sites, respectively. PCR amplification was performed using Taq DNA Polymerase (New England Biolabs, USA) with the following cycle parameters: initial denaturation temperature of 98°C for 30° s, 30 cycles of 98°C for 10s, 65°C for 30s and 72°C for 15s, followed by a final extension of 72°C for 5 min. Amplified PCR products were resolved using 0.8% agarose gel electrophoresis, and amplicon of the 708 bp was purified with a gel extraction kit (Qiagen, USA).
Optimization of recombinant Mrt4 protein expression and purification
Recombinant cells having pET23a (+)-Mrt4 plasmid constructs were screened on LB agar plates supplemented with ampicillin. Positive clone was picked up, and two different IPTG concentrations (0.5 mM and 1.0 mM) and expression temperatures (20°C and 25°C) were tested to optimize the expression, respectively, in 5 mL LB broth containing 50 mg/mL ampicillin. Two different E.coli expression systems BL21 and C41 were used to express recombinant protein according to Mohd. Kashif et al. with varying low temperatures respectively. For pilot protein production, 1% primary culture was inoculated in 400 mL of sterile LB broth supplemented with 50 mg/mL ampicillin and incubated at 37°C with shaking (180 rpm) until the OD600 reached 0.7 followed by overnight induction (0.5 mM IPTG). Grown cells were pelleted down by centrifugation at 4°C. Furthermore, the pellet was resuspended in 20 mL of 300 mM NaCl and 50 mM Tris (pH 8.0) supplemented with protease inhibitor cocktail. These cells were lysed on ice using sonication at an amplitude of 26% for 10s pulse on and 15s off pulse for 20 minutes. The obtained crude lysate was centrifugated at 13,000 rpm for 20 min at 4°C. The supernatant was filtered through a 0.45 μm pore PVDF membrane filter before affinity chromatography. The matrix was extensively washed with 10 bed volumes of equilibration buffer (50 mM Tris (pH 8.0), 300 mM NaCl). The supernatant was passed through the Ni–NTA Agarose matrix (Qiagen, USA). Furthermore, Mrt4 was purified using Ni–NTA (Qiagen, USA) following manual instructions. The concentration of recombinant proteins was calculated through the Bradford method using BSA as a standard [Citation22,Citation23].
Western blotting
Recombinant Mrt4 purified protein and uninduced sample as a control were subjected to 12% polyacrylamide gel and transferred to the PVDF membrane for 90 minutes at 50 mV. The protein-transferred to membrane, was blocked with 5% skimmed milk for 3 hr. Then, the membrane was washed and incubated with the mouse anti-His antibody (1/5000 dilution). After 3 times washing with PBS, pH 7.4, the membrane was then incubated with antimouse IgG alkaline phosphatase (1:10,000) in PBST (PBS, .05% Tween) at pH 7.4. Membranes were then washed 3 times with PBST (PBS, .05% Tween) at pH 7.4. Signals were detected with the liquid substrate (Sigma Aldrich Co, St. Louis, USA) and analyzed using a gel doc system [Citation24].
Biophysical characterization of recombinant Mrt4
Fluorescence measurements
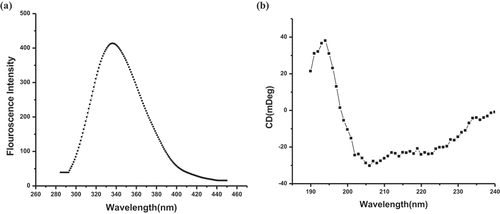
The protein sample (2–4 μmolar) was prepared, and spectra were recorded with a spectro-fluorophotometer (CSIR CDRI, Lucknow) in a quartz cell. Fluorescence spectra were recorded with a 1.0 cm path length at room temperature. Sample was equilibrated for 30 minutes before recording the fluorescence measurements. The excitation wavelength was 280 nm, and the emission was recorded from 300 to 400 nm [Citation22,Citation25].
Circular dichroism measurements
The circular dichroism (CD) spectra were measured with a spectropolarimeter (CSIR CDRI, Lucknow). The instrument was precalibrated with ammonium d-10 camphorsulfonate. A path length of 0.1 cm was implemented for scanning between 250 and 195 nm. A path length of 1.0 cm was implemented for scanning between 300 and 250 nm. The mean residue ellipticity (MRE) in deg.cm2.dmol−1 was defined as
where Θobs is the observed ellipticity (CD) in milli-degrees, Cp is the molar concentration, n is the number of peptide bonds per molecule and ‘l’ is the length of the light path (centimeter) [Citation24,Citation26].
Sequence alignments and phylogenetic analysis
Nowadys, WebLogo is widely used to create graphical representations of the patterns within a multiple sequence alignment and sequence logos. For constructing a phylogenetic tree, a Neighbor-Joining (NJ) tree was constructed based on the National Center for Biotechnology Information (NCBI), U.S. National Library of Medicine, USA, for phylogenetic analysis. It helps in conducting molecular phylogenetics, phylogenetic histories and building sequence alignments [Citation21].
Protein interaction property similarity analysis (PIPSA)
webPIPSA analysis required PDB files with its protein coordinate. These can be achieved as specified by PDB identifier code in the RCSB and user-supplied structures. User-supplied structures can be either generated by comparative modeling techniques using MODELLER or SWISSMODEL or experimentally determined structures from the respective databases, such as MODBASE. The webPIPSA basically calculates similarity indices based on the electrostatic potentials of two or more protein (kcal mol−1 e−1) [Citation27]
Computational studies
Sequence of Mrt4 protein was taken (SGD ID: S000001492) from Saccharomyces cerevisiae genome database (S.G.D). Saccharomyces cerevisiae Mrt4 had no reported structure. We attempted to construct the complete 3Dstructure of Saccharomyces cerevisiae Mrt4. First, suitable templates were searched using the BlastP tool against the protein data bank (RCSB-PDB) and Phyre2 [Citation28]. The PDB structure 3J65 was used as a template to create the structure of yeast Mrt4 protein using Phyre2 online software. UCSF Chimera was used for model visualization and columbic surface analysis [Citation29].
Results
PCR amplification and cloning of mrt4 gene.
S. cerevisiae mrt4 gene was amplified by PCR using gene-specific primers. The PCR product of the expected size (708 bp) was obtained (data not shown). Gene was cloned in pET23a (+) vector, and clone was confirmed by double digestion (). In double digestion, two bands were observed, with the size 3.5 kb correspond to the vector backbone and 708 bp correspond to mrt4 gene (Figure S1 A, lane 2). Positive clones were sequenced and compared with the Mrt4 sequence available in Saccharomyces Genome Database (SGD; http://www.yeastgenome.org/). The clone sequence showed 99.99% similarity with the Mrt4 sequence available in SGD [Citation30].
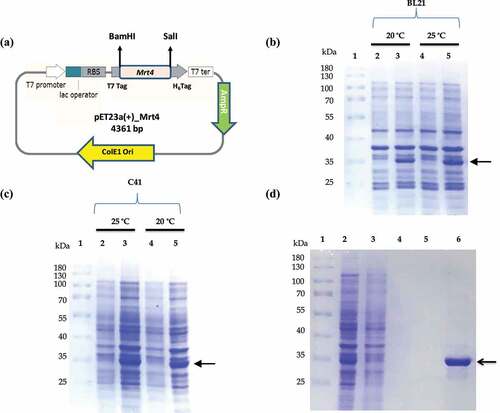
Figure 1. Optimzation of soluble expression and purification of recombinant Mrt4 protein. SDS PAGE profile showing optimization of overexpression of Mrt4 protein in different E.coli expression systems (C41 and BL21) and varying temperatures. (a) Schematic vector map of the pET23a (+)-Mrt4 construct. (b) Lanes 1–5 represent the expression in BL21, protein molecular weight marker (Thermo Scientific PageRuler™ Cat. No. 26,616), uninduced sample, sample after overnight induction at temperature 20°C and uninduced sample, sample after overnight induction at temperature 25°C respectively. (c) Lanes 1–5 represent the expression in C41 protein, molecular weight marker, uninduced sample, sample after overnight induction at temperature 25°C and uninduced sample, sample after overnight induction at temperatures 20°C, respectively. (d) SDS-PAGE profile of purification of Mrt4 protein under native conditions. Lanes 1–6 represent the protein marker, TSP (total soluble protein) fraction after sonication, flow through fraction, wash buffer 1, wash buffer 1 and elution fractions, respectively.
Mrt4 protein expression and solubility optimization
Two different E.coli expression systems BL21 and C41 were implicated to express Mrt4 protein with varying IPTG concentrations and temperatures, respectively ( lane 2–5). Maximum Mrt4 expression was observed in the C41 E.coli expression system followed by BL21. IPTG concentrations (0.5 mM and 1.0 mM) were tested, and both concentrations showed almost the same level of Mrt4 protein expression. Hence, the 0.5 mM IPTG concentration is considered as optimal for Mrt4 expression. Furthermore, two different temperatures (20°C and 25°C) were tested for Mrt4 protein expression and solubility. Soluble expression at 25°C induction was higher than induction at 20°C (). The results showed that the molecular weight of the recombinant protein product is 29 kDa (27.1 kDa Mrt4 + 0.85 kDa His tag+1.1 kDa T7 tag), which corresponds to the predicted size of pET23a (+)-Mrt4. Mrt4 protein was predominantly present in soluble fraction ().
Purification of Mrt4 protein by affinity chromatography
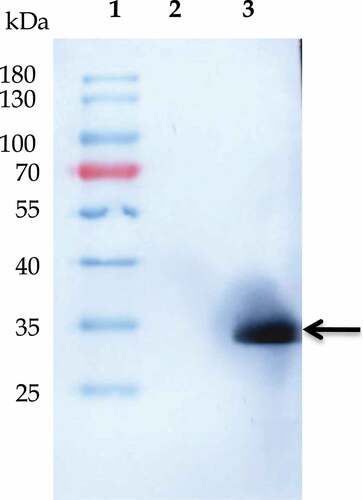
Purification of recombinant Mrt4 protein was performed by immobilized metal affinity chromatography (IMAC) on a Ni–NTA resin column. The SDS-PAGE analysis (, lane 6) represents a single and homogeneous protein band with the expected molecular mass of the Mrt4 protein (~29 kDa). The yield of homogeneously purified recombinant Mrt4 protein was about 3 mg/L. Furthermore, the presence of recombinant protein in E. coli cell lysate was validated through immunoblotting with the anti-His antibody in the induced sample in comparison to the uninduced sample (, lane 3, Figure S1 B). For structural, functional and biochemical characterization, homogeneously purified recombinant protein in ample amount and active form protein is always a prerequisite [Citation31].
Biophysical characterization of recombinant Mrt4
Primary sequence and homology modeling of Mrt4
WebLogo creates graphical representations of multiple sequence alignment, sequence similarty and conversation. Each logo consists of stacks letters, and one stack represents each position of amino acid in the sequence. Letter height represents the conservation of the sequence across the species position (measured in bits), while the color code indicates the type/nature of amino acid in protein. Furthermore, acidic, basic and hydrophobic amino acids are represented with green, red and black, respectively. WebLogo analysis represents that a majority of amino acids of Mrt4 protein are evolutionarily very well conserved (). Phyre2 was used to predict the model of Mrt4 protein, which is based on an alignment generated by HMM-HMM matching [Citation28]. The template known secondary structure (3J65) was used to predict the secondary structure of recombinant Mrt4 protein. Phyre2 secondary structure analysis represented that the basic structure of recombinant Mrt4 protein contains six alpha helices (). The template (3J65) was used to create the Mrt4 columbic surface (Phyre2, Model dimensions (Å): X: 52.355 Y: 59.859 Z: 74.228) coloring through UCSF chimera. White color indicates the hydrophobic patches, blue color indicates the positively charged surface and red color indicates the negatively charged surface (). The online tool conserved domain (CDD server) [Citation32] was used to identify the domain in recombinant Mrt4 protein (Figure-S2). Mrt4 belongs to the ribosomal protein L10 family and P0 and L10e subfamily. These superfamilies mainly contains the archaeal P0 homolog, L10e, eukaryotic 60S ribosomal protein, P0 and the Saccharomyces cerevisiae Mrt4 (Accession: cd05796, E value: 5.98e-79) (Figure-S3).
Figure 3. Primary sequence analysis and homology modeling of Mrt4. (a) WebLogo creates graphical representations of multiple sequence alignment, sequence similarity and conversation. Acidic, basic and hydrophobic amino acids are represented with green, red and black, respectively. (b) Template known secondary structure (3J65) was identified from Phyre2 and used to predict the secondary structure of recombinant Mrt4 protein. (c) The secondary structure of Mrt4 protein generated through Swiss-MODEL homology modeling. (d) The surface view model of Mrt4 having columbic surface coloring using UCSF chimera. White color indicates the hydrophobic patches, blue color indicates the positively charged surface and red color indicates the negatively charged surface.
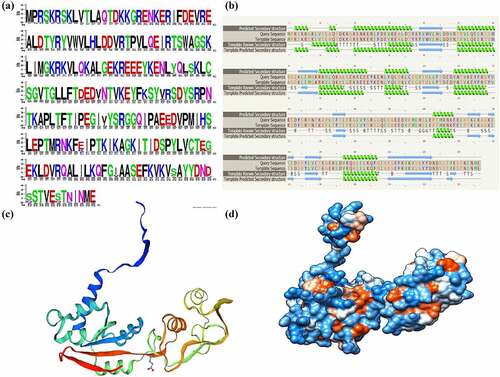
Conserved domain analysis of Mrt4
A multiple sequence alignment represented that the Mrt4 proteins from Chaetomium, Saccharomyces and human are well conserved and demarcated in their rRNA-binding domains, signifying that they have the same interacting site on the 60S subunit. Furthermore, they all have a well-conserved translation factor (TF) binding domain. ClustalW analysis of the Chaetomium, Saccharomyces and human Mrt4 protein sequences using the PSORT program [Citation33] indicated the presence of a bipartite NLS (nuclear localization signal) in the N-terminal part of the Chaetomium, yeast and human proteins, which may contribute to the nuclear import of the Mrt4 protein (Figure-S3).
Recombinant Mrt4 protein is atypical α helical protein
An important characteristic of intrinsic fluorescence of a protein is the very high sensitivity of tryptophan present in that protein to its local environment. Tryptophan fluorescence based on the wavelength is broadly used as a specific tool to examine alteration in proteins to predict the local structure and dynamics [Citation34]. In general, tryptophan has a wavelength of maximum absorption of 280 nm followed by the emission peak solvatochromic range from 300 to 350 nm that depends upon the polarity of the tryptophan local environment. CD is an excellent widely used method for determining the secondary structure of proteins. Mrt4 contains two tryptophan residues (Trp 41 and Trp 60). Hence, protein fluorescence and CD analysis may be used as a diagnosis of the conformational state of a protein. At physiological pH (pH 7.0), both the far-UV CD spectra and fluorescence spectra showed a properly folded secondary structure and a tertiary structure of Mrt4, respectively (). The far-UV CD spectra represent that Mrt4 have a typical six α helix with characteristic negative minima at bands at 222 nm and 208 nm (). In fluorescence emission spectra (λmax~ 337), the peak observed represents the properly folded tertiary structure of recombinant Mrt4 ().
PIPSA (Protein interaction property similarity analysis)
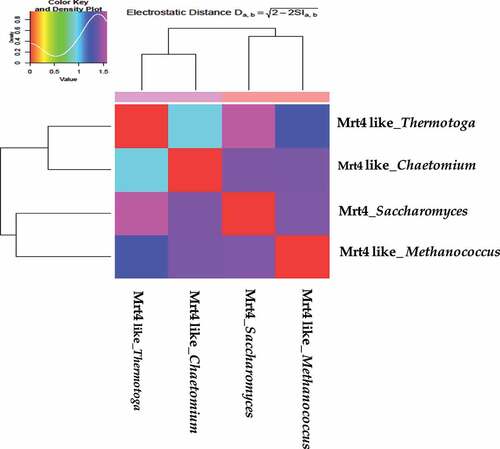
PIPSA analysis widely helps in the estimation of enzyme kinetic studies, functional studies, classification of proteins and protein ligand interaction. The comparisons of electrostatic properties were performed using the PIPSA approach, where the pairwise similarity in the protein electrostatic potentials is calculated in pre-defined analysis regions. Mrt4-like proteins from four different species are clustered according to the all pairwise distances between electrostatic potentials using a color code from red (small distance) to blue (large distance) on an epigram or heat map. Proteins with almost similar electrostatic characteristics are clustered in one group at the graph that signifies their evolutionary relatedness. The Mrt4-like protein forms two subclusters: Chaetomium thermophilum (Thermophilic Ascomycete fungus, 4NWB) and Thermotoga martima (Hyperthermophilic, gram-negative bacterium, PDB-1ZAV) in one subcluster, whereas Saccharomyces cerevisiae and Methanococcus jannaschi (Thermophilic methanogenic Archean, PDB-3JSY) in the distinct second subcluster () [Citation35]. This will bring further insight into Mrt4-like protein-specific regulation with respect to its structural and physico-chemical properties. webPIPSA also provides isoform-specific regulation insights that cannot be obtained from analysis at the sequence level alone [Citation36].
Figure 5. webPIPSA server was used for the comparison of the electrostatic potentials of Mrt4-like proteins from four different species. Protein from species with highly similar electrostatic potentials, such as Chaetomium thermophilum (4NWB) and Thermotoga martima (PDB-1ZAV) in one subcluster, whereas Saccharomyces cerevisiae and Methanococcus jannaschi (PDB-3JSY) in the distinct second subcluster.
Phylogenetic analysis
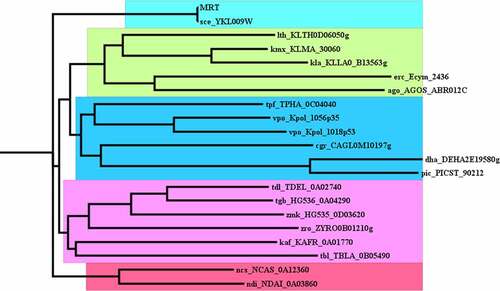
An evolutionary tree or phylogenetic tree is an illustrative depiction of the evolutionary relationships with diverse taxa. It is a diagrammatic representation that has branches and nodes. The branching prototype of a tree is well known as the topology of the tree (). The closer the two groups are located to each other, the more recently they shared a common ancestor. In this analysis, Mrt4 protein closely is related to Kluyveromyces lactis (KLLA0_B13563g) and Lachancea thermotolerans (KLTH0D06050g). Mrt4 protein belongs to biogenesis regulatory (RRS1) family proteins [Citation37]. RRS1 is a nuclear protein that plays a very important role in 25S rRNA maturation and the assembly of the 60S ribosomal subunit in Saccharomyces cerevisiae ().
Discussion
Pure and active recombinant protein is utmost required for structural, functional and biochemical studies. Mrt4 is a 236-amino-acid-long nuclear protein that plays a central role in ribosome biogenesis and mRNA turnover assisting the process of translation. Mrt4 is a kind of ribosomal stalk protein and P0 (or Rpp0) paralog that is critically involved in mRNA turnover and ribosome biogenesis. Mrt4 is well conserved from yeast to human, which shows that the similar functional and structural role of this protein likely exists in humans. Domain-wise Mrt4 belongs to the L10 family (Domain ID: 10,146,578).This family contains uncharacterized eukaryotic proteins like 60S ribosomal protein P0 and Mrt4. Yeast Mrt4 contains the conserved rRNA-binding domain, translation factor (TF) interacting domain and putative NLS homologous to human. At present, homology modeling is the most powerful and widely used method for predicting the tertiary structure of proteins in cases where a query protein has sequence similarity to a protein with a known atomic structure. The template of the known tertiary structure (3J65) was used to create the yeast Mrt4 protein model through UCSF chimera. Mrt4 contains two tryptophan residues (Trp 41 and Trp 60) displaying a properly folded tertiary structure in fluorescence emission spectra under the physiological condition. The far-UV CD spectra represent that Mrt4 has a typical α helix with characteristic negative minima at bands at 222 and 208 nm. Such α-helix proteins are well reported in biological systems like DNA binding proteins (zinc finger motifs) [Citation38] and transmembrane proteins (G protein–coupled receptors (GPCRs) [Citation39]. At physiological pH, both the fluorescence spectra and CD spectra indicate a properly folded tertiary structure and a secondary structure of Mrt4, respectively. The comparisons of electrostatic properties of Mrt4-like proteins were performed using the PIPSA approach. PIPSA analysis provided insights into the electrostatic potential based on the protein sequence and PDB structure. The Mrt4-like protein forms two subclusters, Chaetomium thermophilum (4NWB) and Thermotoga martima (PDB-1ZAV) in one subcluster, whereas Saccharomyces cerevisiae and Methanococcus jannaschi (PDB-3JSY) in the distinct second subcluster. This approach has formerly been used to explore the interaction properties of a number of protein families like human Rab GTPase proteins and blue copper proteins [Citation40].
Conclusions
Mrt4 is a major component of the ribosome assembly machinery and nuclear paralog of the ribosomal protein P0, and it binds to pre-60S subunits at an early stage of assembly in the nucleolus. The molecular structure of Saccharomyces cerevisiae and human Mrt4 is still unknown. Hence, purified Mrt4 protein can be used to study the structure (crystallization) and identify novel protein–protein interactions that involve ribosome biogenesis and mRNA turnover that previously remained elusive in S. cerevisiae and human. Mrt4 is well conserved from yeast to human, and a similar functional and structural role of this protein likely exists in humans.
Author’s contribution
MK conceived the idea. MK, MA and BK performed the experiments. NS and MK analyzed the data. MK wrote the paper. MSA, MIK and SSAS have given critical inputs in the analysis and editing of the manuscript. All the authors have read and approved the manuscript.
Acknowledgements
I greatly acknowledged my supervisor, Dr. Mohd. Sohail Akhtar for his guidance and support. Mohammad Imran Khan, Associate professor, Department of Biochemistry, King Abdulaziz University, Jeddah, Saudi Arabia, greatly acknowledged for professional grammar and revising the language editing in this manuscript. BK acknowledged Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, 201002, India.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Sugiyama M, Nugroho S, Iida N, et al. Genetic interactions of ribosome maturation factors Yvh1 and Mrt4 influence mRNA decay, glycogen accumulation, and the expression of early meiotic genes in Saccharomyces cerevisiae. J Biochem. 2011;150(1):103–111.
- Woolford JL Jr, Baserga SJ. Ribosome biogenesis in the yeast Saccharomyces cerevisiae. Genetics. 2013;195(3):643–681.
- Konikkat S, Woolford JL Jr. Principles of 60S ribosomal subunit assembly emerging from recent studies in yeast. Biochem J. 2017;474(2):195–214.
- Rodríguez-Mateos M, Abia D, García-Gómez JJ, et al. The amino terminal domain from Mrt4 protein can functionally replace the RNA binding domain of the ribosomal P0 protein. Nucleic Acids Res. 2009;37(11):3514–3521.
- Harnpicharnchai P, Jakovljevic J, Horsey E, et al. Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol Cell. 2001;8(3):505–515.
- Zuk D, Belk JP, Jacobson A. Temperature-sensitive mutations in the Saccharomyces cerevisiae MRT4, GRC5, SLA2 and THS1 genes result in defects in mRNA turnover. Genetics. 1999;153(1):35–47.
- Panse VG, Johnson AW. Maturation of eukaryotic ribosomes: acquisition of functionality. Trends Biochem Sci. 2010;35(5):260–266.
- Lo KY, Li Z, Wang F, et al. Ribosome stalk assembly requires the dual-specificity phosphatase Yvh1 for the exchange of Mrt4 with P0. J Cell Biol. 2009;186(6):849–862.
- Geng Q, Xhabija B, Knuckle C, et al. The atypical dual specificity phosphatase hYVH1 associates with multiple ribonucleoprotein particles. J Biol Chem. 2017;292(2):539–550.
- Remacha M, Cruz Díaz JDL, García Gómez JJ, et al. Role and dynamics of the ribosomal protein p0 and its related trans-acting factor Mrt4 during ribosome assembly in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:7519–7532.
- Michalec B, Krokowski D, Grela P, Wawiórka L, Sawa-Makarska J, Grankowski N, Tchórzewski M. Subcellular localization of ribosomal P0-like protein MRT4 is determined by its N-terminal domain. The International Journal of Biochemistry & Cell Biology. 2010 May 1;42(5):736–48.
- Zuk D, Belk JP, Jacobson A. Temperature-sensitive mutations in the Saccharomyces cerevisiae MRT4, GRC5, SLA2 and THS1 genes result in defects in mRNA turnover. Genetics. 1999;153(1):35–47.
- Boocock GR, Morrison JA, Popovic M, et al. Mutations in SBDS are associated with Shwachman–Diamond syndrome. Nat Genet. 2003;33(1):97–101.
- Dror Y, Donadieu J, Koglmeier J, et al. Draft consensus guidelines for diagnosis and treatment of Shwachman‐Diamond syndrome. Ann N Y Acad Sci. 2011;1242(1):40–55.
- Hochkoeppler A. Expanding the landscape of recombinant protein production in Escherichia coli. Biotechnol Lett. 2013;35(12):1971–1981.
- Omidinia E, Mahdizadehdehosta R, Mohammadi HS. Expression, purification and characterization of the proline dehydrogenase domain of PutA from Pseudomonas putida POS-F84. Indian J Microbiol. 2013;53(3):297–302.
- Rosano GL, Ceccarelli EA. Recombinant protein expression in Escherichia coli: advances and challenges. Front Microbiol. 2014;5:172.
- Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10(5):411–421.
- Singh A, Upadhyay V, Upadhyay AK, et al. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact. 2015;14(1):1–10.
- Yuan TZ, Ormonde CF, Kudlacek ST, et al. Shear‐stress‐mediated refolding of proteins from aggregates and inclusion bodies. ChemBioChem. 2015;16(3):393–396.
- Harju S, Fedosyuk H, Peterson KR. Rapid isolation of yeast genomic DNA: bust n’Grab. BMC Biotechnol. 2004;4(1):1–6.
- Kashif M, Bharati AP, Chaturvedi SK, et al. pH and alcohol induced structural transition in Ntf2 a nuclear transport factor of Saccharomyces cerevisiae. Int J Biol Macromol. 2020;159:79–86.
- Bharati AP, Kashif M, Chaturvedi SK, et al. An insight into structural plasticity and conformational transitions of transcriptional co-activator Sus1. PloS one. 2020;15(3):e0229216.
- Bharati AP, Singh N, Kumar V, et al. The mRNA capping enzyme of Saccharomyces cerevisiae has dual specificity to interact with CTD of RNA Polymerase II. Sci Rep. 2016;6(1):1–12.
- Ansari MA, Zubair S, Atif SM, et al. Identification and characterization of molten globule-like state of hen egg-white lysozyme in presence of salts under alkaline conditions. Protein Pept Lett. 2010;17(1):11–17.
- Chandel TI, Afghani M, Masroor A, et al. An insight into the inhibition of fibrillation process verses disaggregation of preformed fibrils of bovine serum albumin by isoprenaline hydrochloride. Int J Biol Macromol. 2020;154:1448–1459.
- Richter S, Wenzel A, Stein M, et al. webPIPSA: a web server for the comparison of protein interaction properties. Nucleic Acids Res. 2008;36(suppl_2):W276–W280.
- Kelley LA, Mezulis S, Yates CM, et al. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10(6):845–858.
- Shukla H, Kumar V, Singh AK, et al. Insight into the structural flexibility and function of Mycobacterium tuberculosis isocitrate lyase. Biochimie. 2015;110:73–80.
- Cherry JM, Hong EL, Amundsen C, et al. Saccharomyces genome database: the genomics resource of budding yeast. Nucleic Acids Res. 2012;40(D1):D700–D705.
- Wingfield PT. Overview of the purification of recombinant proteins. Curr Protoc Protein Sci. 2015;80(1): 6–1.
- Marchler-Bauer A, Derbyshire MK, Gonzales NR, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015;43(D1):D222–D226.
- Horton P, Park KJ, Obayashi T, et al. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007;35(suppl_2):W585–W587.
- Ghisaidoobe AB, Chung SJ. Intrinsic tryptophan fluorescence in the detection and analysis of proteins: a focus on Förster resonance energy transfer techniques. Int J Mol Sci. 2014;15(12):22518–22538.
- Kravchenko O, Mitroshin I, Nikonov S, et al. Structure of a two-domain N-terminal fragment of ribosomal protein L10 from Methanococcus jannaschii reveals a specific piece of the archaeal ribosomal stalk. J Mol Biol. 2010;399(2):214–220.
- Tong R, Wade RC, Bruce NJ. Comparative electrostatic analysis of adenylyl cyclase for isoform dependent regulation properties. Proteins Struct Funct Bioinf. 2016;84(12):1844–1858.
- Thomson E, Ferreira-Cerca S, Hurt E. Eukaryotic ribosome biogenesis at a glance. Journal of Cell Science. 2013;126:4815–4821.
- Luscombe NM, Austin SE, Berman HM, et al. An overview of the structures of protein-DNA complexes. Genome Biol. 2000;1(1):1–37.
- Niitsu A, Heal JW, Fauland K, et al. Membrane-spanning α-helical barrels as tractable protein-design targets. Philos Trans R Soc B. 2017;372(1726):20160213.
- Solomon EI, Hadt RG. Recent advances in understanding blue copper proteins. Coord Chem Rev. 2011;255(7–8):774–789.