ABSTRACT
This study aims to investigate the protective effect of growth differentiation factor 15 (GDF15) in sepsis by regulating macrophage polarization and its mechanism. The mouse macrophages were cultured and treated with lipopolysaccharide (LPS), and some cells were intervened with GDF15 and LY294002. The proinflammatory activated (M1) macrophages and the anti-inflammatory activated (M2) macrophages were measured and observed, and the messenger RNA expression levels of their biomarkers, phosphatidylinositol 3-kinase (PI3K), and protein kinase B (Akt) were detected. The survival rate, cardiac function, and histopathological sections were observed. In the LPS group, after GDF15 intervention, the percentage of M1 macrophages decreased and M2 macrophages increased, the infiltration of monocytes/macrophages into the heart was inhibited, systemic and cardiac inflammation was reduced, and the survival time of the mice was prolonged. GDF15 regulated macrophage polarization and played an anti-inflammatory role by activating the phosphorylation of the PI3K/Akt signaling pathway. In patients with sepsis, the serum GDF15 level increased and was closely related to the severity of the sepsis and the 28-day mortality rate and could be used as a prognostic marker of sepsis. GDF15 regulates macrophage polarization through activating the PI3K/Akt signaling pathway and has a protective effect on survival and the cardiac function of patients with sepsis and sepsis mouse models. The increase in serum GDF15 level is closely related to severity and mortality in patients with sepsis and is therefore a prognostic marker of sepsis.
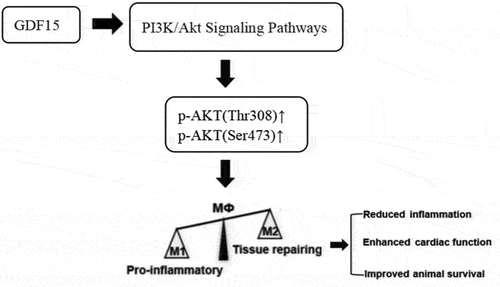
Graphical Abstract
1. Introduction
Sepsis is defined as the syndrome caused by infection when the infection results in host response disorder and leads to circulatory and organ dysfunction (Sepsis 3.0) and is a clinical syndrome with the mortality rate of severe sepsis and septic shock can reach 50%, and the incidence is increasing every year [Citation1]. Sepsis causes an excessive inflammatory response and immunosuppression, leads to organ dysfunction, and threatens life [Citation2]. About 50% of patients with sepsis have myocardial dysfunction [Citation3,Citation4], which is positively correlated with increased mortality [Citation5–7]. Macrophages play a major role in sepsis pathogen defense, inflammatory response regulation, and tissue homeostasis [Citation8]. Macrophage polarization runs through the occurrence, development, and outcome of inflammation-related diseases [Citation9].
Growth differentiation factor-15 (GDF15) has anti-apoptotic, anti-inflammatory, and endothelial protective effects and is involved in tissue repair and the regulation of organ growth and differentiation. In inflammation, trauma, cardiovascular, and cerebrovascular disease, tumor, and other stress states, GDF15 can be vigorously expressed [Citation10–12]. GDF15 is related to an increase in inflammation and involved in the production of anti-inflammatory mediators [Citation13]. In the mouse model of myocardial ischemia–reperfusion injury, GDF15 in myocardial tissue can directly inhibit cardiomyocyte apoptosis through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway [Citation14]. Upregulation of GDF15 expression can reduce oxidative damage to cardiomyocytes by activating the PI3K/Akt signaling pathway [Citation15] and protect endothelial cells from glucose-induced cell damage [Citation16]. The PI3K/Akt signaling pathway is an important signaling pathway for macrophages and plays an important role in macrophage activation and gene expression [Citation17].
The regulation of GDF15 on macrophage polarization in sepsis has not been reported. The researchers speculate that GDF15 regulates macrophage polarization through activating the PI3K/Akt signaling pathway and has a protective effect on the survival and cardiac function of patients with sepsis and sepsis mouse models.
To test the hypothesis that GDF15 protects against sepsis by regulating macrophage polarization, we first measured GDF15 levels in sepsis patients and healthy donors’ sera. Using in vitro mouse peritoneal macrophages and an in vivo mouse model treated with recombinant GDF15 protein (rGDF15), analyses were performed to assess macrophage phenotype, systemic/cardiac inflammation and function, and animal survival after endotoxin LPS excitation. Finally, the underlying mechanisms were investigated, and GDF15 regulates the direction of macrophage polarization through the PI3K/Akt signaling pathway, with protective effects on survival and cardiac function in sepsis patients and septic mouse models.
2. Materials and methods
2.1 Experimental animals
Healthy male C57/BL6 mice aged 6–8 weeks were provided by the Animal Experimental Center of the Hebei Medical University. The methods of animal processing were in accordance with the animal ethical standards.
2.2 Main reagents
Dulbecco’s modified eagle medium (DMEM), a high-glucose medium containing 10% fetal bovine serum (FBS; HyClone, USA); collagenase IV (2 mg/ml; Worthington, Lakewood, NJ, USA); neutral protease II (1.2 U/mL; Sigma-Aldrich, USA); hyaluronidase (Sigma-Aldrich, USA); mouse lymphocyte isolate, Ficoll (Sigma-Aldrich, USA); allophycocyanin anti-F4/80 (BioLegend, USA); anti-CD11b antibody (ab184308; Abcam, UK); Alexa Fluor® 488 anti–CD206 (BioLegend, USA); fluorescein isothiocyanate anti-CD80 (Becton, Dickinson and Company, USA); Ly6C antibody (ab24973; Abcam, UK); Ly6G antibody (ab25377; Abcam, UK); MHC-II antibody (ab180779; Abcam, UK); PI3K inhibitor, LY294002, and corresponding phosphorylated antibody (Cell Signaling Technology, Inc., USA); and recombinant GDF15 protein (rGDF15; PeproTech, USA) were used in this study.
Enhanced chemiluminescence (ECL) detection kit; bicinchoninic acid (BCA); PI3K; anti-PI3Kinase p85 alpha antibody, EPR18702 (ab191606; Abcam, UK); phosphorylated serine/threonine (Ser/Ther) kinase; anti-pan Akt antibody (ab8805; Abcam, UK); anti-Akt (phospho T308) antibody (ab38449; Abcam, UK); anti-AKT1 (phospho S473) antibody, EP2109Y (ab81283; Abcam, UK); PI3Kinase inhibitor, LY294002 (ab20243; Abcam, UK); and rabbit anti-human antibodies against GDF15 (Abcam, UK) were used in this study.
2.3 Isolation and culture of mouse peritoneal macrophages
Ten clean C57/BL6 mice from 6 to 8 weeks were sacrificed by cervical dislocation and soaked in 75% alcohol for 5 minutes. The mouse was lifted by the tail and held upside down. At this time, 5 ml of serum-free DMEM was injected intraperitoneally. The mouse was placed on the back, the abdomen was gently kneaded for 2–3 minutes, and it was left for 5–7 minutes. The abdominal cavity of the mouse was opened under aseptic conditions. When the bowel became flat and the peritoneal fluid was light yellow, 4 ml of peritoneal fluid was withdrawn with a syringe. It was centrifuged and washed, and the cells were counted under a microscope. The cells were cultured in conventional DMEM containing 10% FBS, the cell density was adjusted from 3 × 106 to 4 × 106 /mL, and it was placed in an incubator. After 24 hours, the cells were gently pipetted with serum-free medium to remove the nonadherent cells [Citation18]. Cells were detected using flow cytometry until more than 90% of cells were positive for both CD11b and F4/80, and in this way, the purity of the C57/BL6 mouse macrophages was allowed to reach 99%. C57/BL6 mouse macrophages were cultured in DMEM containing 10% FBS, glutamine, 100 U/mL penicillin, and 100 g/mL streptomycin.
2.4 Isolation and purification of macrophages infiltrating mouse hearts
After euthanasia, the heart was removed, and 10 ml of PBS was perfused through the left ventricle to remove circulating immune cells. The heart was separated and cut into small pieces; digested with collagenase IV, neutral protease II, and 0.9 mM CaCl2; stirred at 37°C; and incubated for 45 minutes. The mixture was filtered with 40 μM cell filters and centrifuged at 4°C and 500 g for 5 minutes. The particles were resuspended in the flow cytometry screening buffer (Hank’s balance salt solution with 1 mM ethylenediaminetetraacetic acid, 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 1% FBS).
2.5 Cells were randomly divided into 4 groups
(1) Control group: treated with 0.9% sodium chloride solution
(2) LPS group: treated with 10 ng/mL LPS
(3) LPS + GDF15 group: after treatment with LPS (10 ng/mL) for 12 hours, cells were treated with rGDF15 (50 ng/mL).
(4) LPS + GDF15 + LY294002 group: after treatment with LPS (10 ng/mL) for 12 hours, cells were treated with rGDF15 (50 ng/mL) and the PI3K inhibitor LY294002.
Peritoneal macrophages and cardiac infiltrating macrophages were collected, and the ratio of proinflammatory activated (M1) macrophages to anti-inflammatory activated (M2) macrophages was detected using flow cytometry (FACScan, Becton, Dickinson and Company, USA). Adopting the standard method [Citation19,Citation20], the proportion of positive cells was analyzed using FCS Express software (ACEA Biosciences, San Diego, CA, USA). Observations were made as to whether GDF15 could play a role in macrophage polarization.
2.6 Real time quantitative polymerase chain reaction analysis
After the macrophages were intervened by group, the total RNA was extracted according to the instructions of the kit, and the purity and concentration of RNA were determined. The messenger RNA (mRNA) of each group was reversely transcribed into complementary DNA according to the operational procedure of the reverse transcription kit. The testing was carried out according to the steps of the reverse transcription polymerase-chain reaction (RT-PCR) kit. Total RNA was extracted from mouse peritoneal macrophages using the total RNA extraction kit, miRNeasy Mini Kit (Qiagen, Hilden, Germany), to determine the mRNA expression of macrophage markers. Blood RNA was extracted with PureLink™ Total RNA Blood Purification Kit (Invitrogen, Carlsbad, CA, USA) to determine GDF15 mRNA, interleukin 6 (IL-6) mRNA, and interleukin 10 (IL-10) mRNA levels in mouse blood. The quantity and quality of RNA was determined using the NanoDrop 2000 system (Thermo Fisher Scientific, Waltham, MA, USA). CDNA was prepared using the MMLV SuperScript III Reverse Transcriptase Kit (Invitrogen, USA), and then each sample underwent double RT-PCR using the SYBR Green Master Mix (Applied Biosystems, USA) and StepOne™ Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA). According to the gene mRNA sequence from the National Center for Biotechnology Information gene library, primers were designed using Primer-BLAST (see for primer sequence), and amplification was carried out. With glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal control, microRNA expression was calculated using the 2−ΔΔCT method.
Table 1. Primer sequences of RT-PCR
After LPS intervention, the mRNA expression of GDF15 in the blood of mice was measured at 0 h, 1 h, 3 h, 6 h, 12 h, and 24 h, respectively. GDF15 mRNA expression was measured at 0 h, 1 h, 3 h, 6 h, and 24 h, respectively.
2.7. Western blot analysis
After 48 hours of transfection, 200 μL of RipA protein lysate was added to extract the total protein. The sample was added with the loading buffer, and the protein was inactivated and denatured by boiling water for 10 minutes. Each lane was loaded with 60 μg of protein, and 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis was conducted. The protein was then transferred from the gel to the polyvinylidene fluoride membrane by wet transfer, blocked using 5% skimmed milk powder for two hours, added with the first antibody, and incubated overnight at 4°C (dilution ratio of the first antibody: PI3K at 1:1500 and Akt at 1:1000). The next day, it was incubated with the second antibody for two hours and then underwent development and exposure using the ECL kit. The final result was expressed as the ratio of the optical density of the target band to the internal control GAPDH.
2.8 Animal model establishment, grouping, and treatment
According to the experimental design, the mice were randomly divided into three groups using the blind method:
(1) Control group (n = 12): the same volume of 0.9% sodium chloride solution was injected intraperitoneally, and measurement was carried out according to the time points.
(2) LPS group (n = 12): LPS (Sigma-Aldrich, USA), 10 mg/kg, was injected intraperitoneally to induce endotoxemia in mice, and measurement was carried out after 24 hours.
(3) LPS + GDF15 group (n = 12): 24 hours after the intraperitoneal injection of LPS, 10 ng/mL, a single dose of mouse rGDF15 protein, 50 ng/kg, was injected intraperitoneally, and measurement was carried out after two hours.
The mice in each group were treated according to the time points, and serum samples were collected, and enzyme-linked immunosorbent assay (ELISA) was conducted to detect tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and interleukin 10 (IL-10; BioLegend ELISA Kit), and cardiac tissue was collected for macrophage isolation and flow cytometry analysis. The septic mice were monitored for 72 hours to observe the survival rate. The cardiac function of the mice was measured by echocardiography, and routine tissue paraffined sections were prepared for histopathology under light microscope.
2.9 Clinical application of serum GDF15 level in patients with sepsis
A total of 40 adult patients were diagnosed by clinical and laboratory examination (20 with sepsis and 20 with septic shock), and 15 healthy donors were recruited. All patients were admitted to or followed up by the emergency department or emergency intensive care unit of the Third Hospital of the Hebei Medical University from January 2019 to December 2020. All patients with sepsis met the Sepsis 3.0 definition and diagnostic criteria jointly issued by the Society of Critical Care Medicine (USA) and the European Society of Intensive Care Medicine in 2016 [Citation1]. The control group included healthy donors recruited from the physical examination center of the Third Hospital of the Hebei Medical University.
Exclusion criteria: Patients who were expected to receive short-term (<72 hour) intensive care treatment; patients with chronic heart, kidney, and liver dysfunction, autoimmune diseases, and cancer; and patients who refused to participate in the study or stopped treatment.
The informed consent of all patients and their families was obtained and signed, and the study was approved by the ethics committee of the hospital. At admission, blood samples were collected to measure liver function, renal function, and inflammatory cytokines. GDF15 levels were determined according to the instructions of the ELISA kit, and the basic clinical data were recorded.
2.10 Statistical method
Count data were expressed as frequency and percentages and were compared using the X2 test. The median interquartile range (IQR) was calculated for continuous variables. Normally distributed data were compared using the independent sample t-test, and non-normally distributed data were compared between the assigned groups using the nonparametric Mann–Whitney U-test. Data were compared between the groups using one-way univariate analysis of variance, and in the case of heterogeneity of variance, the least significant difference multiple comparison method was adopted, and correction was carried out using the Welch method. Spearman correlation analysis was used to analyze the correlation between GDF15 levels using Apache II and SOFA scores. To evaluate the discrimination value, the area under the receiver operating characteristic curve was calculated, and evaluations were performed to identify significant differences between the sepsis and control groups and between the non-survivor and survivor groups. P < 0.05 was considered to be statistically significant. The survival time was expressed as a Kaplan–Meier curve, and the differences were analyzed by a log-rank test in the GraphPad Prism software.
3. Results
To test the hypothesis that GDF15 protects against sepsis by regulating macrophage polarization, we first measured GDF15 levels in the serum of sepsis patients and healthy donors. Using in vitro mouse peritoneal macrophages and an in vivo mouse model treated with recombinant GDF15 protein (rGDF15), we performed a series of analyses. rGDF15 intervention decreased M1-type expression and increased M2-type expression of macrophages in the LPS group, inhibited infiltration of monocytes/macrophages into the heart, reduced systemic and cardiac inflammation, and prolonged survival time in mice. GDF15 exerts anti-inflammatory effects by regulating the direction of macrophage polarization through activation of PI3K/Akt signaling pathway phosphorylation. GDF15 levels are elevated in patients with sepsis and are strongly correlated with the severity of sepsis and 28-day mortality, which can be used as a prognostic marker for sepsis.
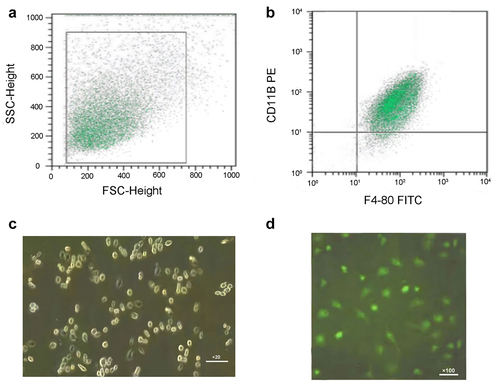
3.1 Identification of mouse peritoneal macrophages
The appropriate flow cytometry recorder was selected to extract the scatter diagram of the cells (see ). In this way, cells with a high expression of F4/80+ and CD11b+ were extracted as macrophages (see ). Mouse peritoneal macrophages were cultured for 24 hours and observed under an ordinary light microscope (20×). The shape of most of the cells was polygonal, and a few cells had pseudopodia and protrusions (see ). Macrophage-specific surface antigen CD68 was positive, confirming that the cultured cells were macrophages (see ).
3.2 Flow cytometry results of mouse peritoneal macrophages (see )
Table 2. Expression of macrophage phenotypic surface markers in mouse peritoneal cavity
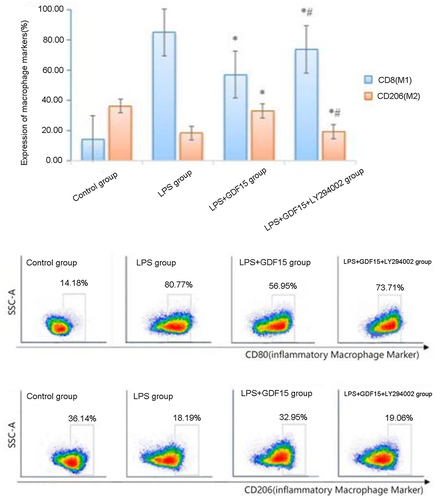
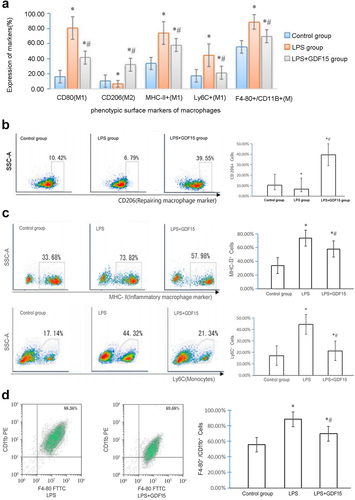
The expression of CD80 in macrophages in the LPS + GDF15 and the LPS group was 56.95% ± 2.96% and 80.77% ± 4.25%, respectively, and the expression of CD206 was 32.95% ± 2.61% and 18.19% ± 1.91%, respectively. RGDF15 inhibited the polarization of M1 macrophages, promoted the polarization of M2 macrophages, and played an anti-inflammatory role. The expression of CD206 in macrophages in the LPS + GDF15 + LY294002 group was 19.06% ± 1.49%, which was significantly lower than the LPS + GDF15 group, suggesting that LY294002 can reduce the polarization of M2 macrophages induced by GDF15 (see ).
3.3 Flow cytometry results of mouse macrophages infiltrating the heart (see )
Table 3. Expression of phenotypic surface markers of macrophages infiltrating into mouse myocardium
When compared with the control group, the percentage of M1 macrophages in the LPS group was significantly higher; the percentage of the MHC-II+, LPS-triggered monocytes (Ly6C+), and M1 macrophages were significantly lower in the LPS + GDF15 group than in the LPS group (see ); and the percentage of CD206+ macrophages in the heart of LPS mice treated with rGDF15 was significantly increased (see ). The percentage of MHC-II+, Ly6C+ and M1 macrophages in the myocardium was significantly lower in the LPS + GDF15 group than in the LPS group, i.e., rGDF15 prevented Ly6C+ (see ) and macrophages (F4-80+/CD11b+ cells) from infiltrating into the heart (see D).
Figure 3. Expression of phenotypic surface markers of macrophages infiltrating into mouse myocardium. A. Expression of phenotypic surface markers of macrophages infiltrating into mouse myocardium. B. Expression of CD206 positive macrophages in mouse myocardium (repairing anti-inflammatory macrophages, M2). C. RGDF15 inhibits the expression of cardiac macrophages/monocytes in LPS-treated mice. D. RGDF15 prevents LPS-triggered macrophages from infiltrating into the heart.
3.4 RT-PCR results
3.4.1 RT-PCR results of macrophage markers and inflammatory factors
In the study of macrophage polarization, inducible nitric oxide synthase is often selected as a marker molecule of the successful polarization of M1 macrophages, and arginase-1 and found in inflammatory zone-1 are often selected as marker molecules of the successful polarization of M2 macrophages [Citation21].
RT-PCR results (see ) revealed that rGDF15 could decrease the polarization of M1 macrophages, increase the polarization of M2 macrophages, decrease the expression of IL-6 mRNA, and increase the expression of IL-10 mRNA. LY294002 could reduce the polarization effect of rGDF15 of the LPS-mediated macrophage on the M2 macrophages and reduce the anti-inflammatory effect of the M2 macrophages (see ).
Figure 4. Expressions of macrophage markers and inflammatory factor mRNA.
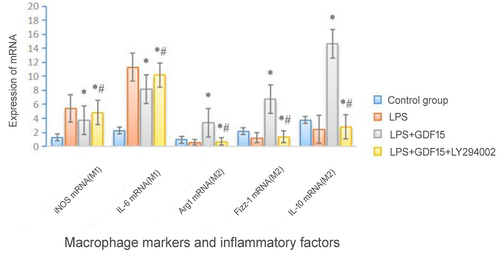
Table 4. Expressions of macrophage markers and inflammatory factor mRNA
3.4.2 GDF15 expression
GDF15 is a member of the transforming growth factor beta (TGF-β) family and is highly expressed in inflammation, trauma, and other cases [Citation10–12]. When compared with zero hours after the LPS injection, the level of serum GDF15 was increased significantly at 1, 3, 6, and 12 hours after the LPS injection (see ). Blood RNA was extracted using the PureLink™ Total RNA blood purification kit to measure changes in the expression of GDF15 mRNA in the blood and hearts of mice after LPS stimulation. GDF15 was highly expressed in the spleen and blood (see ), and the expression of GDF15 mRNA was significantly upregulated in the LPS group at 1 and 3 hours after LPS injection (see ). Notably, after the mice were treated with different doses of LPS for 24 hours, the expression of GDF15 mRNA was significantly decreased (see ). In this study, 10 ng/ml LPS was chosen to measure the change in the expression of GDF15 in the peritoneal macrophages of mice. It was observed that GDF15 mRNA level peaked 3 hours after LPS treatment and gradually decreased at subsequent time points (see ).
Figure 5. The dynamic change of GDF15 expression. A. Serum GDF15 expression in LPS-induced mice (ng/mL) (* p < 0.05,1,3,6,12 h vs.0 h). B. Expression of GDF15 mRNA in blood and tissues. C. Expression of GDF15 mRNA in the heart of mice induced by LPS (* p < 0.05,1,3 h vs.0 h). D. The expression of GDF15 mRNA in macrophages in mice treated with different doses of LPS for 24 hours (* p < 0.05,10,100,1000 ng/ml vs.0 ng/ml). E. The expression of GDF15 mRNA in peritoneal macrophages of mice treated with LPS (10 ng/mL) at different time points.
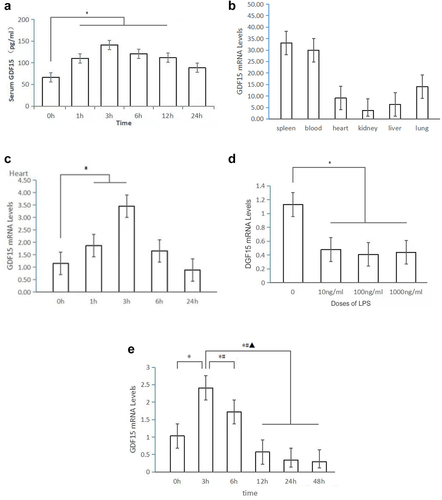
These above results revealed that the expression of GDF15 in the macrophages, whole blood, and hearts of mice changed dynamically after endotoxin injury.
3.5 The results of Western blot (see and ) revealed that the phosphorylation of Akt protein, a downstream molecule of the PI3K/Akt signaling pathway, was enhanced (see ), suggesting that GDF15 can activate the PI3K/Akt pathway, and LY294002, a PI3K inhibitor, can inhibit the activation of GDF15. In the LPS-induced peritoneal macrophages of mice, rGDF15 intervenes in macrophage polarization by promoting the phosphorylation of the PI3K/Akt signaling pathway.
Figure 6. Protein expression of p-PI3K and p-Akt in the PI3K/AKT signaling pathway of macrophages of each group (Figure 6a: * p < 0.05,LPS+GDF15 group vs. LPS group;*# p < 0.05,LPS+GDF15+ LY294002 groupvs.LPS+GDF15 group).
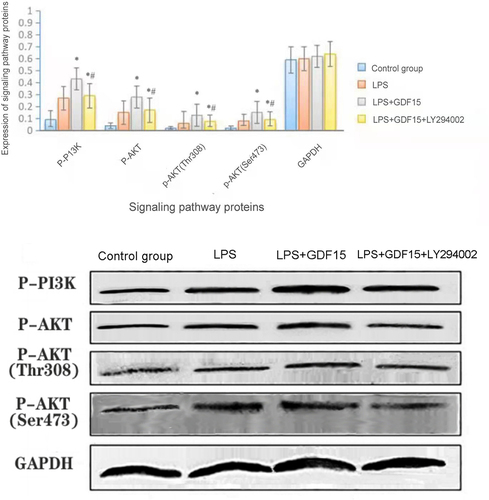
Table 5. Protein expression of p-PI3K and p-Akt in the PI3K/AKT signaling pathway of macrophages of each group
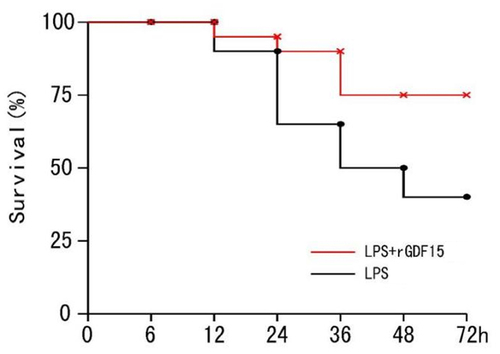
3.4.3. Survival rate of sepsis mice in all groups
The sepsis mice were followed up for 72 hours to monitor the survival rate (see ). The Kaplan–Meier survival curve (see ) revealed that the survival rate of mice was significantly higher in the LPS + rGDF15 (Recombinant growth differentiation factor 15) group than in the LPS group (P = 0.043).
Table 6. Survival rate of sepsis mice in each group (%)
3.4.4. Echocardiographic results
The indexes of systolic and diastolic function of left ventricle can be obtained when mice are intubated through the common carotid artery to the left ventricle. Left ventricular pressure and its change rate are important indexes to reflect and evaluate left ventricular systolic and diastolic functions. Left ventricular systolic pressure (LVSP) and left ventricular diastolic pressure (LVSP) LVDP), left ventricular end-diastolic pressure (LVEDP), Maximum rate of increase of left ventricular pressure (+ DP/DTmax), maximum rate of decrease of left ventricular pressure (-DP/DTmax), heart rate (HR). Among these indicators, LVSP and + DP/DTmax mainly reflect the systolic function of left ventricle. LVDP and -DP/DTMAX mainly reflect the diastolic function of left ventricle.
Echocardiography was performed to evaluate the effect of rGDF15 on cardiac function in LPS-induced sepsis mice (see and ). RGDF15 could significantly improve cardiac systolic function and increase left ventricular end-systolic diameter (LVIDs), left ventricular ejection fraction (EF%), fractional shortening (FS%), maximum velocity of left ventricular pressure increase (+DP/dtmax), and maximum velocity of left ventricular pressure decrease (-DP/dtmax).
Figure 8. The effect of RGDF15 on the cardiac function of LPS-induced sepsis mice (echocardiography) (* p < 0.05, LPS group vs. control group;*# p < 0.05, LPS+GDF15 group vs. LPS group).
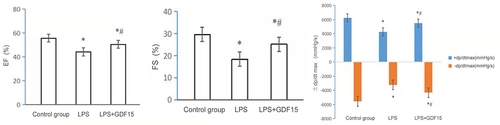
Table 7. The effect of RGDF15 on the cardiac function of LPS-induced sepsis mice (echocardiography)
3.4.5. GDF15 could reduce the production of inflammatory cytokines induced by macrophage endotoxins (. and )
Considering the association between macrophages and sepsis and the important role of macrophages in producing proinflammatory cytokines and mediating the downstream inflammatory response after infection [Citation8], in order to verify the reliability of the in vitro cell culture results in this study, in vivo animal experiments were carried out to determine whether GDF15 could interfere with the secretion of cytokines in sepsis mice under the stimulation of LPS. ELISA results revealed that rGDF15 significantly inhibited the production of proinflammatory cytokines induced by LPS by promoting the increased release of anti-inflammatory cytokines.
Figure 9. Expression of inflammatory cytokines in sepsis mice of each group (* p < 0.05, LPS group vs. control group;*# p < 0.05, LPS+GDF15 group vs. LPS group).
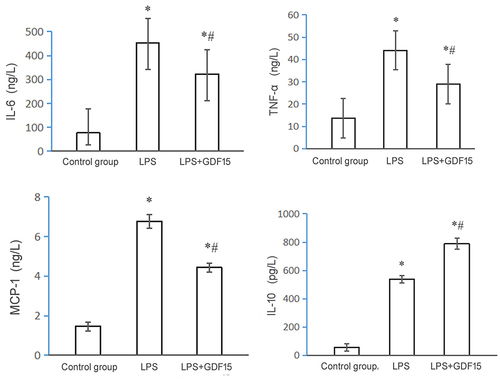
Table 8. Results of cytokines secreted by sepsis mice in each group
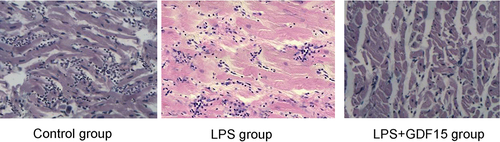
3.4.6. Pathological sections of the heart of sepsis mice in all groups (see )
Control group: The myocardial structure was clear, the myocardial fibers were well arranged with clear transverse lines, and the structure was normal.
LPS group: Myocardial cell edema was obvious, there were extensive myocardial focal hemorrhages and necroses, and there was inflammatory cell infiltration.
LPS + GDF15 group: The myocardial fibers were arranged in waves, and there was inflammatory cell infiltration.
3.5 The serum GDF15 level was closely related to severity and mortality in patients with sepsis
3.5.1. Demographic characteristics and basic clinical data of patients with sepsis (see )
Table 9. Demographic characteristics and basic clinical data of sepsis patients
3.5.1.1. The serum level of GDF15 in patients with sepsis was higher than in healthy controls
The median serum level of GDF15 in patients with sepsis was 1,920.5 ng/L (IQR 1385.6–2438.7 ng/L), which was significantly higher than in healthy blood donors (773.6 ng/L [IQR 512.7–1094.5 ng/L]; P = 0.007; see ). This study also revealed that the median level of GDF15 in the septic shock group (2314.6 ng/L [IQR 1769.4–2614.5 ng/L]) was much higher than in sepsis group (1595.8 [IQR 1187.8–2213.2 ng/L]; see ).
Figure 11. Serum GDF15 level is correlated with poor prognosis in patients with sepsis. A. Scatter plot of serum GDF15 levels in healthy blood donors and septic patients at admission. B. AUC distinguishes sepsis patients from healthy controls. C. Correlation between serum GDF15 level and (c) Apache II score and (d) SOFA score at admission in ICU. D. The serum GDF 15 levels of survival group and non survival group. E. AUROC predicts 28-day mortality.
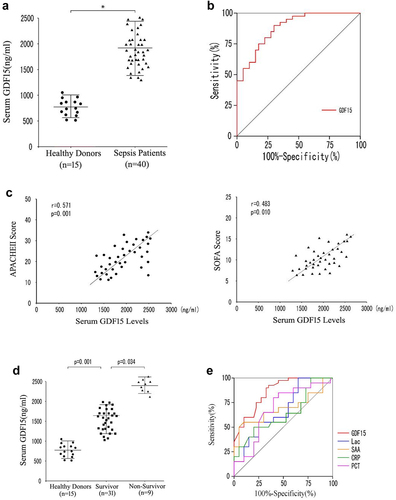
In addition, in this study, the receiver operating characteristic (ROC) curves of GDF15 were drawn to distinguish patients with sepsis from healthy blood donors. The Area under the curve (AUC) of GDF15 was 0.831, the 95% confidence interval (95%CI) was 0.704–0.961 (P = 0.001), and the best cutoff value was 1,785.6 ng/ml (see ).
It is important to note that the serum GDF15 level was positively correlated with the Apache II (r = 0.571, P = 0.001) and SOFA score (r = 0.483, P = 0.010; see ), suggesting that a high serum GDF15 level may be associated with severe multiple organ dysfunction. In this study, we also examined whether the GDF15 level can predict 28-day mortality, and it was observed that the GDF15 level in the survivor group (1643.6 ng/ml) were significantly lower than the non-survivor group (2397.7 ng/ml; P = 0.034), and the higher the serum GDF15 level at admission, the higher the mortality at the 28-day follow-up (see ).
In order to further clarify the predictive ability of serum GDF15 level on mortality in patients with sepsis, an ROC curve analysis was conducted. When compared with other traditional sepsis biomarkers, such as lactic acid, C-reactive protein, procalcitonin, and serum amyloid A, GDF15 levels showed stronger predictive ability. The AUC value was 0.773 (95%CI: 0.572–0.951, P = 0.001; see and ).
Table 10. AUC analysis for 28-day mortality prediction within the derivation
In summary, these experimental data revealed that the increase in serum GDF15 level was closely related to severity and mortality in patients with sepsis.
4. Discussion
Sepsis is caused by infection. In the early stage, the lack of intrinsic anti-inflammatory signals leads to overactivation of immune cells, including monocytes and macrophages, which leads to an inflammatory cytokine storm, causing a variety of clinical symptoms and leading to organ dysfunction. Therefore, the inhibition of pathogens and reduction in the immune response induced by pathogen infection are important means to relieve sepsis.
Macrophage polarization plays an important role in the immune mechanism of sepsis [Citation22]. Macrophages are heterogeneous and plastic and can polarize into different phenotypes in different microenvironments [Citation23]. At present, activated macrophages are divided into two categories [Citation24]: proinflammatory activated macrophages (M1) and inhibit inflammation activated macrophages (M2). The mutual transformation between macrophage phenotypes plays a key role in the development and outcome of inflammatory diseases.
In this study, flow cytometry showed that CD80 was highly expressed in LPS-mediated mouse peritoneal macrophages, suggesting that macrophages were polarized to M1 macrophages under the intervention of LPS. After administration of rGDF15, CD206 could be highly expressed in mouse peritoneal macrophages, suggesting that rGDF15 can decrease the polarization of M1 and increase the polarization of M2 macrophages, thereby playing an anti-inflammatory role. When compared with the LPS + GDF15 group, treatment with LY294002, an inhibitor of the PI3K/Akt signaling pathway, in the LPS + GDF15 + LY294002 group, reduced the polarization of M2 macrophages induced by GDF15 and the anti-inflammatory effect of M2 macrophages. The results of flow cytometry were verified by RT-PCR, showing that rGDF15 can decrease the polarization of M1 macrophages; increase the polarization of M2 macrophages; decrease the mRNA expression of IL-6, a pro-inflammatory factor; and increase the mRNA expression of IL-10, an anti-inflammatory factor.
Given that GDF15 can promote the expression of M2 macrophages, reduce the expression of M1 macrophages, and inhibit the inflammatory response induced by lipopolysaccharide in vitro, the next step was to attempt to determine whether GDF15 has a similar effect in vivo. Therefore, in this study, in vivo animal experiments were conducted to verify this result. ELISA results revealed that when compared with the control group, serum levels of TNF-α, IL-6, and MCP-1 in mice treated with LPS were significantly increased, and serum levels of IL-10 in mice treated with rGDF15 were also significantly increased. When compared with the LPS group, rGDF15 significantly inhibited the production of pro-inflammatory cytokines induced by LPS and caused an increase in the release of anti-inflammatory cytokines. The Kaplan–Meier survival curve revealed that the survival rate of mice was significantly higher in the LPS + rGDF15 group than in the LPS group.
Cardiac dysfunction is the main cause of death in patients with sepsis [Citation25]. Cardiac dysfunction in sepsis is characterized by enlarged heart, decreased EF%, and decreased left ventricular systolic pressure, and the pathological changes are extensive myocardial hemorrhage, capillary congestion, inflammatory cell infiltration, myocardial fibrosis, and necrosis [Citation26]. Echocardiographic findings revealed that rGDF15 could significantly improve cardiac systolic function, which significantly increased EF%, FS%, and LVIDs. Histopathologic section examination revealed that after 24 hours of LPS intervention, a large number of myofilaments in the myocardium were broken, myocardial cells exhibited edema, muscle fiber structure was seriously damaged and disordered, and a large number of inflammatory cells had infiltrated the myocardium. After GDF15 intervention, the pathological damage was significantly reduced, suggesting that in the treatment of sepsis, attention should be paid to protecting and supporting heart function, which can prevent cardiac dysfunction and reduce mortality.
LPS can stimulate monocytes/macrophages to infiltrate the heart and significantly affect the contractile function [Citation27]. Therefore, tests were performed to determine whether rGDF15 affected the phenotype of cardiac macrophages in mice treated with LPS. LPS (10 mg/kg) was injected intraperitoneally to induce endotoxemia in mice. In the LPS group, the percentage of M1 macrophages in the cardiac infiltrating macrophages was significantly higher than in the other two groups. In the LPS + GDF15 group, the percentage of CD206+ macrophages increased significantly when compared with the LPS group, while the percentage of CD80+ macrophages decreased significantly. More importantly, the expressions of F4-80+/CD11b+, MHC-II+, and Ly6C+ were significantly lower in the LPS + GDF15 group than in the LPS group, suggesting that rGDF15 prevented LPS-triggered monocytes (Ly6C+) and macrophages (F4-80+/CD11b+ cells) from infiltrating the heart.
The above results revealed that GDF15 can inhibit the inflammatory response by enhancing the expression of M2 macrophages and decreasing the expression of M1 macrophages in the heart of sepsis mice induced by LPS, which not only inhibits systemic inflammatory responses induced by LPS but also reduces monocyte/macrophage migration to the heart, which improves heart function and survival rates. Therefore, regulating the polarization of macrophages may be an appropriate strategy for the treatment of sepsis, which is very important to improve survival rates.
This study further explored the protective mechanism of GDF15 in sepsis by regulating macrophage polarization. The major pathogenic of sepsis are gram-negative bacteria (G−), and LPS is the main component of the cell wall. As immune cells, macrophages can induce, phagocytize, and eliminate pathogens. The PI3K/Akt signaling pathway plays an important role in macrophage activation and gene expression, which is involved in the regulation of macrophage polarization; is widely distributed in various cells; and can regulate cell survival, growth, proliferation, differentiation, and apoptosis and cytoskeleton rearrangement [Citation28,Citation29]. PI3K in the PI3K/Akt signaling pathway is a family of phosphatidylinositol kinases with Ser/Thr protein kinase activity [Citation30]. Akt is also known as protein kinase B (PKB); belongs to the Ser/Thr protein kinase, which is a downstream target protein of PI3K; and is also the most significant effector of PI3K [Citation31]. Akt can regulate a variety of cell functions after phosphorylation including cell activity, proliferation, differentiation, and intermediate metabolism [Citation32,Citation33].
GDF15 is a member of the TGF-β super family that was first discovered by Bootcov in 1997 [Citation33] and is a stress response protein. In ischemia–reperfusion injury [Citation14], heart failure [Citation34], and atherosclerosis [Citation35], GDF15 is highly expressed in cardiomyocytes and regulates their structure and apoptosis [Citation34]. Inflammation-induced GDF15, and that GDF15 was necessary for surviving both bacterial and viral infections, as well as sepsis [Citation36].
GDF15 is secreted in an inactive manner, and after being activated by TGF-β kinase, it pays a role by forming a heteromer with Ser/Thr kinase receptors, which mainly activates Smad proteins, and Smad proteins transduce the signal into the nucleus to regulate the expression of the target gene [Citation37]. As a member of the TGF-β super family, the GDF15 receptor can phosphorylate Smad proteins and mediate the intracellular signal pathway. Furthermore, GDF15 can activate other Smad-independent signaling pathways, such as PI3K-PKB (PKB, Akt), Ras-ERK, p38, and c-Jun N-terminal kinase [Citation38]. Therefore, the mechanism of GDF15 involves a variety of signaling pathways, and as a stress response protein, it is widely involved in various disease processes ().
Figure 12. GDF15 mediates the activation of intracellular Smad signaling pathway and Smad-independent signaling pathway.
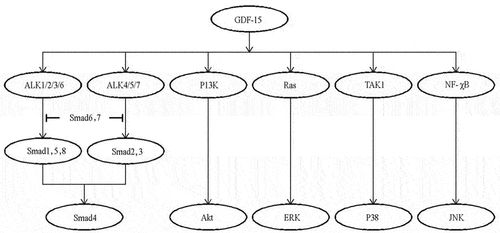
At present, it remains unclear whether GDF15 can mediate macrophage polarization through the PI3K/Akt signaling pathway. In this study, the results of Western blot revealed that the phosphorylation of Akt protein, a downstream molecule of the PI3K/Akt signaling pathway, was enhanced, which suggests that GDF15 can activate the PI3K/Akt pathway, and LY294002, a PI3K inhibitor, can inhibit the activation of GDF15, reduce the polarization of M2 macrophages induced by GDF15, and reduce the anti-inflammatory effect of M2 macrophages. This suggests that rGDF15 intervenes in macrophage polarization by promoting the phosphorylation of the PI3K/Akt signaling pathway to produce a protective effect in sepsis (see ).
This study reveals a new mechanism of macrophage polarization regulation, enriches the basic research on macrophages, and provides a new way of thinking and an experimental basis for eliminating inflammation in inflammatory diseases that present with a polarization disorder of the macrophages.
Subsequently, the investigators analyzed the clinical applications for GDF15. In the majority of hospitalized patients with COVID-19, GDF15 levels were increased, and the higher concentration was associated with SARS-CoV-2 viremia, hypoxemia, and poor prognosis [Citation39]. There was a high level of GDF15 in the peripheral blood of patients with sepsis [Citation40]. These findings suggest that GDF15 is a potential therapeutic target for sepsis secondary to bacterial infection. Kempf et al. [Citation41] reported the serum GDF15 levels of 429 healthy individuals with an average age of 65 years and obtained the GDF15 level standard that is generally accepted at present: the normal range is <1200 ng/L, the slightly increased range is 1200–1800 ng/L, and the obviously increased range is >1900 ng/L. Similar to foreign research reports, the results of this study revealed that the serum levels of GDF15 were significantly higher in severe, hospitalized patients, and the serum levels of GDF15 in patients with sepsis was higher than in healthy controls. This result indicates that GDF15 has a potentially compensatory effect in host immune response. The increase in serum GDF15 levels is closely related to severity and mortality in patients with sepsis and is a powerful prognostic marker.
This study has the following limitations: First, because of the small sample size, the current clinical data provide only preliminary observations. Second, although endotoxin is the main pathogenic factor of G− bacteria-induced sepsis, it will be interesting to study whether GDF15 has a similar protective effect on real microbe-induced sepsis.
5. Conclusions
GDF15 regulates macrophage polarization through activating the PI3K/Akt signaling pathway and has a protective effect on survival and the cardiac function of patients with sepsis and sepsis mouse models. High levels of serum GDF15 are closely related to the severity of the sepsis and the mortality. Therefore, GDF15 is a powerful prognostic marker for sepsis.
Regulation of macrophage polarization as a target may be a new idea for the treatment of sepsis. GDF15 plays a protective role in regulating sepsis macrophage polarization and endotoxin-induced cardiac injury in sepsis. The mechanism of GDF15 regulating the direction of macrophage polarization in sepsis: GDF15 regulates the direction of macrophage polarization through PI3K/Akt signaling pathway and has protective effects on survival and cardiac function in sepsis patients and sepsis mouse models. Elevated serum GDF15 levels are closely associated with the severity and mortality of patients with sepsis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016 Feb 23; 315(8):801–810.
- Sly LM, Rauh MJ, Kalesnikoff J, et al. LPS-induced upregulation of SHIP is essential for endotoxin tolerance. Immunity. 2004 Aug;21(2):227–239.
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Intensive Care Med. 2008 Jan;34(1):17–60.
- Hochstadt A, Meroz Y, Landesberg G. Myocardial dysfunction in severe sepsis and septic shock: more questions than answers? J Cardiothorac Vasc Anesth. 2011 Jun;25(3):526–535.
- Landesberg G, Gilon D, Meroz Y, et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012 Apr;33(7):895–903.
- Sanfilippo F, Corredor C, Fletcher N, et al. Diastolic dysfunction and mortality in septic patients: a systematic review and meta-analysis. Intensive Care Med. 2015 Jun;41(6):1004–1013.
- Palmieri V, Innocenti F, Guzzo A, et al. Left ventricular systolic longitudinal function as predictor of outcome in patients with sepsis. discussion e003865. Circ Cardiovasc Imaging. 2015 Nov;8(11):e003865.
- Peiseler M, Kubes P. Macrophages play an essential role in trauma-induced sterile inflammation and tissue repair. Eur J Trauma Emerg Surg. 2018 Jun;44(3):335–349.
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008 Dec;8(12):958–969.
- Lambert JR, Whitson RJ, Iczkowski KA, et al. Reduced expression of GDF-15 is associated with atrophic inflammatory lesions of the prostate. Prostate. 2015 Feb 15;75(3):255–265.
- Xu XY, Nie Y, Wang FF, et al. Growth differentiation factor (GDF)-15 blocks norepinephrine-induced myocardial hypertrophy via a novel pathway involving inhibition of epidermal growth factor receptor transactivation. J Biol Chem. 2014 Apr 4;289(14):10084–10094.
- Jakubowska K, Pryczynicz A, Dymicka-Piekarska V, et al. The growth differentiation factor-15 (GDF-15) can be useful in the detection of distant metastases in sera of colorectal cancer patients. Prog Health Sci. 2016;6(1):40–49.
- Conte M, Martucci M, Mosconi G, et al. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness. Front Immunol. 2020 May 12;11:915.
- Kempf T, Eden M, Strelau J, et al. The transforming growth factor-beta superfamily member growth-differentiation factor-15 protects the heart from ischemia/reperfusion injury. Circ Res. 2006 Feb 17;98(3):351–360.
- Pan YK, Liu QP. Neutrophil infiltration and dynamic expression of GDF15 in rat myocardial dissection without reflow phenomenon. Chinese J Pathophysiol. 2013;29(1):155–158.
- Adela R, Banerjee SK. GDF-15 as a target and biomarker for diabetes and cardiovascular diseases: a translational prospective. J Diabetes Res. 2015;2015:490842.
- Zhou D, Huang C, Lin Z, et al. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Signal. 2014 Feb;26(2):192–197.
- Zhang Z, Xu J, Liu Y, et al. Mouse macrophage specific knockout of SIRT1 influences macrophage polarization and promotes angiotensin II-induced abdominal aortic aneurysm formation. J Genet Genomics. 2018 Jan 20; 45(1):25–32.
- Peng J, Li Y, Wang X, et al. An Hsp20-FBXO4 axis regulates adipocyte function through modulating PPARγ ubiquitination. Cell Rep. 2018 Jun 19; 23(12):3607–3620.
- Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014 Jan 16; 40(1):91–104.
- Wang Y, Liao X, Sun J, et al. Characterization of HIF-1α/glycolysis hyperactive cell population via small-molecule-based imaging of mitochondrial transporter activity. Adv Sci (Weinh). 2018 Jan 9; 5(3):1700392.
- Shen LZ, Li L, Yan J. The role of macrophage polarization in the immune mechanism of sepsis. Chinese Electronic J Intensive Care Medicine. 2019May;5(2):185–186.
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012 Mar;122(3):787–795.
- Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005 Oct;23(4):344–346.
- Fattahi F, Ward PA. Complement and sepsis-induced heart dysfunction. Mol Immunol. 2017 Apr;84:57–64.
- Zhang X, Dong S, Qin Y, et al. Protective effect of erythropoietin against myocardial injury in rats with sepsis and its underlying mechanisms. Mol Med Rep. 2015 May;11(5):3317–3329.
- Shahid F, Lip GYH, Shantsila E. Role of monocytes in heart failure and atrial fibrillation. J Am Heart Assoc. 2018 Feb 1; 7(3):e007849.
- Chang L, Zhao D, Liu HB, et al. Activation of sonic hedgehog signaling enhances cell migration and invasion by induction of matrix metalloproteinase-2 and −9 via the phosphoinositide-3 kinase/AKT signaling pathway in glioblastoma. Mol Med Rep. 2015 Nov;12(5):6702–6710.
- Green BD, Jabbour AM, Sandow JJ, et al. Akt1 is the principal Akt isoform regulating apoptosis in limiting cytokine concentrations. Cell Death Differ. 2013 Oct;20(10):1341–1349.
- Liang H, Zheng QL, Fang P, et al. Targeting the PI3K/AKT pathway via GLI1 inhibition enhanced the drug sensitivity of acute myeloid leukemia cells. Sci Rep. 2017 Jan 18; 7:40361.
- Ruan JY, Chen BC, Zhang XL, et al. Progress in signaling pathways of macrophage M1/2 polarization. J Iimmunol. 2015;31:911–917.
- Maroulakou IG, Oemler W, Naber SP, et al. Akt1 ablation inhibits, whereas Akt2 ablation accelerates, the development of mammary adenocarcinomas in mouse mammary tumor virus (MMTV)-ErbB2/neu and MMTV-polyoma middle T transgenic mice. Cancer Res. 2007 Jan 1; 67(1):167–177.
- Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc Natl Acad Sci U S A. 1997 Oct 14; 94(21):11514–11519.
- Xu J, Kimball TR, Lorenz JN, et al. GDF15/MIC-1 functions as a protective and antihypertrophic factor released from the myocardium in association with SMAD protein activation. Circ Res. 2006 Feb 17;98(3):342–350.
- Brown DA, Breit SN, Buring J, et al. Concentration in plasma of macrophage inhibitory cytokine-1 and risk of cardiovascular events in women: a nested case-control study. Lancet. 2002 Jun 22; 359(9324):2159–2163.
- Luan HH, Wang A, Hilliard BK. GDF15 is an inflammation-induced central mediator of tissue tolerance. Cell. 2019 Aug 22; 178(5):1231–1244.
- Xu YY, Guang XF. Application of growth differentiation factor-15 in cardiovascular disease. Chinese J Cardiovascular Res. 2010 Dec;8(12):950–953.
- Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in TGF-beta family signalling. Nature. 2003 Oct 9; 425(6958):577–584.
- Myhre PL, Prebensen C, Strand H, et al. Growth differentiation factor 15 provides prognostic information superior to established cardiovascular and inflammatory biomarkers in unselected patients hospitalized with COVID-19. Circulation. 2020 Dec;142(22):2128–2137.
- Santos I, Colaço HG, Neves-Costa A, et al. CXCL5-mediated recruitment of neutrophils into the peritoneal cavity of Gdf15-deficient mice protects against abdominal sepsis. Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):12281–12287.
- Kempf T, Horn-Wichmann R, Brabant G, et al. Circulating concentrations of growth-differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007 Feb;53(2):284–291.