ABSTRACT
Ovulation-inducing drugs such as endogenous steroids could reduce endometrial receptivity during the implantation window, resulting in lower clinical pregnancy rates and higher miscarriage rates. The present study employed electroacupuncture therapy along with different frequencies on elevating impaired endometrial receptivity to elucidate the mechanism therein. The rats were split up into seven groups of normal, model, low-frequency electroacupuncture (LF-EA), high-frequency electroacupuncture (HF-EA), LF-EA+anti-miRNA, HF-EA+anti-miRNA, and anti-miRNA. PCR assays were used to detect miR-223-3p expressions. The effects of electroacupuncture and miR-223-3p on rats’ endometrial membrane cell-drinking process in a manner of scanning electron microscopy were recorded. After that we observed on the electroacupuncture effects on the conditions of adhesion molecules and LIF/STAT3 signaling pathway with assays of immunofluorescence and Western Blot. This study was end up with dual luciferase assay, where combination of miR-233-3p onto the 3’-UTR sequence of LIF was determined. PCR assay demonstrated that HF-EA procured an inhibition in miR-223-3p expression, whereas scanning electron microscopy turned out that both electroacupuncture and miR-223-3p were capable of raising the amount of intrauterine pinocytosis and the number of blastocyst implantation in rats. Additionally, assays of Western Blot and immunofluorescence showed that therapy of electroacupuncture brought about decreasing expressions in adhesion molecules of E-cadherin, β-catenin and claudin-1 (CLDN1). We found that both electroacupuncture and miR-223-3p were able to fortify LIF/STAT3 signaling pathway, then the fact of miR-223-3p combination to LIF 3’-UTR sequence was validated via our dual-luciferase assay. Electroacupuncture therapy inhibited the miR-223-3p expression upon LIF/STAT3 signaling pathway to elevate endometrial receptivity.
Graphical Abstract
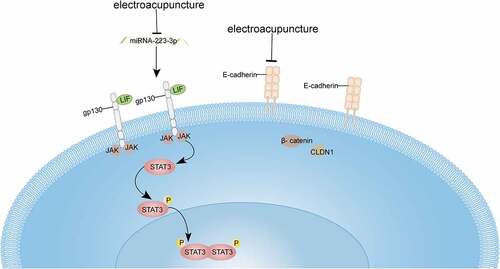
Highlights
1. Electroacupuncture therapy effectively reduce miRNA-223-3p expression in COH rats.
2. Electroacupuncture and electroacupuncture together with anti-miRNA-223-3p could increase the number of pinopodes and endometrial blastocyst implantation count.
3. Electroacupuncture and electroacupuncture together with anti-miRNA-223-3p reduce the protein expression levels of E-cadherin, β-catenin and CLDN1 adhesion molecules in COH rats.
4. Electroacupuncture and electroacupuncture together with anti-miRNA-223-3p could markedly enhance the LIF/STAT3 signaling pathway in COH rats.
Introduction
Embryo implantation is the most important event in mammalian reproduction referring to a process that an activated blastocyst establishes its connection with receptive endometrium in ways of localization, adhesion, and invasion. Receptivity of the endometrium to implanted embryos is termed as endometrial receptivity, where a successful pregnancy depends on [Citation1–3]. It has been revealed by previous studies that lower clinical pregnancy rates together with higher miscarriage rates were resulted from the ovulation induction drugs utilized in the period of hyperovulation for a possible interference begetting within the physiological regulation of endogenous steroid hormones on endometrium might lead to negative impacts on the endometrial receptivity of implantation window period [Citation4–6]. Conventional clinical therapies to elevate endometrial receptivity currently are the administrations of regulatory hormone and traditional Chinese medicine and the applications of improving microcirculation, mechanical stimulation, and surgery. Besides, acupuncture therapy is proved highly efficient in elevating endometrial receptivity as well as in clinical pregnancy rates [Citation7–10].
Most of the endometrial receptivity studies in last decades were scattered around aspects of genes, proteins, cytokines, adhesion molecules, microRNAs, and endometrial floras. E-cadherin, β- catenin, and claudin-1 (CLDN1) are the adhesion molecules upon the cell membrane and cytoplasm and are possibly dual-functional, exerting their abilities in adhering and closely connecting within epithelial cells of the endometrial gland [Citation11]. Those adhesion molecules are expressed at early stage of implantation to secure the adhesion, then to be down-regulated at the later stage to provide advantages for epithelial cells’ dissociation and blastocysts implantation [Citation12–14].
Leukemia inhibitory factor (LIF), known as a member to Interleukin-6 family, is a multifunctional cytokine coming in multiple biological activities under different surroundings of tissues and cells [Citation15]. LIF is an active player in many sections of reproduction, which are the development of follicles and embryos, embryo implantation and pregnancy maintenance and so on. Function of LIF therein comes into effect in a way of binding to the receptor LIFR on the target cell membrane, which is subsequently combined with gp130 to form a dimer; and this dimer is the trigger for janus kinase (JAK) to phosphorylate transcription activator signal transducer and activator of transcription (STAT3) protein [Citation16–20]. miR-223-3p has been validated as a up-stream regulator to LIF mRNA.
We found that expression patterns of LIF mRNA and miRNA-223-3p were distinct in the endometrium of pregnant mice that the former was high whereas the later was low, while that condition was reversed in non-pregnant mice.
Previous study presented an outcome in pregnant rats that LIF mRNA was high-expressed when miR-223-3p low-expressed, while this situation in non-pregnant rats was opposite suggesting a possible miR-223-3p targeting regulation towards LIF mRNA. This hypothesis was validated in a recent report, where miR-223-3p targets LIF 3’-UTR sequence to silence this gene and to manipulate the expressions of its down-stream genes so that the endometrial receptivity is established, and the embryos become implanted [Citation21,Citation22].
In this study, we aim to investigate the different frequencies electroacupuncture therapy on elevating impaired endometrial receptivity to elucidate the mechanism therein. It is reported that the effect of electroacupuncture in improving the endometrial receptivity of patients undergoing freeze-thaw embryo transfer [Citation23]. Then, we had our observation on effects of different frequencies of electroacupuncture on rats’ endometrial receptivity; then we performed the research into the effects of electroacupuncture and miR-223-3p toward LIF/STAT3 signaling pathway. And finally, we run a test on hypothesis that the electroacupuncture might affect the LIF/STAT3 signaling pathway through miR-223-3p. The present study explored the molecular mechanism of electroacupuncture in improving endometrial receptivity in COH rats, and provided new insights for acupuncture in improving IVF, endometrial receptivity and clinical pregnancy rate.
Method
Experiments were performed to explore the efficacy and mechanism of different frequencies electroacupuncture on endometrial receptivity of medicine induced COH rats at the cellular and molecular level. Initially, COH rats model was established which were split up into seven groups of normal, model, LF-EA, HF-EA, LF-EA+anti-miRNA, HF-EA+anti-miRNA, and anti-miRNA and given the corresponding treatment. PCR assays were used for miR-223-3p expressions detection. The electroacupuncture and electroacupuncture along with anti-miR-223-3p on apical vesicle process of endometrial epithelial cells was observed under a scanning electron microscope. The expression of adhesion molecules like E-cadherin, β-catenin and CLDN1, and LIF/STAT3 signaling pathway within different treatment groups were detected by assays of immunofluorescence and Western Blot. This study was end up with our dual luciferase assay, where combination of miR-233-3p onto the 3’-UTR sequence of LIF was determined.
Experimental animals
The animal experiments in this study were approved by the Attitude of the Animal Care Welfare Committee of Guizhou University of Chinese Medicine (20,210,018). Subjects of female SD rats were randomly split up into seven groups, which were the ones of normal, model, LF-EA, HF-EA, LF-EA+anti-miRNA, HF-EA+anti-miRNA, and anti-miRNA, with 20 rats in each.
Model establishment: It was started on the third day of rats’ estrus that they were given a daily intraperitoneal injection in the dose of GnRHa 1.5 μg/100 g based on body weight. At the early stage of the estrus that the 7th day of model establishment, rats were simultaneously administrated with PMSG 40IU/100 g body weight at 9 o<apos;>clock am of that day and HCG injection of 100 U/100 g body weight [Citation24]. At meanwhile, group of normal were treated with an eight-day continuous equal normal saline injection. A successful model establishment was obtained when vaginal secretions of rats increased notably, and numerous anucleated keratinocytes in secrete smears were observed under the optical microscope.
Specimen harvest: After the last injection, male and female rats were cooped up, then an observation to female rats’ vaginal plug was made on the 2nd day. The rats were defined as pregnant when a visible female rats’ vaginal plug was found, and the day was the first day of pregnancy (hereinafter referred to pd1). Pregnant rats were anesthetized on the second day (pd2) and exposed to the uterus after laparotomy, injected bilaterally with 10 nmol miRNA antagomir. The rats were sacrificed on day 9 by laparotomy to check the actual number of implantations in both uteruses.
Electroacupuncture therapy
The electroacupuncture is a therapy on the basis of quality regulation theory of acupuncture and moxibustion. All the groups with electroacupuncture intervention were given pre-treatments 20 days before our model establishment. Referring to the experimental animal acupuncture atlas developed by the Experimental Acupuncture and Moxibustion Research Association of the National Society of Acupuncture and Moxibustion, we pinpointed Guanyuan and Zusanli points, and made the rats wear self-made rat fixed clothes. Stainless steel millineedle of 0.30 × 13 mm was selected, and the depth of acupuncture was 3–5 mm, in which Guanyuan (CV4) was stabbed oblique to the direction of xijian process and Zusanli (ST36) was directly stabbed. After acupuncture, a pair of wires were used to connect Guanyuan and Zusanli, respectively, and Zusanli points were selected alternately. Electroacupuncture treatment was stopped after modeling. Electroacupuncture therapy was lasted for 7 days as a course of treatment, one day was suspended between the two courses, and the treatment lasted for four courses (a total of 31 days). In contrast, we only took grasping and fixation toward the other groups of rats instead of electroacupuncture.
(1) Stimulation parameters of low-frequency electroacupuncture treatment group: continuous wave, 2 Hz, 1 mA, with local muscle tremor as the degree, leaving the needle for 20 min.
(2) Stimulation parameters of high-frequency electroacupuncture treatment group: continuous wave, 50 Hz, 1 mA, local muscle tremor as the degree, and the needle was left for 20 min.
(3) Stimulation parameters of low frequency +miRNA antagonist group: same as those of the low-frequency electroacupuncture group. After the successful female rats of estrus period modeling, which were caged together with male mice, and the day of discovery to vaginal plug was recorded as the first day of pregnancy (pd1). Female rats’ uterus was exposed after laparotomy after pd2 anesthesia, and the uterus was given 10 nmol miRNA Antagomir for bilateral uterine horn injection.
(4) Stimulation parameters of the high frequency +miRNA antagonist group: same as those of the low frequency electroacupuncture group. The administration method of miRNA antagonist was same as above.
HE staining
In the course of the histological steps, endometrial tissues were fixed with 4% paraformaldehyde for 24 hours at least. Paraffin-embedded tissues were dehydrated and sectioned (thickness: 5 μm) and embedded with paraffin [Citation10]. The pathological changes in endometrial tissues were estimated by hematoxylin and eosin staining (HE).
Scanning electron microscopy observation
All samples were fixed with 2.5% glutaraldehyde at room temperature for 10 min; which were then dehydrated via 50%, 70%, 90%, and 100% acetone gradient for 10 min each, and replaced through 50%, 70%, 90%, and 100% isoamyl acetate for 10 min each. Scanning electron microscopy was applied for observation on the development of pinocytosis on endometrial epithelial cell membrane surface [Citation25]. The pinocytosis consists of three stages, which are development, maturation, and decline: in the developmental stage, the pinocytosis microvilli are slender, dense, and upright, and the membranous protrusions on the top gradually form and develop to the top of the whole cell, and then the microvilli become shorter and fewer, and merge with each other; the microvilli of mature pinocytosis processes disappear completely, the surface becomes smooth, and the membranous processes come to be larger, higher than the ciliated cells, shaped mushrooms; pinocytosis membrane-like protrusions within the declining period appear atrophy, and microvilli reappear on the surface, on the basis of the percentage of pinocytosis area covering the surface of the endometrium (>50%, 20%-50%, <20%), the number of pinocytosis can be divided into three types: rich, medium, and small.
Immunofluorescence
The cells were rinsed with phosphate buffer saline (PBS), TritonX-100 was added dropwise to the slides, 10% serum was added dropwise after PBS immersion, and blocked, each slide was dripped with primary antibody and placed in a wet box, and incubated overnight; the wet box was then taken out, the sample was rewarmed at room temperature for 30 minutes, and the diluted fluorescent secondary antibody was added dropwise after immersion in PBS, and then incubated in the wet box again for 60 minutes; the samples therein were immersed in PBS and then DAPI was added dropwise, and the observation was performed through a fluorescence microscopy imaging system [Citation26].
Western blot assay
In the first step of the test, 1/3 of the remaining fresh uterus was quickly frozen and stored for examination, and the expression of E-cadherin, β-catenin and CLDN1 in the epithelium of the endometrial cavity was detected via immunofluorescence. In addition, conventional methods were used to extract and quantify the total protein in the quick-frozen endometrium samples in Test 1.
Western blot assay was performed with sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis toward protein samples, and the proteins were then transferred onto the membranes, commenced with blocking, primary antibody incubation, HRP-labeled secondary antibody hybridization, etc [Citation27]. Chemiluminescence was used to develop color, and the optical density was determined with a gel image analysis system. Each sample was repeated three times for mean analysis. β -actin was used as an internal reference.
qPCR assay
On pd4, half of the SD rats in each group were sacrificed, and one side of the endometrial tissue was taken out by the extrusion method, and then quickly frozen in liquid nitrogen at −80°C for inspection. We extracted the total RNA from the sample with Trizol reagent (Takara, Japan). We obtained cDNA by using PrimeScript TMR T kit (Takara, Japan). Response system as well as procedures for qRT-PCR are shown in the instructions of the TB Green Premix Ex Taq II (Takara, Japan), and all the procedures were accomplished on the platform of CFX96 Real-Time system (Bio-Rad, USA). Relative expression of the genes was calculated in a manner of 2−∆∆CT algorithm [Citation22].
Primer sequences of the genes were shown:
rno-miR-223-3p-F: (TGTCAGTTTGTCAAATACCCC),
rno-miR-223-3p-RT:(CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGGGTATT)rno-miR-R-(GTGGAGTCGGCAATTCAGTT),LIF-F(CCAACAACGTGGATAAGCTATG),LIF-R(GTTTTTCTGATCCCAGGTGATG),JAK-F(AGGAATCTTCTTACCAGGATGCG),JAK-R(ATGCTTGGTTAAGGTTTCCAAGGT),STAT3-F(GCTCAGTGGAACCAGCTCCA),STAT3-R(CTTGGCTCTCAATCCAAGGCG).
Immunohistochemistry
The samples were sectioned, fixed with fresh 4% paraformaldehyde, repaired and dissected, dewaxed and hydrated. After antigen repair treatment, another triton-100 was added to block endogenous peroxidase, primary antibody was incubated, secondary antibody was added, and staining solution was added at room temperature [Citation28].
Dual luciferase assay on promoter activity
Cells were implanted into the culture plate and transfected with plasmids, add Opti-MEM and Lipofectamine 3000, then pGL-LIF3ʹUTR -WT-promoter-Luc and pGL-LIF3ʹUTR -mut-promoter-Luc were added, which contained Renilla fluorescence Plasmid pRL-TK of the enzyme gene, then continuing to be cultured in a 37°C incubator. Lysis Buffer (PLB) was added after the cell culture medium was replaced for luciferase detection: culture supernatant was taken, which was kept from light and added with LARII to measure firefly luciferase activity, and Stop solution was added to measure Renilla luciferase activity; the ratio of firefly luciferase activity/renilla luciferase activity was to be calculated [Citation29].
Statistical analysis
With the help of SPSS 23.0, the data were analyzed. Mean values of two independent sample groups were calculated with the algorithm of t test; mean values of multiple sample groups were analyzed by one-way ANOVA, P < 0.05. All the experiments were repeated three times independently, and the results indicated that the mean value ± standard deviation.
Results
1. Electroacupuncture therapy effectively reduced miR-223-3p expression
It has been verified that miR-223-3p is a up-stream molecule to LIF mRNA. They present a distinctly opposite trend in pregnant rats that is high-expressed LIF mRNA on the surface of pregnant rats’ endometrium versus low-expressed miR-223-3p in non-pregnant rats’ endometrium. Those facts suggested that low-expressed miR-223-3p provide an advantage for the down-regulation of LIF mRNA. Recent study elucidated this regulatory mechanism that the miR-223-3p makes a complementary pairing onto LIF mRNA 3’ UTR, which leads to the silencing in this gene; expression and brings about changes in regulation of its down-stream genes, and those changes would allow endometrial receptivity to be established and embryo implanted.
This paper was committed to revealing the effects of LF-EA and HF-EA to expression of miR-223-3p in rats. In this study, the SD rats were grouped into the ones of normal, model, LF-EA, HF-EA, LF-EA+anti-miRNA, HF-EA+anti-miRNA, and anti-miRNA. Expression of miR-223-3p in each group was determined through qPCR assay, then it turned out that that expression in model group was notably higher than normal group, the miR-223-3p expression in HF-EA group was significantly lower than the one of model, besides that, when it was compared with the anti-miRNA group, the miR-223-3p expression in HF-EA+anti-miRNA group showed a trend of greatly decline. All together, we were reasonable to believe that the level of miR-223-3p in rats’ endometria is capable of being effectively reduced after electroacupuncture therapy, and this outcome would be more perceptibly after anti-miRNA addition ().
Figure 1. Estrus cycle and relative miR-223-3p expression in rats (A) Relative miR-223-3p expression in rats of different treatments. (B) The vaginal secretions of rats were tested via HE staining. Results from real time PCR are expressed as (2−ΔΔCt; arbitrary units) ± SD relative to GAPDH. Data were presented in a manner of mean ± SD (n ≥ 3 experiments). Outcomes of *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 were determined via Student<apos;>s t-test (two groups) or one-way ANOVA, subsequently with Tukey<apos;>s test (more than two groups).
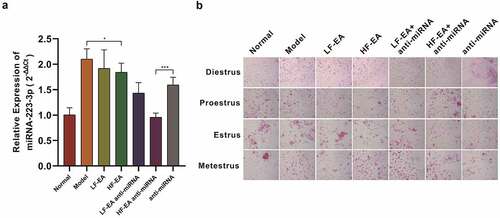
We also made another observation on rats’ estrus, which comprised of rats’ diestrus, proestrus, estrus, metestrus. The rats in each group of pre-estrus showed a large number of nucleated epithelial cells and a small number of keratinocytes. The estrus period was manifested by full-field keratinizing epithelial cells, a small number of nucleated epithelial cells were keratinizing epithelial cells and white blood cells in the late estrus period, and a large number of white blood cells and a small amount of mucus are presented in the inter-estrous period ().
2. Electroacupuncture therapy notably elevated the implantation ability of blastocyst in rats
Pinocytoid process is a large, smooth membranous process formed by endometrial epithelial cells, also known as pinocytoid vesicles. Pinocytoid vesicles replace the missing microvilli on the surface of endometrial epithelial cells during implantation. It appears about 1 week after ovulation, and its existence is very brief, subsiding after about 48 hours. There is a positive correlation between the number of pinocytoid processes and blastocyst implantation in normal human and animal models. Fully developed pinocytosis has become one of the most representative morphological and structural markers in the evaluation of endometrium and embryo implantation window. In our experiment, six rats were randomly selected from each group and sacrificed, the uteri of which were removed for electron microscope observation. We found that the number of pinocytosis decreased significantly in model group versus normal group, and the number of pinocytosis increased significantly in electroacupuncture group when compared with model group, and the number of pinocytosis increased drastically after anti-miRNA addition at the same time ().
Figure 2. Effects of electroacupuncture and miR-223-3p toward rats’ endometrial blastocyst implantation ability (A) Observation with Scanning Electron Microscope toward Rats’ Pinopodes of Endometrial Epithelial Cells (B) Effects of electroacupuncture and miR-223-3p on the number of rats’ blastocyst implantation in each group. Data were presented in a manner of mean ± SD (n ≥ 3 experiments). Outcomes of *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 were determined via Student<apos;>s t-test (two groups) or one-way ANOVA, subsequently with Tukey<apos;>s test (more than two groups).
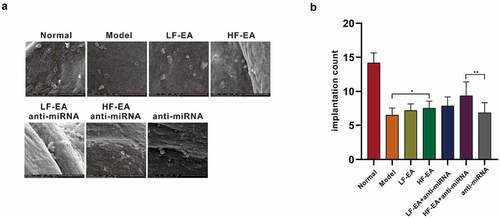
We randomly selected six rats in estrus from each group and reared them in cages at a ratio of 1:1 female to male, and checked in the next morning. Female rats with vaginal plugs were recorded as the first day of conception. The rats were sacrificed on the 8th day after the co-cage, the uterus was taken out, and the uterus on both sides were cut open to count the number of implantation. We found that compared with normal group, the number of implantation of blastocysts in rats’ endometrium in model group was evidently reduced, and the number of implantation of blastocysts in HF-EA group was significantly increased in contrast to model group. At the same time, after anti-miRNA addition, it was found that the number of blastocyst implantation presented significant increase in HF-EA group. In conclusion, electroacupuncture therapy is capable of increasing the number of pinocytoid processes on endometrium and enhancing the implantation capacity of blastocysts by decreasing miRNA-223-3p expression, thus, the endometrium receptivity is elevated ().
3. Electroacupuncture therapy and miRNA-223-3p had effective impacts on the expression of adherence factor
E-cadherin, β-catenin, and tight junction protein CLDN1 play a key role in endometrial glandular epithelial cells, which is adhesion and tight junction. CLDN1, E-cadherin, and β-catenin are located in the cell membrane and in the cytoplasm. They all have a possible dual role. In the early stage, they are expressed on the cell surface to ensure adhesion, and their expression levels are down-regulated in the later stage, allowing epithelial cell dissociation and blastocyst invasion. The decrease in the expression of cell surface adhesion molecules is to reduce the mechanical barrier of blastocyst implantation.
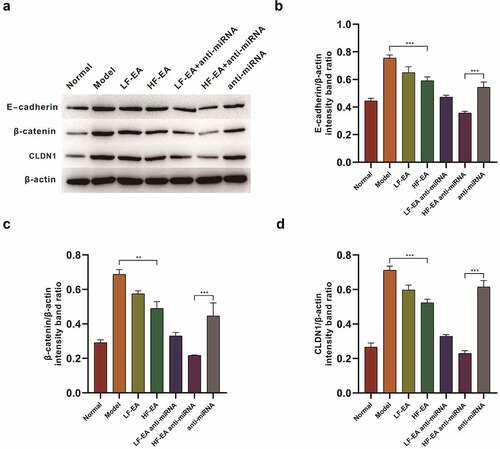
In this section, the effects of electroacupuncture therapy and miR-223-3p on E-cadherin, β-catenin and tight junction protein CLDN1 of rat endometrial cells in each group were investigated. We performed a detection on the expressions of E-cadherin, β-catenin and CLDN1 through Western Blot, and found that the expressions of E-cadherin in model group were obviously increased to normal group. In addition, expression of E-cadherin in LF-EA and HF-EA groups showed a significant decrease after our therapy. After anti-miRNA addition at the same time, it was found that the E-cadherin expression was largely decreased after electroacupuncture therapy; compared with the normal group, β-catenin expression in model group showed a significant upward trend. After electroacupuncture, the expression of β-catenin presented a potent decrease; at the same time, after adding anti-miRNA, it was found that electroacupuncture reduced β-catenin expression prominently. We also found that when compared with normal group, the CLDN1 expression in model group increased significantly. After treatments with LF-EA and HF-EA, CLDN1 expression turned decreased significantly. After electroacupuncture with anti-miRNA addition at the same time, a significant decrease in CLDN1 expression showed up ().
Figure 3. Western bolt assay on the effects of Electroacupuncture and miRNA-223-3p to adhesion factors (A) Expressions of E-cadherin, β-catenin and CLDN1 in rats’ endometrium of each group were measured by Western bolt.
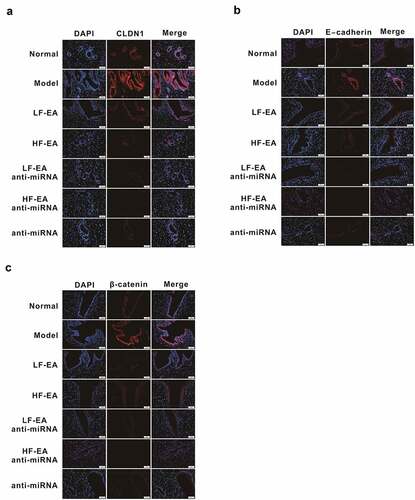
Moreover, we detected the expressions of E-cadherin, β-catenin, and CLDN1 by immunofluorescence assay, confirming that these adhesion molecules located in the cell membrane and cytoplasm. Compared with the normal group; expression of E-cadherin in model group was conspicuously increased, and the expression of E-cadherin in LF-EA and HF-EA groups was notably decreased after the therapy; after the simultaneous addition of anti-miRNA, we found that electroacupuncture therapy further reduced E-cadherin expression effectively (). Compared with the normal group, the expression of β-catenin in model group was significantly increased; the expression of β-catenin was evidently decreased after treatments with LF-EA and HF-EA; then the addition of anti-miRNA in electroacupuncture therapy significantly reduced β-catenin expression (). Compared with normal group, expression of CLDN1 in model group was significantly increased; the expression of CLDN1 in LF-EA and HF-EA groups decreased notably after treatment; the simultaneous addition of anti-miRNA in electroacupuncture therapy greatly reduced CLDN1 expression ().
Figure 4. Effects of Electroacupuncture and miR-223-3p on Adhesion molecules via immunofluorescence assay Immunofluorescence assay on the E-cadherin expression in rats’ endometrium Immunofluorescence assay on the β-catenin expression in rats’ endometrium (C) Immunofluorescence assay on the CLDN1 expression in rats’ endometrium Data were presented in a manner of mean ± SD (n ≥ 3 experiments). Outcomes of *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 were determined via Student<apos;>s t-test (two groups) or one-way ANOVA, subsequently with Tukey<apos;>s test (more than two groups).(B) Protein quantitative map on the E-cadherin expressions in rats’ endometrium of each group (C) Protein quantitative map on the β-catenin expression in rats’ endometrium of each group (D) Protein quantitative map on the CLDN1 expression in rats’ endometrium of each group Data were presented in a manner of mean ± SD (n ≥ 3 experiments). Outcomes of *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 were determined via Student<apos;>s t-test (two groups) or one-way ANOVA, subsequently with Tukey<apos;>s test (more than two groups).
4. Electroacupuncture therapy and miRNA-223-3p impinged on rat<apos;>s LIF/ STAT3 signaling pathway
The leukemia inhibitory factor LIF belongs to the interleukin six families and is a pleiotropic cytokine that maintains a variety of biological activities in different tissues and cells. The leukemia suppressor LIF is a key factor in embryo implantation and participates in the regulation of many aspects of reproductive activities, including follicles, embryo development, embryo implantation and pregnancy maintenance. Its mechanism of action is to bind the receptor LIFR on the target cell membrane and to bind it to the transmembrane glycoprotein GP130 to form a dimer to activate the JAK enzyme and phosphorylate the downstream signal transduction and transcriptional activation factor STAT3 protein. p-STAT3 enters the nucleus to regulate the downstream transcription to complete the signal transduction, so as to achieve its biological role.
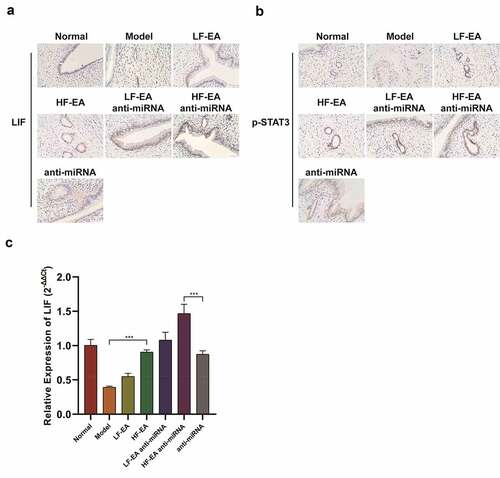
In this section, we were to elucidate the effects of electroacupuncture therapy and miRNA-223-3p to LIF/STAT3 signaling pathway by performing immunohistochemistry and qPCR assay. Our outcomes show that the levels of LIF and p-STAT3 in model group were significantly lower than those in normal group; the levels of LIF and p-STAT3 in low-frequency electroacupuncture group and the high-frequency electroacupuncture group were significantly increased versus the model group. After adding anti-miRNA at the same time, the levels of LIF and p-STAT3 in electroacupuncture group increased significantly (). The results of qPCR assay also showed that the LIF level of model group was greatly lower than the normal group, and the LIF levels in LF-EA and HF-EA groups were significantly higher than that of model group (). After adding anti-miRNA at the same time, the level of LIF in electroacupuncture group presented a significant increase. This indicated that electroacupuncture and miRNA-223-3p regulate the LIF/STAT3 signaling pathway.
Figure 5. Electroacupuncture and miR-223-3p fortified LIF/STAT3 signaling pathwayImmunohistochemical staining on LIF expression in rats’ endometrium Immunohistochemical staining on P-STAT3 expression in rats’ endometrium qPCR assay on LIF expression in rats’ endometrium Data were presented in a manner of mean ± SD (n ≥ 3 experiments). Outcomes of *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 were determined via Student<apos;>s t-test (two groups) or one-way ANOVA, subsequently with Tukey<apos;>s test (more than two groups). Results from real time PCR are expressed as (2−ΔΔCt; arbitrary units) ±SD relative to GAPDH.
5. Effect of miRNA-223-3p on LIF/STAT3 signaling pathway in rat endometrium
We divide the rats into three groups: miRNA-233-3p mimics-NC+Vector, miRNA-233-3p mimics+Vector, and miRNA-233-3p mimics+LIF. Western Blot assay was used to determine level of LIF/STAT3 signaling pathway. The results showed that the levels of LIF, JAK, STAT3 and p-STAT3 in miRNA-233-3p mimics +Vector group were significantly decreased compared with those in mirNA-233-3p mimics-NC +Vector group (). Besides, we used qPCR experiments to verify that miRNA-223-3p is capable of reducing the levels of LIF, JAK, and STAT3. This indicates that miRNA-223-3p is able to reduce the level of LIF/STAT3 signaling pathway in the rat endometrium ().
Figure 6. miR-223-3p inhibits LIF/ STAT3 signaling pathway in rats’ endometriumWestern Blot results of miRNA-223-3p<apos;>s efficacy on LIF/ STAT3 signaling pathway qPCR assay on miRNA-223-3p<apos;>s effect to LIF expression qPCR assay on miRNA-223-3p<apos;>s effect to JAK expression qPCR assay on miRNA-223-3p<apos;>s effect to STAT3 expression (E) miRNA-223-3p<apos;>s efficacy on luciferase activity of LIF Data were presented in a manner of mean ± SD (n ≥ 3 experiments). Outcomes of *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 were determined via Student<apos;>s t-test (two groups) or one-way ANOVA, subsequently with Tukey<apos;>s test (more than two groups). Results from real time PCR are expressed as (2−ΔΔCt; arbitrary units) ±SD relative to GAPDH.
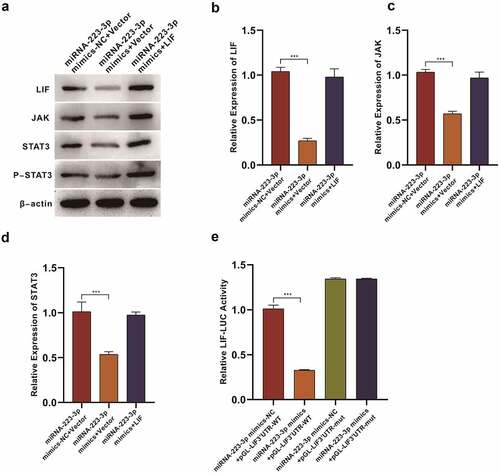
We used dual luciferase activity test to verify the LIF 3’-UTR<apos;>s interaction with miRNA-223-3p. And it turned out that miRNA-233-3p mimics could reduce the activity of luciferase. After the mutation in LIF 3’-UTR sequence was made, the activity of luciferase rose sharply indicating the miRNA-233-3p capacity to bind to the 3’-UTR sequence of LIF ().
Discussion
In vitro fertilization-Embryo transfer (IVF-ET) is an assisted technology of reproduction, whose controlled ovarian hyperstimulation (COH) ensures the number and quality of follicles, but the embryo implantation rate is only 20%-30% [Citation30]. Current studies on the mechanism of embryo implantation have revealed that in addition to the quality of the embryo, decreased endometrial receptivity is also a cause of implantation failure [Citation31]. More and more researchers believed that other than ovarian steroid hormones, a series of other cytokines, transcription factors, and lipid messengers participate in the implantation process through self-secretion [Citation32].
Electroacupuncture has a good clinical effect on low endometrial receptivity [Citation33], but the molecular mechanism of electroacupuncture therapy towards low endometrial receptivity has not been fully studied. Frequency is one of the key parameters of electroacupuncture stimulation. It has been reported that low frequency electroacupuncture improves endometrial receptivity in hyperovulation cycle [Citation34], further, Wei Chen et al. reported that low-frequency electroacupuncture facilitates implantation by activating the VEGFR2/PI3K/AKT and VEGFR2/ERK signaling pathways to enhance endometrial angiogenesis in a rat model of ovarian hyperstimulation [Citation35], but that of high-frequency electroacupuncture to endometrial receptivity in hyperovulation cycle remains unknown.
miRNA-223-3p is an up-stream miRNA of LIF mRNA that is low-expressed in endometrium in pregnant rats, whereas the situation of LIF mRNA is inverse in non-pregnant rats. This fact suggests a relationship within LIF mRNA and miRNA-223-3p in rats’ endometrium. We first conducted our research on electroacupuncture therapy to endometrial miRNA-223-3p in rats during hyperovulatory cycle, and it turned out that the miRNA-223-3p expression in HF-EA group showed significant decreasing in contrast to the model group; besides that, in HF-EA+ anti-miRNA group turned alike when compared with the anti-miRNA group. The expression of miRNA-223-3p in HF-EA group was slightly decreased versus the LF-EA.
Our research on the electroacupuncture therapy on implantation ability of blastocyst in rats showed that when compared with the model group, implantation number of blastocyst in HF-EA group was significantly increased. After simultaneous addition of anti-miRNA, the number of blastocyst implantation in HF-EA group reached a significant increase. All together, we drew a conclusion that electroacupuncture therapy elevated the number of pinocytoid processes in endometrium and fortified the implantation ability of blastocyst by down-regulating miRNA-223-3p expression; moreover, the implantation effect of the HF-EA was better than that of LF-EA group.
Given that adhesion molecules such as E-cadherin, β-catenin and CLDN1 are essential players in embryo transplantation, we instituted our assays towards electroacupuncture therapy and miRNA-223-3p on adhesion factors. And the results showed that the E-cadherin, β-catenin and CLDN1 expressions in hyperovulatory cycle rats’ endometrium of LF-EA and HF-EA groups decreased significantly after electroacupuncture therapy, suggesting that electroacupuncture dissociates epithelial cells and promotes the transplantation capacity of blastocyst via decreasing the expressions of adhesion molecules.
Finally, we extended our study towards electroacupuncture therapy and miRNA-223-3p on LIF/ STAT3 signaling pathway, then immunohistochemistry and qPCR were performed. And it showed that the LIF and p-STAT3 expressions in LF-EA and HF-EA groups were significantly elevated when compared with the model group. After the simultaneous addition with anti-miRNA, both LIF and p-STAT3 expressions in electroacupuncture group were largely up-regulated. These results suggested that electroacupuncture therapy and anti-miRNA-223-3p could enhance the level of LIF/ STAT3 signaling pathway in rat uterus.
At present, the mechanism of low endometrial receptivity in recurrent implantation failure- in vitro fertilization (RIF-IVF) has not been fully elucidated, but it is certain that the factors such as gene and epigenetic modification play an important role in the occurrence and development of RIF-IVF [Citation36].
Epigenetic mechanisms include DNA methylation, histone modification, chromatin remodeling, and non-coding RNA regulation, among which non-coding RNA regulation is one of the most basic epigenetic mechanisms [Citation37,Citation38]. MicroRNAs (miRNAs), a category of non-coding single-stranded endogenous small RNA molecules, they are the ones of high conservation without an open reading frame, made from about 22 nucleotides. They participate in epigenetic regulation in a way of binding to the 3 ‘non-coding region of the target gene through complete and/or partial complementation, or directly shearing target genes’ mRNA for degradation [Citation39–41]. We found that miRNA-233-3p mimics reduced luciferase activity by double-luciferase activity assay, suggesting the interaction amid miRNA-233-3p and the 3’-UTR sequence of LIF. This assay proved that miRNA-233-3p is indeed a regulatory miRNA upstream of LIF.
Conclusions
Our study has proved that the electroacupuncture therapy with different frequencies is capable of elevating the endometrial receptivity of rats, and the efficacy of HF-EA is better than LF-EA. Further, we also found that electroacupuncture therapy and anti-miRNA-223-3p could reduce the expressions of adhesion molecules such as E-cadherin, providing conditions for blastocyst implantation. Finally, we demonstrated that both electroacupuncture therapy and anti-miRNA-223-3p are able to enhance LIF/STAT3 signaling pathway, then our next study will focus on different frequencies of electroacupuncture therapy on other factors of endometrial receptivity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Liu H, Huang X, Mor G, et al. Epigenetic modifications working in the decidualization and endometrial receptivity [J]. Cell Mol Life Sci. 2020;77(11):2091–2101.
- Ashary N, iwari A, Modi D. Embryo implantation: war in times of love [J]. Endocrinology. 2018;159(2):1188–1198.
- Lessey BA, Young SL. What exactly is endometrial receptivity? [J]. Fertil Steril. 2019;111(4):611–617.
- Sahar N, Mujihartini N, Pudjianto DA, et al. Increased progesterone on the day of administration of hCG in controlled ovarian hyperstimulation affects the expression of HOXA10 in primates’ endometrial receptivity [J]. Biomedicines. 2019;7(4):83.
- Haouzi D, Assou S, Dechanet C, et al. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocol effects [J]. Biol Reprod. 2010;82(4):679–686.
- Teh WT, McBain J, Rogers P. What is the contribution of embryo-endometrial asynchrony to implantation failure?[J]. J Assist Reprod Genet. 2016;33(11):1419–1430.
- Zhong Y, Zeng F, Liu W, et al. Acupuncture in improving endometrial receptivity: a systematic review and meta-analysis [J]. BMC Complement Altern Med. 2019;19(1):61.
- von Grothusen C, Lalitkumar S, Boggavarapu NR, et al. Recent advances in understanding endometrial receptivity: molecular basis and clinical applications [J]. Am J Reprod Immunol. 2014;72(2):148–157.
- Miravet-Valenciano JA, Rincon-Bertolin A, Vilella F, et al. Understanding and improving endometrial receptivity [J]. Curr Opin Obstet Gynecol. 2015;27(3):187–192.
- Chi Y, He P, Lei L, et al. Transdermal estrogen gel and oral aspirin combination therapy improves fertility prognosis via the promotion of endometrial receptivity in moderate to severe intrauterine adhesion [J]. Mol Med Rep. 2018;17(5):6337–6344.
- Liu B, Li X, Li C, et al. miR-25 mediates metastasis and epithelial–mesenchymal-transition in human esophageal squamous cell carcinoma via regulation of E-cadherin signaling [J]. Bioengineered. 2019;10(1):679–688.
- Zhou X, Xu B, Zhang D, et al. Loss of CDYL results in suppression of CTNNB1 and decreased endometrial receptivity [J]. Front Cell Dev Biol. 2020;8:105.
- Rahnama F, Thompson B, Steiner M, et al. Epigenetic regulation of E-cadherin controls endometrial receptivity [J]. Endocrinology. 2009;150(3):1466–1472.
- Bellati F, Costanzi F, De Marco MP, et al. Low endometrial beta-catenin and cadherins expression patterns are predictive for primary infertility and recurrent pregnancy loss [J]. Gynecol Endocrinol. 2019;35(8):727–731.
- Alfano R, Youngblood BA, Zhang D, et al. Human leukemia inhibitory factor produced by the ExpressTec method from rice (Oryza sativa L.) is active in human neural stem cells and mouse induced pluripotent stem cells [J]. Bioengineered. 2014;5(3):180–185.
- Shokrzadeh N, Alivand MR, Abedelahi A, et al. Calcitonin administration improves endometrial receptivity via regulation of LIF, Muc-1 and microRNA Let-7a in mice [J]. J Cell Physiol. 2019;234(8):12989–13000.
- Shariati MBH, Niknafs B, Seghinsara AM, et al. Administration of dexamethasone disrupts endometrial receptivity by alteration of expression of miRNA 223, 200a, LIF, Muc1, SGK1, and ENaC via the ERK1/2-mTOR pathway [J]. J Cell Physiol. 2019;234(11):19629–19639.
- Kim EY, Choi HJ, Chung TW, et al. Water-extracted Perilla frutescens increases endometrial receptivity through leukemia inhibitory factor-dependent expression of integrins [J]. J Pharmacol Sci. 2016;131(4):259–266.
- Chung TW, Park MJ, Lee H, et al. Enhancement of endometrial receptivity by cnidium officinale through expressing LIF and integrins [J]. Evid Based Complement Alternat Med. 2019;2019:7560631.
- Klentzeris LD. The role of endometrium in implantation [J]. Hum Reprod. 1997;12(11 Suppl):170–175.
- Hesam Shariati MB, Seghinsara AM, Shokrzadeh N, et al. The effect of fludrocortisone on the uterine receptivity partially mediated by ERK1/2-mTOR pathway [J]. J Cell Physiol. 2019;234(11):20098–20110.
- Dong X, Sui C, Huang K, et al. MicroRNA-223-3p suppresses leukemia inhibitory factor expression and pinopodes formation during embryo implantation in mice [J]. Am J Transl Res. 2016;8(2):1155–1163.
- Shuai Z, Lian F, Li P, et al. Effect of transcutaneous electrical acupuncture point stimulation on endometrial receptivity in women undergoing frozen-thawed embryo transfer: a single-blind prospective randomised controlled trial [J]. Acupuncture Med. 2015;33(1):9–15.
- Yu N, Yan W, Wang Y, et al. Effect of Zhuyun recipe on endometrial pinopode expression in mice with embryonic implantation dysfunction and ovulation stimulation [J]. Exp Ther Med. 2015;9(2):488–492.
- Sm P, Ss P, Rs P, et al. Electron microscopy of human endometrium during window of implantation [J]. Journal of Cytology & Histology. 2018;9(4).
- Im K, Mareninov S, Diaz MFP, et al. An introduction to performing immunofluorescence staining [J]. Methods Mol Biol. 2019;1897:299–311.
- Yang H, Wang S, Kang Y-J, et al. Long non-coding RNA SNHG1 predicts a poor prognosis and promotes colon cancer tumorigenesis [J]. Oncol Rep. 2018;1(40):261–271.
- Wu F, Chen X, Liu Y, et al. Decreased MUC1 in endometrium is an independent receptivity marker in recurrent implantation failure during implantation window [J]. Reprod Biol Endocrinol. 2018;16(1):60.
- Clément T, Salone V, Rederstorff M. Dual luciferase gene reporter assays to study miRNA function [J]. Methods Mol Biol. 2015;1296:187–198.
- Nyboe Andersen A, Goossens V, Bhattacharya S, et al. Assisted reproductive technology and intrauterine inseminations in Europe, 2005: results generated from European registers by ESHRE: ESHRE. The European IVF Monitoring Programme (EIM), for the European Society of Human Reproduction and Embryology (ESHRE) [J]. Hum Reprod. 2009;24(6):1267–1287.
- Ruiz-Alonso M, Blesa D, Díaz-Gimeno P, et al. The endometrial receptivity array for diagnosis and personalized embryo transfer as a treatment for patients with repeated implantation failure [J]. Fertil Steril. 2013;100(3):818–824.
- Fox C, Morin S, Jeong JW, et al. Local and systemic factors and implantation: what is the evidence? [J]. Fertil Steril. 2016;105(4):873–884.
- Shuai Z, Lian F, Li P, et al. Effect of transcutaneous electrical acupuncture point stimulation on endometrial receptivity in women undergoing frozen-thawed embryo transfer: a single-blind prospective randomised controlled trial [J]. Acupunct Med. 2015;33(1):9–15.
- Xi J, Cheng J, Jin CC, et al. Electroacupuncture improves pregnancy outcomes in rats with thin endometrium by promoting the expression of pinopode-related molecules [J]. Biomed Res Int. 2021;2021:6658321.
- Chen W, Chen J, Xu M, et al. Electroacupuncture facilitates implantation by enhancing endometrial angiogenesis in a rat model of ovarian hyperstimulation† [J]. Biol Reprod. 2019;100(1):268–280.
- Pei CZ, Kim YJ, Baek KH. Pathogenetic factors involved in recurrent pregnancy loss from multiple aspects [J]. Obstet Gynecol Sci. 2019;62(4):212–223.
- Choi Y, Kim HR, Lim EJ, et al. Integrative analyses of uterine transcriptome and MicroRNAome reveal compromised LIF-STAT3 signaling and progesterone response in the endometrium of patients with recurrent/repeated implantation failure (RIF) [J]. PLoS One. 2016;11(6):e0157696.
- Chu B, Zhong L, Dou S, et al. miRNA-181 regulates embryo implantation in mice through targeting leukemia inhibitory factor [J]. J Mol Cell Biol. 2015;7(1):12–22.
- Wu M, Wang G, Tian W, et al. MiRNA-based Therapeutics for Lung Cancer [J]. Curr Pharm Des. 2018;23(39):5989–5996.
- Paul ABM, Sadek ST, Mahesan AM. The role of microRNAs in human embryo implantation: a review [J]. J Assist Reprod Genet. 2019;36(2):179–187.
- Yao Q, Chen Y, Zhou X. The roles of microRNAs in epigenetic regulation [J]. Curr Opin Chem Biol. 2019;51:11–17.
