ABSTRACT
Development of ultra-high strength and corrosion-resistant aluminum (Al) alloys is demonstrated by a combination of suitable alloying elements and processing technology able to cause extended solid solubility and nanocrystalline structure. Binary Al-transition metal (M: Cr, Ni, Mo, Si, Ti, Mn, V, Nb) alloys, produced by high-energy ball milling and subsequent cold compaction, have exhibited significantly high hardness and corrosion resistance compared to any commercial Al alloy. The cyclic potentiodynamic polarization tests revealed a significant improvement in pitting and repassivation potentials. X-ray diffraction analysis revealed the grain refinement <100 nm and extended solid solubility.
GRAPHICAL ABSTRACT
IMPACT STATEMENT
High-energy ball-milled Al alloys, owing to excellent corrosion resistance and high hardness, are expected to be a new class of Al alloys and initiate a multidisciplinary research direction.
Pure Al exhibits a good corrosion resistance but poor mechanical properties and therefore is alloyed with other elements to increase strength [Citation1]. Solid solution strengthening, precipitation strengthening, grain refinement, and work hardening are the main strengthening mechanisms for Al alloys [Citation1]. However, precipitation and work hardening introduce electrochemical heterogeneities which cause localized corrosion. Therefore, a tradeoff between mechanical and corrosion properties exists in Al alloys [Citation1–3]. Characteristics of the matrix and secondary phases (i.e. composition, number, morphology, and distribution) play a vital role in determining the corrosion performance [Citation4,Citation5]. Moreover, the maximum achievable yield strength of Al alloys is currently limited to ∼560 MPa [Citation1].
While precipitation and work hardening introduce electrochemical heterogeneities and cause localized corrosion, solid solution strengthening does not deteriorate the corrosion performance necessarily; rather, suitable alloying elements may increase the corrosion resistance of Al alloys. We hypothesize- if a significant amount of suitable alloying element (e.g. Cr, V, Mo, Nb, and Ti) could be dissolved in Al, the corrosion resistance and strength could be improved simultaneously. However, since solubility of most alloying elements in Al is extremely limited, formation of secondary phases which cause localized corrosion [Citation3,Citation6] is inevitable through conventional processing methods. For example, Al-5at.%Cr and Al-5at.%Ti, produced by casting, showed the presence of coarse intermetallics and high corrosion rate without a significant increase in strength [Citation7]. Therefore, non-conventional alloy production methods capable of increasing the solid solubility of alloying elements are desirable. Pulse electrodeposition [Citation8,Citation9], sputter deposition [Citation10–13], high-energy ball milling (HEBM), and rapid solidification techniques [Citation14–16] are some of the examples of non-conventional alloy production methods. Sputter deposition has been reported to cause a significant increase in the solid solubility and therefore increased corrosion resistance [Citation11–13,Citation17–19]. For instance, the pitting potential (Epit) of sputter-deposited Al-6at.%Mo alloy in 0.1 M KCl was ∼400 mV nobler than that of pure Al [Citation20]. Several alloying elements, e.g. Cr [Citation21], Ta [Citation22], Zr [Citation22], Ti [Citation23], Nb [Citation23], V [Citation23], and W [Citation12], have been reported to improve/ennoble Epit. However, the sputter-deposited alloys were synthesized in the form of thin films, their mechanical properties were not well investigated, and up-scaling the production for bulk alloy production is challenging. Literature suggests that HEBM is also capable of producing alloys with extended solid solubility, nanocrystalline structure, and high strength [Citation24–27]. For instance, solid solubility of Fe in Al was reported to increase to 4.5 wt.% (from ) due to HEBM [Citation28].
The authors’ previous work has shown that ball-milled Al–Cr alloys, owing to the extended solid solubility of Cr in and Al grain refinement <100 nm, exhibited ultra-high strength and corrosion resistance [Citation26,Citation29]. Similar to Cr, several other alloying elements (M: Mo, V, Cr, Ti, Mn, Si, Nb), owing to improved passivation, are also deemed capable of increasing corrosion resistance and mechanical properties when produced by HEBM. Therefore, studying the properties of ball-milled Al alloys is of great merit. The corrosion resistance and hardness of the Al–M (M: Mo, V, Cr, Ti, Mn, Nb) alloys presented in this paper are superior to any commercial Al alloy. This work also presents a comparison of the effectiveness of various alloying elements in improving the corrosion performance and hardness of HEBM Al alloys.
Al-5at.%M alloys were synthesized via HEBM followed by cold compaction. Aluminum powder (purity 99.7%, size −50/+100 mesh) and the alloying elements powder (purity <99.8%, size −100 mesh) were loaded in hardened steel jars with hardened steel balls (10 mm diameter). Stearic acid (1.5 wt.%) was used as a process-controlling agent. Steel jars were loaded and sealed in a glove box (high-purity Ar atmosphere, O2 < 25 ppm) to maintain an inert atmosphere. HEBM was performed in a planetary mill at a speed of 280 RPM for 100 h. The milling was interrupted for 30 min after every one hour of milling. The HEBM powder was consolidated using an auto pellet press in a tungsten carbide die under uniaxial pressure. The load was progressively increased in 16 steps of 187 MPa for 15 s until reaching a final pressure of 3 GPa, which was held for 5 min. The cold compacted test specimen was of 7 mm diameter and 2.5 mm thickness. Commercial alloys (AA7075-T651, AA5083-H32, AA-6063-T6, and AA2024-T3l) were used for comparing the properties as needed.
Powder X-ray diffraction (XRD) analysis was performed in the 2-θ range of 37.5–39.5 degrees using a Cu K-alpha radiation. This region was chosen for detailed analysis of the peak corresponding to the (111) plane. The scanning speed was 0.167 deg/min with a step size of 0.0012 degrees. The grain size was calculated using Scherrer equation after subtracting the instrumental broadening [Citation30]. The procedure for calculation of the solid solubility and grain size is described in the supplementary appendix (A1). The XRD analysis was also performed in 2-θ range of 10–80 degrees with a scanning speed of 1 degree/minute and a step size of 0.02 degrees to observe the formation of any intermetallic or unalloyed elements (Figure S1).
Vickers hardness was measured under the applied load of 25 g and dwelling time of 10 s. For each alloy, a total of 10 tests were performed and the average value was reported.
Corrosion behavior of the produced alloys and four commercial alloys (AA7075-T651, AA5083-H32, AA-6063-T6, and AA2024-T3l) was studied by cyclic potentiodynamic polarization (CPP) using a VMP-300 potentiostat. All samples were polished to 1200 grit SiC sandpaper followed by rinsing with copious amounts of distilled water, ethanol and drying. CPP tests were carried out in a conventional three-electrode electrochemical cell, using a platinum mesh as a counter electrode and a saturated calomel electrode (SCE) as a reference electrode. All tests were performed in 0.01 M NaCl. The open circuit potential (OCP) of the samples was monitored for 20 min before commencing the CPP tests. Potential scans started from −250 mV vs. OCP, and upwards with a scan rate of 1 mV/s until an anodic current of 200 µA/cm2 was reached followed by a reverse scan to −250 mV vs. OCP. CPP tests were performed at least five times. The most representative CPP curve for each alloy tested is shown in Figure S2. The pitting potential (Epit) and transition potential (Etrans) were determined from CPP as described in [Citation31–33] (supplementary appendix A2).
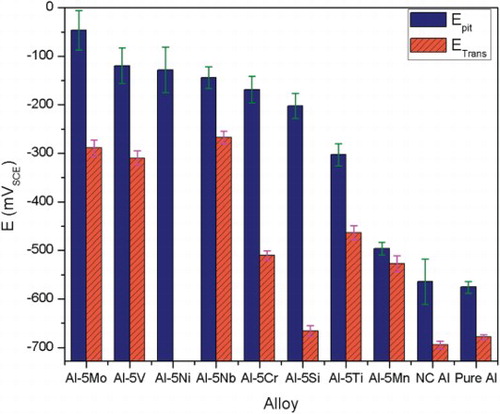
The Epit and Etrans for all the produced alloys along with that of pure Al are presented in . Higher Epit indicates higher pitting corrosion resistance. Etrans indicates repassivation capability in case of passive film breakdown [Citation3,Citation17]. Epit of the HEBM alloys was significantly nobler than that of pure Al and any commercial Al alloy. It should be noted that Epit of most of the commercial alloys is lower than that of pure Al [Citation3]. Epit for Al-5at.%Mn alloys was 77.8 mV higher than that of pure Al. Mn was the least effective in increasing the Epit (). Al-5at.%Mo alloy exhibited most noble Epit; 527.6 mV higher than that for pure Al. Etrans for all the alloys, except Al-5at.%Si and Al-5at.%Ni, was also significantly higher than that of pure Al. Etrans for Al-5at.%Si was 464 mV below its Epit. Al-5at.%Ni had no observable transition potential, indicating poor repassivation behavior. Influence of the alloying elements in ennobling the Epit was in the order of Mo > V > Ni > Nb > Cr > Si > Ti > Mn, whereas the effectiveness of the alloying elements in ennobling Etrans was in the order of Nb > Mo > V > Ti > Cr > Mn > Si > Ni. Considering ennoblement of Epit and Etrans Mo, Nb and V were deemed most effective elements in improving the pitting corrosion resistance. Excellent corrosion behavior of the HEBM alloys was further confirmed by the visual inspections and surface analysis after constant immersion tests in 0.01 M NaCl for 180 days. The average pit depth in the HEBM alloys was significantly lower (∼an order of magnitude) than that for commercial alloys (supplementary appendix A3, Figures S3 and S4).
Figure 1. Epit and Etrans for the HEBM Al-5at.%M alloys. ‘NC Al’ is the HEBM Al and ‘Pure Al’ is the coarse grain pure Al.
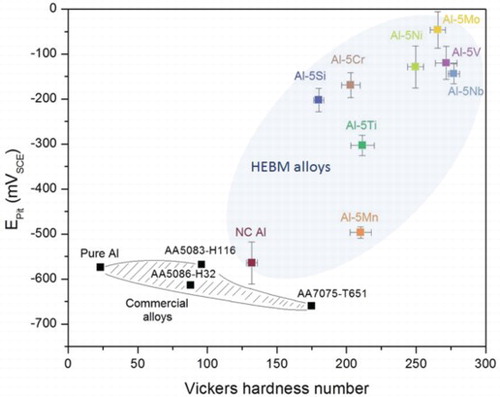
The hardness of the alloys produced herein was significantly higher than many commercial Al alloys. The effectiveness of the alloying elements in increasing the hardness was Nb > V > Mo > Ni > Ti > Mn > Cr >Si. shows the Epit (representative of corrosion resistance) vs. Vicker’s hardness (representative of strength) plot for HEBM alloys. The dashed region in the plot shows a commonly noticed relationship between hardness and corrosion performance in commercial alloys, i.e. a decrease in corrosion resistance with increasing strength. Contrary to commercial alloys, HEBM alloys produced in this study exhibited significantly higher pitting potential and hardness. Mo, Nb, and V appear to be most effective in improving the corrosion performance and hardness simultaneously.
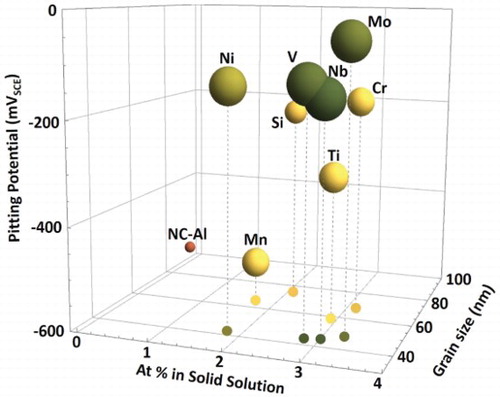
Excellent corrosion behavior and hardness of the produced alloys () are attributed to the concurrent influence of grain boundary and solid solution strengthening. The grain size and solid solubility, determined from XRD analysis, are presented on the X–Y plane in . The Epit is indicated on the Z axis and the size of the sphere indicating each alloy scales with the strength (larger radius is higher hardness). The grain size of all the alloys was <100 nm and dependent upon the alloying element. Alloying with Ni was most effective in refining the grain size (32 nm) followed by V, Nb, Mo, Ti, Mn, Cr, and Si. All of the alloying elements showed high solid solubility—significantly higher than the thermodynamically predicated values. clearly shows that Mo had the highest solid solubility in Al followed by Nb, Cr, V, Ti, Si, Ni, and Mn. However, none of the alloying elements were completely soluble at 5%. XRD scans (Figure S1) revealed peaks corresponding to the unalloyed alloying elements and intermetallics which indicated that part of the added alloying element was also present as unalloyed element and intermetallic. XRD analysis was supported by SEM/EDXS, indicating refined secondary phases and increased solid solubility of the alloying elements (supplementary appendix A4, Figure S5).
Figure 3. Influence of the alloying element (M) on pitting potential, grain size, and solid solubility in HEBM Al–M alloys.
HEBM caused remarkable grain refinement and solubility of alloying elements (), which are deemed to be the main contributors to improved corrosion and mechanical properties. Estimates of the relative contributions of grain refinement, solid solution strengthening, and dispersion strengthening are made in supplementary appendix (A6). The grain boundary contribution was in excess of half the measured strength in all alloys (∼57–67%) and solid solution strengthening contributed anywhere from 12% to 25%. Based on the limited information available on precipitate size and distribution available from the SEM images (Figure S5), the Orowan strengthening is a maximum contribution of ∼2.5%; further TEM analysis will likely reveal more, finer second phases and may slightly increase this contribution. However, even if all the remaining unallocated components to the measured hardness were due to these possible finer precipitates, the contribution of second phases to the strength would still be on the order of solid solution strengthening and far less than grain boundary strengthening. This tracks with other work, for instance, the contribution of Orowan strengthening in a ball milled Al-20at.%Cr alloy was estimated to be only 6.9% of the total strength [Citation26]. It should be noted that the influence of each alloying element is complex and a detailed study will be required to understand the efficiency of an individual alloying element in improving properties, grain refinement, and solid solubility.
It is interesting to notice that HEBM did not cause a significant ennoblement in Epit and Etrans of pure Al () which clearly indicated the prominent role of the chemical composition and processing technology over grain refinement in improving the corrosion resistance. It should be noted that there is no consensus on the role of grain refinement on the corrosion behavior and the present study supports our previous view that the processing method and chemical composition play a critical role in determining the corrosion behavior [Citation34,Citation35]. Improvement in corrosion behavior observed in the Al-5at.%M alloys in this study should be attributed to the increased solid solubility of the alloying elements () because of one or more of the following mechanisms:
Enrichment of the passive film with the alloying elements and therefore improved passivity and decreased passive film breakdown [Citation22,Citation36].
Increased repassivation tendency causing high corrosion resistance. It should be noted that the pit growth depends upon the formation of the critical pit solution [Citation3]. Pit growth stops and repassivation occurs in the absence of the critical pit solution. Hydrolysis of metal ions (e.g. Al3+ and Cr3+) causes acidification and leads to critical pit solution. However, some of the metal ions possess smaller hydrolysis constants and therefore do not facilitate critical pit solution formation [Citation37]. Furthermore, particular alloying elements (e.g. V, Mo, and Nb) may reduce the rate of active dissolution occurring within pits, depleting the availability of metal cations to hydrolyze, therefore, decreasing the likelihood of critical pit solution formation [Citation23,Citation38].
Release of the oxyanions (i.e. molybdate and vanadate) able to inhibit corrosion in initiation stages. The presence of some of the alloying elements is expected to form corrosion products (i.e. molybdate, vanadate, and chromate) that are well-known inhibitors [Citation39].
The corrosion behavior of Al alloys depends upon the nature of secondary phases and their interaction with the matrix [Citation3]. Formation of the super sutured solid solution is expected to decrease the difference in the electrochemical potential of the matrix and intermetallics which should moderate the deleterious influence of the intermetallics (supplementary appendix A5, Figure S6).
In summary, Al-5at.%M alloys (M: Mo, Nb, V, Ni, Cr, Ti, Si, Mn), exhibiting extended solid solubility of the alloying elements and grain size <100 nm, were successfully produced by HEBM and subsequent compaction. Ni was found most effective in refining the grain size. Hardness and corrosion resistance (represented by pitting potential and repassivation tendency) of the high-energy ball-milled Al-5at.%M alloys were significantly higher than any commercial Al alloy. Mo, V, and Nb were most effective in increasing corrosion resistance and hardness simultaneously.
Supplement_Material
Download MS Word (61.2 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
R. K. Gupta http://orcid.org/0000-0003-2684-1994
References
- Polmear I, John DS. Light alloys: from traditional alloys to nanocrystals. Oxford: Butterworth-Heinemann; 2005.
- Davis JR, editor. ASM speciality book: aluminum and aluminum alloys. Materials Park: ASM International; 1993.
- Davis JR. Corrosion of aluminum and aluminum alloys. ASM International; 1999.
- Ralston KD, Birbilis N, Weyland M, et al. The effect of precipitate size on the yield strength-pitting corrosion correlation in Al-Cu-Mg alloys. Acta Mater. 2010;58:5941–5948. doi: 10.1016/j.actamat.2010.07.010
- Gupta RK, Deschamps A, Cavanaugh MK, et al. Relating the early evolution of microstructure with the electrochemical response and mechanical performance of a Cu-rich and Cu-lean 7xxx aluminum alloy. J Electrochem Soc. 2012;159:C492–C502. doi: 10.1149/2.062211jes
- Lifka B. Corrosion of aluminum and aluminum alloys. In: Schweitzer P, editor. Corros. Eng. Handb. Schweitzer: Marcel Dekker; 1996. p. 99–155.
- Esquivel J, Gupta RK. Corrosion behavior and hardness of Al-M (M: Mo, Si, Ti, Cr) alloys. Acta Metall Sin (English Letters). 2017;30:333–341. doi: 10.1007/s40195-017-0550-2
- Tsuda T, Hussey CL, Stafford GR. Electrodeposition of Al-Mo alloys from the Lewis acidic aluminum chloride-1-ethyl-3-methylimidazolium chloride molten salt. J Electrochem Soc. 2004;151:C379–C384. doi: 10.1149/1.1704611
- Tsuda T, Hussey CL. Electrochemistry of vanadium (II) and the electrodeposition of aluminum-vanadium alloys in the aluminum chloride-1-ethyl-3-methylimidazolium chloride molten salt. J Min Metall Sect B Metall. 2003;39:3–22. doi: 10.2298/JMMB0302003T
- Sanchette F, Billard A. Main features of magnetron sputtered aluminium–transition metal alloy coatings. Surf Coatings Technol. 2001;142-144:218–224. doi: 10.1016/S0257-8972(01)01197-5
- Moshier WC, Davis GD, Ahearn JS, et al. Corrosion behavior of aluminum-molybdenum alloys in chloride solutions. 1986;134:2677–2684.
- Shaw BA, Davis GD, Fritz TL, et al. The influence of tungsten alloying additions on the passivity of aluminum. J Electrochem Soc. 1991;138:3288–3295.
- Frankel GS. Pitting of sputtered aluminum alloy thin films. J Electrochem Soc. 1989;136:1243–1244.
- Polesya AF, Stepina AI. Formation of supersaturated ternary solid solutions from melts cooled at high rates. PHYS Met Met. 1969;27:122–126.
- Shechtman D, Schaefer RJ, Biancaniello FS. Precipitation in rapidly solidified Al–Mn alloys. Metall Trans A. 1984;15:1987–1997.
- Tonejc A, Ročák D, Bonefačić A. Mechanical and structural properties of Al–Ni alloys rapidly quenched from the melt. Acta Metall. 1971;19:311–316. doi: 10.1016/0001-6160(71)90097-6
- Natishan PM, McCafferty E, Hubler GK. The corrosion behavior of Mo–Al, Cr–Al and Cr–Mo–Al surface alloys produced by ion beam mixing and ion implantation. Corros Sci. 1991;32:721–731.
- Sanchette F, Billard A, Frantz C. Mechanically reinforced and corrosion-resistant sputtered amorphous aluminium alloy coatings. Surf Coatings Technol. 1998;98:1162–1168. doi: 10.1016/S0257-8972(97)00231-4
- Goldman ME, Ünlü N, Shiflet GJ, et al. Selected corrosion properties of a novel amorphous Al–Co–Ce alloy system. Electrochem Solid-State Lett. 2005;8:B1–B5. doi: 10.1149/1.1848261
- Moshier WC, Davis GD, Ahearn JS, et al. Influence of molybdenum on the pitting corrosion of aluminum films. J Electrochem Soc. 1986;133:1063–1064. doi: 10.1149/1.2108709
- Moshier WC, Davis GD, Cote GO. Surface chemistry of sputter-deposited Al–Mo and Al–Cr alloys polarized in 0.1 N KCl. J Electrochem Soc. 1989;136:356–362. doi: 10.1149/1.2096635
- Davis GD, Moshier WC, Fritz TL, et al. Evolution of the chemistry of passive films of sputter-deposited, supersaturated Al alloys. J Electrochem Soc. 1990;137:422–427.
- Frankel GS, NewMan RC, Jahnes CV, et al. On the pitting resistance of sputter-deposited aluminum alloys. J Electrochem Soc. 1993;140:2192–2197. doi: 10.1149/1.2220794
- Suryanarayana C. Mechanical alloying and milling. Prog Mater Sci. 2001;46:1–184. doi: 10.1016/S0079-6425(99)00010-9
- Suryanarayana C, Ivanov E, Boldyrev V. The science and technology of mechanical alloying. Mater Sci Eng A. 2001;304-306:151–158. doi: 10.1016/S0921-5093(00)01465-9
- Gupta RK, Fabijanic D, Dorin T, et al. Simultaneous improvement in the strength and corrosion resistance of Al via high-energy ball milling and Cr alloying. Mater Des 2015;84:270–276. doi: 10.1016/j.matdes.2015.06.120
- Darling KA, Roberts AJ, Armstrong L, et al. Influence of Mn solute content on grain size reduction and improved strength in mechanically alloyed Al–Mn alloys. Mater Sci Eng A. 2014;589:57–65. doi: 10.1016/j.msea.2013.09.047
- Mukhopadhyay DK, Suryanarayana C, Froes FHS. Structural evolution in mechanically alloyed Al–Fe powders. Metall Mater Trans A. 1995;26:1939–1946.
- Gupta RK, Fabijanic D, Zhang R, et al. Corrosion behaviour and hardness of in situ consolidated nanostructured Al and Al–Cr alloys produced via high-energy ball milling. Corros Sci. 2015;98:643–650. doi: 10.1016/j.corsci.2015.06.011
- Patterson AL. The Scherrer formula for X-ray particle size determination. Phys Rev. 1939;56:978–982.
- Pride ST, Scully JR, Hudson JL. Metastable pitting of aluminum and criteria for the transition to stable pit growth. J Electrochem Soc. 1994;141:3028–3040. doi: 10.1149/1.2059275
- Yasuda M, Weinberg F, Tromans D. Pitting corrosion of Al and Al–Cu single crystals. J Electrochem Soc. 1990;137:3708–3715. doi: 10.1149/1.2086291
- Trueba M, Trasatti SP. Study of Al alloy corrosion in neutral NaCl by the pitting scan technique. Mater Chem Phys. 2010;121:523–533. doi: 10.1016/j.matchemphys.2010.02.022
- Frankel GS, Chen X-B, Gupta RK, et al. Effect of vacuum system base pressure on corrosion resistance of sputtered Al thin films. J Electrochem Soc. 2014;161:C195–C200. doi: 10.1149/2.056404jes
- Gupta RK, Birbilis N. The influence of nanocrystalline structure and processing route on corrosion of stainless steel: a review. Corros Sci. 2015;92:1–15. doi: 10.1016/j.corsci.2014.11.041
- Davis GD, Moshier WC, Long GG, et al. Passive film structure of supersaturated Al–Mo alloys. J Electrochem Soc. 1991;138:3194–3199. doi: 10.1149/1.2085392
- Martell AE. Stability constants, Pt. 2, Inorganic ligands, revised from Bjerrum J, Schwarzenbach G, Sillen LG. 1958. London: Chem. Soc. London Spec. Pub. 1964; 7.
- Frankel GS, Li T, Scully JR. Perspective—localized corrosion: passive film breakdown vs pit growth stability. J Electrochem Soc. 2017;164:C180–C181. doi: 10.1149/2.1381704jes
- Jakab MA, Scully JR. On-demand release of corrosion-inhibiting ions from amorphous Al–Co–Ce alloys. Nat Mater. 2005;4:667–670. doi: 10.1038/nmat1451