?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Neutron flux measurement has a critical role in reactor safety. A potential novel sensor development is through the use of fissionable and fissile isotopes doped into yttrium aluminum garnets (e.g. U:YAG). To develop this sensor, the material must be fabricated to allow for the emission of photons upon excitation. Due to the complex processes required for the fabrication, a review that is focused on solid-state reaction, co-precipitation, and pressureless sintering is provided. This review aims at revealing the relationship between processing parameters and their effects on resultant microstructure and light transmittance to yield the optimal processing for the material.
IMPACT STATEMENT
The paper is significant at providing insights on the fabrication of transparent polycrystalline doped YAG and accelerate the development of the advanced neutron flux sensor.
1. Introduction
Measuring neutron flux over time in nuclear experiment reactors, especially transient reactors, presents a difficult challenge. This is because power burst occurs over a short period and reactor power needs to be coupled to the experiment phenomenon with high precision. Similarly, the emergence of autonomous small or micro reactor systems will require accurate active power measurement. Moreover, the neutron flux sensor instrumentation needs to be small due to the compact reactor core size as well as robust in extreme environments. Current neutron flux sensors do not meet these requirements. To develop the desired neutron flux sensor, the sensor material is critical.
Fission energy produced from fissionable (e.g. U-238) and fissile (e.g. U-235) isotopes is suspected to provide sufficient energy to cause luminescence in a host material. The intensity of luminescence may be correlated to the neutron flux, therefore providing a method of measuring reactor power with a rapid response time. Yttrium aluminum garnet (YAG) is one such host material that can accommodate the fissionable/fissile isotopes into its crystal lattice. YAG has been used widely as a gain material for solid-state lasers in the form of neodymium (Nd) doped YAG (Nd:YAG) as well as a scintillator in the form of cerium (Ce) doped YAG (Ce:YAG). The Nd:YAG crystals are used in medicine, dentistry, manufacturing, and defense [Citation1]. The Ce:YAG crystals are used as a highly effective gamma-ray scintillator [Citation2]. The quantum properties of YAG allow light emission of specific wavelengths when energy is provided to the material. The high thermal stability, chemical stability, and mechanical strength of YAG, make the material an ideal candidate for neutron flux sensor in extreme environments. Therefore, fissionable or fissile isotope doped YAG (mainly U:YAG) is a promising candidate scintillator material to measure neutron flux over time in extreme environments.
The YAG or doped YAG has been successfully fabricated in both single crystal and polycrystalline forms. Single crystal is generally made by the crystal growth method, which takes weeks to months and the allowable concentration of dopant in the crystal is limited. While a higher concentration of dopant is desired as it extends the scintillator’s life. Conversely to the crystal growth method, the synthesis and processing method can make transparent polycrystalline YAG or doped YAG in a short period of time and allows a higher concentration of dopant. Transparent polycrystalline exhibits same light transmittance as single crystal, and thus has many advantages over single crystal, including reduced production cost and time. Moreover, a higher concentration of dopant can be added to the polycrystalline form than the single crystalline form.
Fabrication of transparent polycrystalline U:YAG is critical to support the development of advanced neutron flux sensor, however, the fabrication methods are fairly unexplored. The first report of successful fabrication of U:YAG powder is from Zhang et al. [Citation3], for which the co-precipitation method was used. The same group also sintered transparent U:YAG using co-precipitation and vacuum sintering methods [Citation4]. Gong et al. [Citation5] sintered transparent U:YAG using solid-state reaction (SSR) and vacuum sintering methods. Although literature resource of U:YAG is rare, fabrication of transparent polycrystalline YAG has a long history, and the experience is useful for the development of U:YAG. Fabrication of transparent polycrystalline Nd:YAG with comparable quality to that of single crystal Nd:YAG was first reported in 1995 [Citation6]. Since this initial report, there have been a significant number of publications regarding the fabrication of doped YAG through various methods. From these reports, it is known that YAG can be doped with a variety of lanthanide series elements (Nd, Yb [ytterbium], Er [europium], and Ce) [Citation7].
The fabrication of transparent polycrystalline U:YAG is a complex process. Microstructure and light transmittance properties are affected by each processing step, from powder synthesis to sintering. Each of these steps has multiple parameters which must be optimized. To find the optimal processing parameters for U:YAG, this work reviews the techniques and methods that have been used to fabricate transparent polycrystalline Nd:YAG, Ce:YAG and others, with a focus of analyzing the difference in results obtained by method variations, and suggesting the appropriate methods and parameters to fabricate transparent polycrystalline U:YAG. The review would provide insights on the fabrication of transparent polycrystalline U:YAG and accelerate the development of the advanced neutron flux sensor.
2. Transparent ceramic vs. single crystal
Single crystals are formed through crystal growth processes which require extended periods at high temperatures. Crystal growth has numerous difficulties associated with the process, including high temperature, slow growth rates, non-uniform composition, brittle products, and extensive optimization of growth parameters [Citation8]. Consequently, General Electric was the first to develop transparent polycrystalline ceramic fabrication methods through sintering of powders to high-density [Citation9]. Sintering powders to form solid structures is a well-established technology that has been employed for thousands of years to fabricate various ceramic materials. However, the ability to sinter ceramics to a sufficient density for light transmittance is relatively recent. When highly optimized, the polycrystalline synthesis method has many advantages over the crystal growth process, primarily, reduced time and cost, increased mechanical strength, production scalability, and complex shape formation. As an example, the traditional method for producing single crystalline Nd:YAG material uses the Czochralski crystal growth process. Fabrication of the single crystal Nd:YAG requires about one month (the growth rate of Nd:YAG is approximately 0.2–0.5 mm/h) at a temperature of 1970°C [Citation8,Citation10]. Due to the difference in ionic radius of Nd compared to Y, the segregation coefficient of Nd in YAG is approximately 0.2. This restricts the maximum concentration of Nd that can be doped into YAG single crystal to approximately 1–1.5 at. % [Citation8,Citation10]. An additional complication of the Czochralski crystal growth process is the extensive post-processing as the grown crystal has an amorphous shape.
In comparison, transparent polycrystalline YAG ceramics can be produced in a few days with increased dopant concentration and in controlled geometries. The procedure follows three general steps: powder preparation, green body formation, and sintering. The exact parameters of the sintering procedure are highly dependent on the powder preparation and sintering method. In general, the sintering profile for YAG with sintering aids in a vacuum furnace requires a temperature of 1750°C for 8 h to achieve transparency. Other sintering methods, such as spark plasma sintering (SPS), hot isostatic press (HIP), and two-step sintering (TSS), can often reduce the sintering time required. As mentioned before, higher dopants can be achieved by the sintering method than crystal growth method because a solid–liquid interface which promotes segregation is not present during the process. A dopant concentration of 9 at. % in the Nd:YAG still remains transparent [Citation11]. This concentration is significantly higher than the ∼1 at. % maximum of crystal growth method.
Producing transparent polycrystalline ceramics is challenging because the elimination of defects is critical to prevent light scattering. A theoretical model for the scattering of light in translucent alumina (Al2O3) by Apetz and van Bruggen [Citation12] provides a framework to understand the impact of defects on transparency. The transmission of light through a material is affected by four phenomena: intrinsic scattering between grain boundaries (birefringence), surface scattering from nonuniform surfaces, scattering due to pores in microstructure, and light absorption due to secondary phases. The intrinsic scattering of the sample can be mitigated by selecting a material that has an optically isotropic crystalline structure. A cubic lattice does not experience light scattering due to birefringence because the crystallographic orientation of grains does not affect the index of refraction. Surface effects can severely reduce transmittance as the incoming light is scattered prior to entering the material. This effect can be eliminated by polishing the material to a high degree. Secondary phases contained in the microstructure can result in scattering or absorption. These phases can form due to contamination, improper stochiometric ratios, and additives. The use of additives is of high importance during fabrication as they can often aid in eliminating pores, preventing grain growth, and reducing sintering temperature or holding time. However, only small quantities are permitted, to prevent secondary phase formation. Other sources of secondary phases may be prevented through careful experimental procedures. Finally, the most significant source of light scattering in a high-purity sample is the presence of pores in the microstructure. Because of the difference in the refractive index between ceramic materials and the gas-filled pores, they are highly effective at scattering light. For polycrystalline alumina, a porosity (one minus relative density) of 0.1% decreases the real in-line transmission (RIT) from 86% to 1% when the pore diameter is similar to the wavelength of the light [Citation12]. Therefore, to obtain transparent polycrystalline ceramics, the elimination of pores is critical.
The four conditions which affect light transmittance are shown in . In this figure, the red line indicates the light traveling through the material; the line thickness corresponds to the total amount of light that is able to be transmitted. At the surface, light is scattered due to the change in the index of refraction and the nonuniform surface. The light then encounters a pore which results in significant scattering due to the multiple changes in the index of refraction. Next, a secondary phase is encountered, which may either scatter or absorb the light. Finally, only a small amount of the initial light passes through the material because of the defects which are encountered. This diagram does not show the effects of birefringence. If this were shown, the light would refract each time a grain boundary is crossed.
Figure 1. Schematic of light scattering while traveling through isotropic polycrystalline ceramic. Adapted from Tsabit et al. [Citation13].
![Figure 1. Schematic of light scattering while traveling through isotropic polycrystalline ceramic. Adapted from Tsabit et al. [Citation13].](/cms/asset/9ddb1385-04f6-456f-bec2-ead371204d5e/tmrl_a_2109441_f0001_oc.jpg)
3. YAG properties and crystal structure
YAG is a well-established gain material for solid-state lasers as it can be doped with a variety of fluorescent elements. The wide variation in atomic substitution allows for fine control over the wavelength of the laser. The material is selected as an excellent laser host due to its high efficiency attainable due to the mechanical strength, thermal conductivity, and chemical stability. A selection of the thermal, optical, physical, and mechanical properties is listed in .
Table 1. Selection of thermal, optical, physical, and mechanical properties of YAG. Adapted from Kochawattana [Citation14].
The YAG phase is a stochiometric line in the phase diagram of Y–Al–O, as shown in . The garnet phase is labeled as YAG, at which the ratio of Al:Y is 5:3. There are three ternary compounds in the system: YAG, YAP (YAlO3), and YAM (Y4Al2O3). The two phases, YAP and YAM, may be created as intermediate phases. The exact variation in stoichiometry that would still produce the YAG phase was determined by Singh et al. [Citation15]. For an excess of 1.0 at. % yttria, secondary phases cannot be detected by X-ray diffraction (XRD), so transparent samples can still be sintered. However, the presence of any excess Al2O3 is detrimental to transparency despite no evidence of a secondary phase being found through XRD analysis. Patel et al. [Citation16] investigated the amount of excess Al2O3 that could be held in the YAG lattice, the results indicate that only an excess of 0.2 at. % could be contained before the formation of secondary phases.
Figure 2. Phase diagram for Y–Al–O system, adapted from [Citation17].
![Figure 2. Phase diagram for Y–Al–O system, adapted from [Citation17].](/cms/asset/0927e701-3e15-4df8-bcac-6b1962abcaaf/tmrl_a_2109441_f0002_oc.jpg)
The unit cell of YAG is cubic, a model is shown in . The isotropic nature of the cubic unit cell ensures high transparency is obtainable as effects of birefringence are not present. The high transparency of the material increases the efficiency of photon emission as little light is lost due to absorption or scattering. For each unit cell in the Y3Al5O12 lattice, there are eight formula units containing a total of 160 atoms [Citation18]. The representative formula for the crystal is C3A2D3O12, in which the Al3+ ions are located at the A and D sites. These sites correspond to the trigonally distorted octahedral and tetrahedral locations, respectively. The dodecahedral site, C, contains the Y3+ ion. These cation positions have no degrees of positional freedom. This crystal lattice promotes the substitution of lanthanide or actinide series ions with a 3+ ionic state. The substitutions occur at the dodecahedral sites of the Y3+ ions for which a variety of elements, Nd3+, Ho3+, Yb3+, Eu3+, etc., have been successfully doped [Citation7].
Figure 3. Unit cell of YAG, adapted from [Citation19,Citation20].
![Figure 3. Unit cell of YAG, adapted from [Citation19,Citation20].](/cms/asset/8d560bfa-84d7-4ace-bce7-a8cd7d1818fe/tmrl_a_2109441_f0003_oc.jpg)
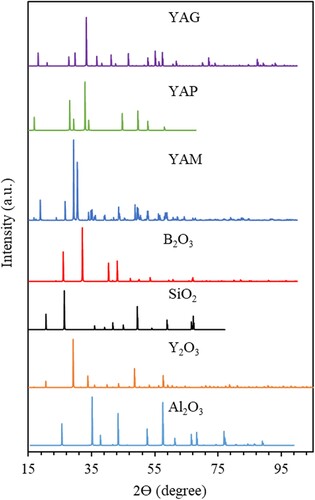
For the reader’s reference, is added to show the XRD patterns of YAG, YAP, YAM, B2O3, SiO2, Y2O3, and Al2O3 as these phases may be found in the YAG-based polycrystalline, in particular if the polycrystalline is made by SSR method. These references are gathered from the International Center for Diffraction Data (ICDD) database [Citation21]. The PDF card and citations are as follows: YAG PDF 00-033-0040, YAP PDF 00-054-0621, YAM PDF 00-034-0368, B2O3 PDF 01-084-9720, SiO2 PDF 00-046-1045, Y2O3 PDF 00-041-1105, and Al2O3 PDF 00-046-1212. The spectra of the phases indicate that they are not overlapped with each other, particularly the main peaks of the phases are not overlapped, and thus it is not challenging to identify the phase(s) in a processed sample.
4. YAG ceramic processing
Since 1990s, there have been several reported methods for the fabrication of doped YAG samples. The production of powders, selection of sintering aid, precursor calcination, green body formation, and sintering procedure are each critical for determining the final transparency of samples. Each step has multiple methods by which it may be accomplished, each having its own set of parameters. This section is a review of the published literature regarding the conventional sintering (pressureless sintering) of transparent YAG.
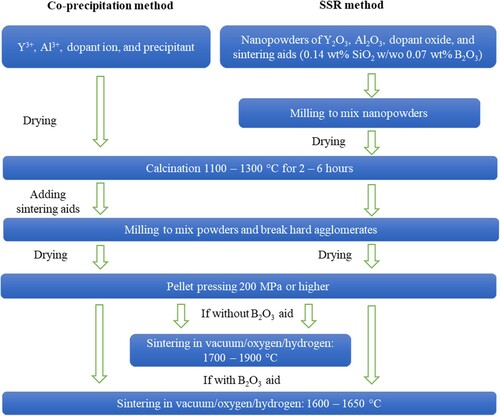
Conventional sintering of YAG is often conducted in a vacuum furnace, however, an oxygen atmosphere is also feasible [Citation22]. Two designs of high temperature vacuum furnaces exist. The difference between these furnace designs is the selection of heating material. The tungsten vacuum furnace and graphite vacuum furnace have similar capabilities but use different materials in the hot zone. The former is constructed of tungsten and the latter is constructed of graphite. Post processing is often required when using a graphite vacuum furnace due to the carbon contamination on the sample surface. On the other hand, the oxygen furnace is heated using molybdenum heating elements surrounding an alumina tube filled with flowing oxygen [Citation22]. summarizes the parameters of powder synthesis and sintering which have been applied to produce YAG-based polycrystalline ceramics. The fabrication steps of all the reviewed literatures are different and the parameters such as time, temperature, rotatory rate, pressure and so on are different to some extent. No consensus appears to be made regarding the exact steps and parameters, while the reviewed literatures obtained good transparency. The commonly applied steps of the fabrication processes are summarized in the fabrication route map in . The most critical steps and parameters and their effects are discussed in the following context.
Table 2. Summary of powder synthesis and conventional sintering parameters of YAG-based polycrystalline ceramics.
4.1. Powder source
Powder purity and morphology have a significant impact on the ability to sinter transparent ceramic. High purity ensures that no secondary phases are present in the material. As previously stated, the YAG lattice can accommodate at most a 0.2 at. % excess of Al2O3 and an excess of 1 at. % Y2O3 before the formation of secondary phases [Citation15,Citation16]. To obtain highly transparent ceramics, nearly all pores must be removed during the sintering process. The initial porosity of the sample is dependent on the morphology of the powder. Ideally, the powder is expected to have a narrow particle size distribution with an average diameter below 300 nm and consist of spherically shaped powders to promote a high particle packing density in the green body. depicts how the powder size and shape impact the initial size of pores within a structure. The open volume within the region bounded by the triangle formed by connecting the centers of the particles will be minimized as the powder size is reduced. Ensuring that the initial pore size is small is critical for obtaining high-density ceramic. A pore larger than the average grain size is thermodynamically stable till grain growth has increased the grain size to destabilize the pore [Citation47]. Nonuniform powder containing hard agglomerates (particle groups held together by forces stronger than van der Waals forces) can significantly increase the initial pore size as a high packing density is unattainable.
Figure 6. Schematic of particle packing prior to sintering (a) and after partial sintering which has resulted in a reduced pore size (b). X is the distance between the center of the particles, this distance is reduced as sintering occurs. Replotted from L. F. Francis [Citation48].
![Figure 6. Schematic of particle packing prior to sintering (a) and after partial sintering which has resulted in a reduced pore size (b). X is the distance between the center of the particles, this distance is reduced as sintering occurs. Replotted from L. F. Francis [Citation48].](/cms/asset/624456ab-4281-4e61-a3d3-9c1c790edd31/tmrl_a_2109441_f0006_ob.jpg)
YAG powders may be acquired through two methods. The first method is SSR which mixes Al2O3 and Y2O3 nanopowders followed by sintering to convert the oxides to the YAG phase [Citation5,Citation6,Citation32–35,Citation40,Citation41,Citation46,Citation22–27,Citation30,Citation31]. As such, phase transition and sintering occur synchronously. The second method is through chemical synthesis processes (e.g. co-precipitation [Citation4,Citation15,Citation44,Citation45,Citation28,Citation29,Citation36–39,Citation42,Citation43], sol–gel [Citation49–51], flame spray [Citation52], etc.) which create either a YAG powder or a precursor that will be calcinated to form YAG. Searching open literature, it is found that SSR and co-precipitation methods are applied more often than other methods.
4.1.1. Solid-state reaction
The SSR method starts from the as-received high-purity nanopowders of Al2O3 and Y2O3 combined in proper stoichiometric ratios. A dopant oxide powder (Nd2O3, Yb2O3, etc.) may also be added at this stage while adjusting the stoichiometric ratio to account for the dopant. The powders are mixed by ball milling to ensure adequate homogeneity and prevent the formation of secondary phases in isolated regions. The phase transformation of Al2O3 and Y2O3 to YAG occurs synchronously with sintering or during calcination prior to sintering.
Kochawattana [Citation14] investigated the diffusion mechanism between Al2O3 and Y2O3 which inter-diffuse and react to form YAG during heating. As significant heat is applied, the Al3+ atoms are capable of diffusing into the Y2O3 particles. By reducing the powder size, the diffusion distance is decreased, resulting in more rapid phase formation and removal of secondary phases. Specifically, the size of the Y2O3 particle is critical for these effects to occur. A series of reactions occur during this diffusion, converting the Al2O3 and Y2O3 powders first to the YAM phase, then the YAP phase, and finally the YAG phase [Citation53]:
(1)
(1)
(2)
(2)
(3)
(3)
The formation of these phases is accompanied by large volume changes, an expansion of 5.97% for the YAM phase, a shrinkage of 17.44% for the YAP phase, and an expansion of 11.46% for the YAG phase [Citation6,Citation14]. These volume changes are significant and may adversely affect the densification; therefore, a calcination step that forms the YAG is implemented prior to sintering [Citation26,Citation31,Citation35]. Bagayev et al. [Citation35] reported on three methods for sintering SSR powders: no calcination, calcination of powder, and calcination of powder compact (i.e. green body). Calcination of the powder prior to green body and sintering achieves the sample with the highest transparency. The transmittance at 1064 nm-wavelength is 83.28% using the second method, compared to 75.98% the third method and 68.47% the first method. After calcination, a ball milling step is required to break apart agglomerates that are formed during calcination. However, this additional step significantly increases processing time and potentially introduces contaminants into the powder.
The advantage of the SSR method over chemical synthesis methods is that high purity nanoparticles of Al2O3 and Y2O3 are widely available in various particle size distributions. Additionally, changes in dopant concentration and edits to the stoichiometric ratios of the Al2O3 and Y2O3 are relatively easy compared to long chemical procedures required for other powder synthesis methods. The disadvantage of using the SSR method is that maintaining stoichiometry during processing may be difficult as the material may absorb water resulting in mass changes. Therefore, working in controlled atmospheric environments is preferable when creating a batch of SSR powder.
4.1.2. Co-precipitation
The alternative method to the SSR is through the chemical synthesis of YAG powders. Multiple methods that have been reported for achieving YAG and doped YAG powders, including co-precipitation [Citation4,Citation15,Citation28,Citation29,Citation36–39,Citation42–45], sol–gel [Citation49–51], and flame spray [Citation52]. The co-precipitation method is discussed in the following paragraphs.
The co-precipitation method has multiple parameters that can be adjusted to produce YAG precursor powders of different morphologies and degrees of homogeneity. In general, a cation solution of deionized (DI) water, yttrium nitrate (Y(NO3)3), and aluminum ammonium sulfate (NH4Al(SO4)2) is mixed. To produce doped YAG, the dopant is added at this stage by adjusting the stoichiometric ratio between Y(NO3)3 and a dopant nitrate such as neodymium nitrate (Nd(NO3)3), uranyl nitrate (UO2(NO3)2), cerium nitrate (Ce(NO3)3), etc. After mixing, a precipitant solution, consisting of ethanol, DI water, and ammonium hydrogen carbonate ((NH4)HCO3), is addedFootnote1 to the cation solution for the precipitation purpose and obtain the precipitates of Y2(CO3)3, Y(OH)CO3, AlOOH, Al(OH)3, and NH4Al(OH)2CO3 with a diameter of less than 300 nm [Citation54]. The solution is then filtered using a Buchner filter apparatus, or a centrifuge to obtain the precipitates. The precipitate powders are then washed repeatedly and dried in an oven.
The effects of adding ethanol to the precipitant solution were reported in Tong et al. [Citation55]. The ratio of 0.2–1.2 alcohol to water is beneficial to YAG phase formation when the powder is calcinated at 1000°C. The reason for adding alcohol to the solution is to act as a dispersant to promote a more homogeneous mixture of the precipitants. Another discovery is that the chemical reaction should remain below 25°C to prevent secondary phases from forming.
Important parameters to consider when employing this technique are the molar concentration of the cations in the solution and the ratio between ethanol and DI water in the precipitant solution. Wang et al. [Citation56] investigated the effects of cation concentration on the formation of YAG phase. Higher concentration (1.5 M) of cations is not conducive to the formation of the YAG phase during calcination due to the formation of YAM, as detected by XRD analysis. Instead, a moderate concentration of 0.5 M of Al3+ promotes the formation of YAG phase at a lower calcination temperature. The high concentration results in large Y2O3 particles, increasing the diffusion distance for the Al3+ ions. Thus, longer calcination times and higher temperatures would be required to remove the excess YAM phase. While using the lower concentration, 0.5 M Al3+, the particle formation is uniform between the two materials. The particle size is approximately 100 nm, which promotes YAG phase formation at a temperature of 1050°C.
4.1.3. Effect of powder characteristics
Powder morphology is known to have a significant impact on the transparency of samples after sintering. As shown in , initial porosity in a sample prior to sintering is greatly affected by powder uniformity and size. Chen et al. [Citation57] studied the effect of ball milling parameters on the transparency of Yb:YAG using the co-precipitation method. A ball milling time of 12 h is determined to be optimal for reducing agglomerated particles. Extending ball milling past the 12 h results in the creation of some large particles despite the average particle size continuing to decrease. These large particles are formed due to small particles becoming attached to the surface of larger particles. The change in particle size and the effect of large agglomerates results in a change in transmittance, as shown in . The light transmittance of Yb:YAG increases about 20% after the 12-hour ball milling compared to no ball milling. The impact of powder morphology on transmittance is significant.
Figure 7. Change in transmittance of Yb:YAG sintered at 1750°C for 15 h as a result of ball milling time (black solid: approximate particle size; red circle: 600-nm wavelength; blue triangle: 1100-nm wavelength). Plotted based on datasets from Chen et al. [Citation57].
![Figure 7. Change in transmittance of Yb:YAG sintered at 1750°C for 15 h as a result of ball milling time (black solid: approximate particle size; red circle: 600-nm wavelength; blue triangle: 1100-nm wavelength). Plotted based on datasets from Chen et al. [Citation57].](/cms/asset/ac7cbb58-ef68-425b-9001-bed1d19f878b/tmrl_a_2109441_f0007_oc.jpg)
Powder crystallinity was also observed to have a significant effect on the transmittance, as reported by Ma et al. [Citation43]. Crystallinity refers to the degree of structural order in a solid. The degree of crystallinity is varied by changing the calcination temperature and time of co-precipitated powders. In general, the degree of crystallinity in the Nd:YAG powder could be increased by increasing calcination time and temperature; however, this also increases particle size. shows the change in transmittance for samples of different crystallinity degrees. Sample S6, which has the highest degree of crystallinity due to the calcination parameters 1250°C for 4 h, has the highest transmittance at 81% for 1064 nm despite having the largest particle size of approximately 260 nm. In comparison, sample S1 has a powder size of approximately 60 nm but only has a transmittance of 52% at 1064 nm. Calcination was conducted at 1100°C for 2 h for this sample. Transmission electron microscopy (TEM) images reveal that the S1 powder contains a 4-nm thick amorphous or defect-rich layer while the S6 powder has no such layer. This layer was undetectable by XRD; therefore, the importance of crystallinity may not always be reported.
Figure 8. Effect of powder crystallinity on transmittance of Nd:YAG samples sintered at 1780°C for 10 h. S1–S3: calcination at 1100°C for 2, 4, 6 h, respectively. S4–S6: calcination at 1150, 1200, 1250°C respectively for 4 h. The sample S6 has the highest degree of crystallinity. Reprinted with permission from Ma et al. [Citation43].
![Figure 8. Effect of powder crystallinity on transmittance of Nd:YAG samples sintered at 1780°C for 10 h. S1–S3: calcination at 1100°C for 2, 4, 6 h, respectively. S4–S6: calcination at 1150, 1200, 1250°C respectively for 4 h. The sample S6 has the highest degree of crystallinity. Reprinted with permission from Ma et al. [Citation43].](/cms/asset/a00d47a2-a2d1-47e6-91a2-97be66c62aa2/tmrl_a_2109441_f0008_ob.jpg)
4.2. Calcination
Calcination is a critical step to convert the precursor powders obtained from chemical synthesis into the YAG phase. The procedure is also often implemented when performing sintering using SSR powders to prevent large volume expansion and contraction during sintering. The effect of calcination on the formation of the YAG phase has been reported in several publications [Citation26,Citation28,Citation42,Citation43], though no consensus appears to be made regarding the exact temperature and time which should be applied to the precursor powders. Therefore, the calcination procedure is likely entwined with the powder synthesis procedure, which would account for the variation in results.
Sang et al. [Citation28] investigated the effects of calcination temperature on the ability of co-precipitated powders to be sintered, concluding that the ideal calcination procedure is 2 h in air at a temperature between 1100°C and 1300°C. The XRD results of the calcination study are shown in . From these XRD patterns, it is apparent that as the calcination temperature raises the peaks are sharper and narrower, indicating a higher degree of crystallinity. Similar conclusions were also found in others’ work, including Tong et al. [Citation58]. However, the method of YAG powder formation does have an impact on the calcination temperature that should be applied. Using the SSR, Fang et al. [Citation26] investigated the effects of calcination temperature on YAG transparent ceramics. It was reported that a calcination temperature higher than 800°C improved the mechanical strength of the ceramic, while a calcination temperature of 1100°C had a negative effect on the optical transmittance of the ceramic. Thus, the best calcination temperature was reported to be 1000°C for 6 h in air. Between the work of Fang et al. [Citation26] and Sang et al. [Citation28], both the temperature and time of calcination were impacted by the powder process. Therefore, careful consideration must be made when choosing a calcination temperature in combination with the powder fabrication method.
Figure 9. XRD results of powders calcinated at various temperatures. Reprinted with permission from Sang et al. [Citation28].
![Figure 9. XRD results of powders calcinated at various temperatures. Reprinted with permission from Sang et al. [Citation28].](/cms/asset/e4372e1e-5505-4857-8533-407c9d064a43/tmrl_a_2109441_f0009_oc.jpg)
Sang et al. [Citation28] also investigated the impact of calcination on microstructure development during sintering. It was stated that powders calcinated at a low temperature of 900°C or 1000°C had excessively high sintering activity resulting in abnormal grain growth leading to pore formation, as shown in (a,b). While those produced at a temperature higher than 1100°C were found to have a uniform grain distribution, increased transparency, and fewer pores, as shown in (c–e).
Figure 10. SEM images of grain structure of sintered samples calcinated at different temperatures: (a) 900°C, (b) 1000°C, (c) 1100°C, (d) 1200°C, (e) 1300°C, (f) in-line transmittance plot of the sample shown in (e). Reprinted with permission Sang et al. [Citation28].
![Figure 10. SEM images of grain structure of sintered samples calcinated at different temperatures: (a) 900°C, (b) 1000°C, (c) 1100°C, (d) 1200°C, (e) 1300°C, (f) in-line transmittance plot of the sample shown in (e). Reprinted with permission Sang et al. [Citation28].](/cms/asset/1f45b2b1-6a50-4f32-9e3e-f2e331e864c7/tmrl_a_2109441_f0010_ob.jpg)
To produce U:YAG powders, the calcination atmosphere, hold time, and temperature were inconsistent between the works of Zhang et al. [Citation3], Zeng et al. [Citation4], and Gong et al. [Citation5]. A calcination procedure of 8 h at a temperature of 800°C in an oxygen atmosphere after green body formation was used by Gong et al. [Citation5] for SSR powder. Sintering was then conducted at 1900°C for 10 h resulting in transparent U:YAG with the U4+ ion substituting into the Y3+ lattice position. The difference in calcination procedure between Gong et al. [Citation5] and the other works is accounted for due to the use of SSR powder compared to co-precipitation powder. Zhang et al. [Citation3] reported a detailed study on how the calcination procedure effects the charge state of the U ion substitution. It was reported that a U4+ substitution into the Y3+ lattice position was attainable by calcinating in an atmosphere of N2 with 3% H2 at 1000°C for 4 h. A following work by the same group [Citation4] performed calcination in an air atmosphere at 1250°C for 4 h. The samples were then sintered in a graphite heated vacuum furnace at 1800°C for 15 h resulting in transparent U:YAG with the U6+ ion substituting into the Y3+ lattice position. The reason for the change in calcination procedure was not stated though the difference did affect the resultant charge state of the U ion.
4.3. Green body formation
A high-density green body indicates a low initial porosity, thus leading to a highly densified sample at lower temperatures and shorter times during sintering. As residual pores drastically reduce the transparency of ceramic, a low initial porosity is desired. depicts how the density of a green body is altered by the morphology of powder and the distribution of powder sizes within the green body. To obtain a high-density green body, the morphology of powder is the most critical factor, followed by the compaction method. To produce transparent YAG ceramics, pores need to be eliminated. To eliminate pores, powders need to be nanometer size and uniform in shape.
Figure 11. Illustration of packing structures within a green body ceramic as a result of powder morphology. Reprinted with permission from Uematsu [Citation59].
![Figure 11. Illustration of packing structures within a green body ceramic as a result of powder morphology. Reprinted with permission from Uematsu [Citation59].](/cms/asset/68e20b2c-1e38-49cc-9c30-6c08d50c0179/tmrl_a_2109441_f0011_ob.jpg)
In addition to the powder property, the pressure that densifies powders into solid compact is another critical factor. The formation of YAG green bodies can be accomplished through various methods, including uniaxial pressing (dry pressing), cold isostatic pressing (CIP), and colloidal processing. A schematic depicting many consolidation methods is shown in . Uniaxial pressing or dry pressing uses a set of cylinders with a false bottom that allows for the formation of a pellet under pressure. Uniaxial pressing is often reported in combination with CIP but can also be used independently. A pressure in the range of 200 MPa is reported for independent usage, while 20 MPa followed by 200-MPa CIP is reported when used in combination. A CIP consists of a chamber of fluid in which the sample is placed while the fluid is compressed, leading to a uniform pressure applied to the sample in all directions. Careful attention should be taken to ensure that contamination of the sample does not occur while in the fluid chamber. A pressure of 200 MPa is commonly reported in procedures for fabricating transparent YAG samples. Colloidal processing refers to multiple methods involving the use of a suspension of particles in a fluid to form the desired shape. These methods are known to produce a denser and more uniform green body. This is because the immersion in a fluid reduces the van der Waals attractions and allows for better packing. Additionally, colloidal processing is better able to remove agglomerates as the solution can be easily filtered prior to consolidation. Methods of colloidal processing include slip casting, tape casting, gelcasting, etc.
Figure 12. Schematic of consolidation methods for producing ceramic green bodies. Replotted from Crouch et al. [Citation60].
![Figure 12. Schematic of consolidation methods for producing ceramic green bodies. Replotted from Crouch et al. [Citation60].](/cms/asset/5e3a1186-9c9f-4a79-a48a-03f28f3c88d3/tmrl_a_2109441_f0012_oc.jpg)
4.4. Sintering
Sintering is the process of consolidating the powder compact into a solid by reducing the porosity from the initial value of 40–50%. For transparent polycrystalline YAG, the final porosity must be less than 0.1%. The reduction of porosity is accomplished through the application of heat and/or pressure, which facilitates densification through grain rearrangement and growth [Citation61]. Sintering temperature is often 50–80% of the materials melting point to ensure that geometric shape can be obtained while also promoting densification. Sintering techniques primarily apply heat to the samples to promote densification; however, pressure and electric current are often applied in combination with heat. YAG is often treated with pressure during sintering through either hot isostatic press (HIP), hot press (HP), or spark plasma sintering (SPS), as pressure is highly effective at eliminating pores. Sintering of transparent polycrystalline YAG has been achieved through various techniques including vacuum furnace [Citation4–6,Citation15,Citation23–45], SPS [Citation7,Citation62–76], HIP [Citation77,Citation78], microwave [Citation79], and two-step sintering [Citation80]. However, the most common method for YAG production is the use of conventional furnaces. These have the benefit of producing samples of unrestricted dimensions as complicated pressure chambers are not required. Conventional sintering of YAG is conducted in a vacuum to promote the diffusion of gas out of the sample pores; however, recent work by Stevenson et al. [Citation22,Citation81] and Huang et al. [Citation46] reported that sintering in oxygen is also feasible. This review focuses on conventional sintering in both oxygen and vacuum.
The mechanisms of sintering have been described in numerous literatures [Citation61,Citation82,Citation83], a brief summary of these mechanisms is provided based on these literatures. Sintering is a thermodynamically irreversible process and is accompanied by the reduction of free energy in the system. The driving forces for the reduction in free energy are curvature of particle surfaces, externally applied forces, and chemical interactions. The particles within the green body undergo geometric change during sintering which is divided into three stages. In the initial stage, necks form between adjacent particles, increasing the contact area of the particles and improving relative density to ∼60%. The intermediate stage of sintering begins when grain growth first occurs. The microstructure of the material during this stage consists of a pore phase and a grain phase. Pores remain connected throughout the material and are bounded by grain boundaries which results in irregularly shaped pores. As the connections between the pores are closed, the final stage of sintering has begun. Pores that are connected to grain boundaries can then continuously shrink until eliminated. However, a second version of the final sintering stage can occur if non-uniform grain growth is present. In this process, pores may become isolated from grain boundaries and become unable to shrink in size. This is because pores are a collection of vacancies in the lattice; therefore, without access to grain boundaries, which act as sinks for the vacancies, elimination cannot occur.
Two parameters are often used to describe the microstructural evolution of a material during the sintering process. These are the average grain size and the relative/theoretical density. Relative/theoretical density is commonly stated as a percentage of the theoretical maximum density of a material. The converse of relative density is the porosity of the sample, which is related as 1 minus the relative density. Additionally, linear shrinkage or volume shrinkage can be measured during heating to determine the stage of sintering.
4.4.1. Sintering in vacuum atmosphere
The first report of successful fabrication of Nd:YAG polycrystalline transparent ceramics with qualities similar to that of single crystals was made by Ikesue et al. [Citation6] in 1995. The samples were fabricated using conventional sintering in a vacuum at 1750°C for 20 h. A sintering aid of 0.14 wt. % SiO2 was added to the SSR powders used in the fabrication. The in-line transmittance of the sample at 1064 nm was approximately 80%. Sintering in vacuum atmosphere has continued to be the most prominent method in literature for sintering transparent YAG. Sintering in vacuum is common for transparent ceramics as the pressure difference between gas trapped in pores and the vacuum leads to more rapid pore elimination. summarizes the results of many reports using conventional sintering in vacuum with either a tungsten furnace or graphite furnace. A few of these works are discussed in detail in the proceeding paragraphs.
Fabrication of transparent polycrystalline U:YAG was reported by Zeng et al. [Citation4] and Gong et al. [Citation5] using conventional sintering in a vacuum. Both works used separate fabrication methods, Zeng et al. [Citation4] using co-precipitation and Gong et al. [Citation5] using SSR, though similar results were obtained. In addition to the difference in powder processing, both groups used different furnace types. Zeng et al. [Citation4] used a graphite heated vacuum furnace to sinter powder compacts at 1800°C for 15 h, obtaining a sample with a maximum transmittance of 78% in the visible spectrum. Gong et al. [Citation5] instead used a tungsten heated vacuum furnace at 1900°C for 10 h, obtaining in a sample with a transmittance of 79.04% at 714 nm. Both groups used CaO as a sintering aid, which requires higher sintering temperature and longer time than the sintering aid of SiO2 (see section 4.5).
Fabrication of thick YAG is often difficult as inner pores may be closed from the surrounding atmosphere much sooner than the pores closer to the surface. Fang et al. [Citation26] reported the successful fabrication of thick YAG samples through sintering in a vacuum. Small pellets, 5 mm in thickness, were fabricated to transparency. Additionally, large dome measuring 120 mm in diameter by 15 mm in thickness was also sintered to transparency. This work represents a significant breakthrough in YAG transparent ceramics as the complex shape and thickness had yet to be reported. Sintering of the pellets required 1750°C for 10 h in a tungsten heated vacuum furnace with sintering aid of 0.14 wt. % SiO2. Sintering of the dome required 1750°C for 30 h. The powder used was produced by SSR with a critical calcination step occurring after green body formation. The temperature of calcination was 1000°C for 6 h; this step was stated as the reason the thick samples could be fabricated at high transparencies. The in-line transmittances at 1000 nm for the pellet and dome were 82.9% and 80.1%, respectively.
4.4.2. Sintering in oxygen atmosphere
High-temperature vacuum furnace is more time- and cost-consuming than non-vacuum furnace, and thus, a more affordable and efficient method is desired. Huang et al. [Citation46] sintered polycrystalline Nd:YAG in an oxygen atmosphere furnace and obtained transparent Nd:YAG. The powder was made by SSR method, with sintering aid of 0.14 wt. % SiO2, and ball milled for 12 h, The Nd:YAG was sintered in oxygen atmosphere at 1710°C for 3 h, obtaining in-line transmittance of 80% at 1064 nm and a relative density of 99.5%. This relative density is equivalent to samples sintered in a vacuum furnace. Therefore, it is feasible to achieve transparent YAG-based ceramics in the oxygen atmosphere. Stevenson et al. [Citation22,Citation81] also obtained transparent polycrystalline Nd:YAG ceramics through sintering in oxygen atmosphere. In addition to the discovery as Huang et al., this work found that using a combined sintering aids of SiO2 and B2O3 (more discussion see section 4.5), the sintering dwell temperature can be reduced from > 1700°C to 1600°C. The 1600°C can be carried out in a less expensive furnace and thus lowers the manufacturing cost. Using the combination of sintering aids, the Nd:YAG samples sintered in different atmospheres (flowing oxygen and vacuum) at 1600°C for 20 h had comparable in-line transmittance plots, as shown in . Both samples are highly transparent with in-line transmissions approaching 84% from 400 to 1100 nm. The light transmission at 250–400 nm of the sample sintered in flowing oxygen is relatively lower than the one sintered in vacuum.
Figure 13. In-line transmission plot of polycrystalline 1 at. % Nd:YAG samples with the same sintering aids of SiO2 and B2O3 in flowing O2 compared to vacuum atmosphere. Reprinted with permission from Stevenson et al. [Citation22].
![Figure 13. In-line transmission plot of polycrystalline 1 at. % Nd:YAG samples with the same sintering aids of SiO2 and B2O3 in flowing O2 compared to vacuum atmosphere. Reprinted with permission from Stevenson et al. [Citation22].](/cms/asset/112764c6-4750-4ec4-82f2-7f9641704a91/tmrl_a_2109441_f0013_oc.jpg)
It shall be noted that though vacuum and non-vacuum atmosphere are both feasible methods, the non-vacuum atmosphere is constrained by the types of gas. The atom size of gas molecular must be small to rapidly diffuse out of the material. Using the same synthesis and sintering parameters to make the Nd:YAG samples, the argon atmosphere furnace yielded a relative density of only 98.1% while the oxygen atmosphere furnace yielded 99.5% [Citation46]. This is because solid-state diffusion is required during the final stage of sintering. When pores become isolated, the difference in relative density is a result of the slow diffusion rates of the large argon atoms compared to the oxygen atoms. This finding is consistent with Coble [Citation84] which investigated the effect of the atmosphere on the densification of Al2O3. It was reported that atmospheres of hydrogen, vacuum, and oxygen could be used to sinter pore free Al2O3 while nitrogen, argon, and helium inhibited densification.
Another example is from the air-atmosphere sintering work of YAG by Mohammadi et al. [Citation27]. Solid-state powder was formed by ball milling for 16 h with 0.14 wt. % SiO2 added as a sintering aid. Sintering was then performed at 1710°C for 12 h. The air sintered samples were reported to have a relative density of 97.3%, while in a vacuum the samples would be 99.8%. shows the change in relative density for samples sintered in a vacuum compared to air as a function of sintering temperature with a dwell time of 6 h. The reason for the difference in relative density was determined to be a result of the slow diffusion of air (primarily nitrogen) out of the crystal once the pores were closed to the surrounding atmosphere in the final stages of densification. Solid-state diffusion is required to eliminate the final pores in the material; however, the slow diffusivity of nitrogen resulted in slow diffusion out of the material and the inability to be incorporated into the lattice. A similar conclusion was drawn by Huang et al. [Citation85] when investigating the sintering of transparent Y2O3 in oxygen.
Figure 14. Relative density of polycrystalline YAG sintered in vacuum vs. air at given temperatures. Reprinted with permission from Mohammadi et al. [Citation27].
![Figure 14. Relative density of polycrystalline YAG sintered in vacuum vs. air at given temperatures. Reprinted with permission from Mohammadi et al. [Citation27].](/cms/asset/32d2c44d-7ac3-45b5-a73a-46fa560641d2/tmrl_a_2109441_f0014_oc.jpg)
Despite the remarkable discovery, there have been no further investigations into the sintering of doped YAG using an oxygen environment. Based on the reviewed works, it is suspected that a hydrogen atmosphere may also work for sintering transparent YAG as the large diffusivity of hydrogen would allow for more rapid diffusion out of the material.
4.5. Sintering aids
Sintering aids are often critical to obtaining highly dense ceramic through both pressureless and pressure-assisted sintering methods. The addition of sintering aid has multiple desired effects on the sintering process, including reduced sintering time, decreased sintering temperature, and increased density [Citation47,Citation86]. The maximum amount of sintering aid that can be added to enhance sintering is limited by the formation of secondary phases, which may reduce material’s transparency. During heating, sintering aids melt to form a liquid phase, which facilitates diffusion of atoms of YAG toward pores and thus promotes densification. At the onset of liquid formation, the liquid wets the powder surface, spreading to fill the pores and dissolving bonds that are formed during initial heating. The liquid phase is soluble in YAG solid, which prevents the formation of secondary phases. As such, the additive chosen must ensure mutual solubility between the solid and liquid. Pores that remain in the sample past the wetting and diffusion stages must be removed by solid-state sintering.
Due to the high temperatures and long periods required to sinter YAG-based polycrystalline ceramics, sintering aids are used to support sintering mechanics and increase the densification rate and/or limit grain growth. The sintering aid is typically mixed with YAG powder in quantities of less than 1 wt. % through ball milling to ensure homogenous distribution. Sintering aids that have been used for YAG include calcium oxide (CaO) [Citation4,Citation5,Citation23,Citation25,Citation40,Citation87], silicon oxide (SiO2) [Citation6,Citation10,Citation34,Citation36,Citation40–46,Citation81,Citation22,Citation88,Citation26–28,Citation30–33], boron oxide (B2O3) [Citation22,Citation81], and magnesium oxide (MgO) [Citation23–25,Citation30,Citation31,Citation41].
Using SiO2 for YAG was first reported in Ikesue et al. [Citation6]. It was determined that the sintering aid is critical in producing fully transparent samples with laser slope efficiencies comparable to the Czochralski growth method. The use of SiO2 as the sintering aid has become standard in the fabrication of transparent YAG. Often, the SiO2 is added in the form of tetraethyl orthosilicate (TEOS). TEOS is preferred because it is a liquid, therefore promoting a more homogenous distribution of sintering aid throughout the powder. TEOS breaks down into SiO2 during the heating process. Multiple investigations have been conducted on the effects of SiO2 during sintering [Citation22,Citation36,Citation81,Citation88]. It’s concluded that a doping concentration of 0.28 wt. % increases the density of the samples at all sintering times and temperatures [Citation88]. It was reported that the Nd:YAG with 0.14 wt. % SiO2 samples sintered in a vacuum furnace could reach a relative density of 99.9% in 2 h at temperatures higher than 1650°C. However, the issue with SiO2, which has promoted further study into sintering aids, is excessive grain growth during sintering. The effects are shown in ; an increase in SiO2 increases the grain growth rate. Grain size, as long as grains are uniform in size, does not affect light transmittance, though ceramics with finer grains are preferred in applications that require robustness in high strength.
Figure 15. Grain size increases with the increase of SiO2 concentration at given temperatures. Reprinted with permission from Stevenson [Citation81].
![Figure 15. Grain size increases with the increase of SiO2 concentration at given temperatures. Reprinted with permission from Stevenson [Citation81].](/cms/asset/022fc148-f517-4584-b3f7-8ac658a85568/tmrl_a_2109441_f0015_ob.jpg)
A solution to the issue of grain growth is to use a second sintering aid MgO in addition to SiO2. There is not much research regarding the independent impacts of MgO on the sintering of YAG except for a recent study by Vorona et al. [Citation24]. The investigation was performed using the SSR method. The reported effect of MgO on YAG ceramics is that grain growth for samples without aid is abnormal due to the low homogeneity of powders, while the addition of MgO makes grain boundary mobility more uniform, resulting in a narrow distribution of grain sizes. The suggested amount of MgO is 0.03 wt. %, an increase from this value has a detrimental effect on grain size and distribution. This sintering aid also has an impact on the transparency of samples when sintered at 1750°C for 10 h, as the YAG sample containing no MgO was found to be non-transparent, while any addition of MgO below the solubility limit lead to a transparent sample. The addition of MgO at or above the solubility limit reduces transparency due to the formation of secondary phase. Commonly, both SiO2 and MgO are used together to promote densification and limit grain growth. Publications by Yang et al. [Citation30] and Li et al. [Citation31] both investigate the grain structure of the YAG using MgO and SiO2 as sintering aids. As noted previously, SiO2 is the dominant driver for the densification mechanics of sample and can result in excessive grain growth. Magnesium oxide is then used to limit grain growth during sintering. SEM images of Nd:YAG samples sintered with various compositions of the two sintering aids are shown in . It is evident that 0.145 wt. % SiO2 + 0.10 wt. % MgO would result in smaller grain size and mitigate pores compared to the single addition of SiO2 or MgO, respectively. The composition of 0.145 wt. % SiO2 and 0.10 wt. % MgO was determined to be optimal by Yang et al. [Citation30]. Similarly, Li et al. [Citation31] concluded that the optimal combination of sintering aids was 0.112 wt. % SiO2 + 0.08 wt. % MgO.
Figure 16. SEM images of mirror polished (top) and fractured (bottom) polycrystalline YAG with sintering aids of (A) 0.14 wt. % SiO2, (B) 0.145 wt. % SiO2 + 0.10 wt. % MgO, and (C) 0.10 wt. % MgO. Reprinted with permission from Yang et al. [Citation30].
![Figure 16. SEM images of mirror polished (top) and fractured (bottom) polycrystalline YAG with sintering aids of (A) 0.14 wt. % SiO2, (B) 0.145 wt. % SiO2 + 0.10 wt. % MgO, and (C) 0.10 wt. % MgO. Reprinted with permission from Yang et al. [Citation30].](/cms/asset/32a80751-c505-47ec-85cf-e1f0696a0fec/tmrl_a_2109441_f0016_ob.jpg)
Calcium oxide is another sintering aid to replace the SiO2 sintering aid. Zhang et al. [Citation87] studied the effects of CaO on the sintering of YAG ceramics. The optimal concentration of CaO is suggested to be 0.045 wt. % as which promotes densification without excessive grain growth. The advantage of CaO compared to SiO2 is that CaO can increase the densification rates while also limiting the grain growth to prevent the formation of large grains, which occurs when doping with only SiO2. However, the stronger effects of SiO2 on the densification rate of the samples lead to significantly reduced sintering temperatures and hold times. Because of this effect, CaO is used less often as a higher temperature furnace is required to obtain transparent YAG. In addition, Chaika et al. [Citation40] suggest that a combination of CaO and SiO2 should not be used because the two additives interact and form a secondary phase in the grain boundaries.
Boron oxide (B2O3) is another sintering aid aimed at further reducing the sintering temperature for transparent Nd:YAG ceramics [Citation22,Citation81]. The method came from the experience of sintering cordierite (Mg2Al4Si5O18), in which co-doping with SiO2 is known to reduce the sintering temperature and time. The investigation successfully fabricated transparent Nd:YAG at temperatures as low as 1600°C. The optimal doping concentration was suggested to be 1.35% mol (equivalent to 0.14 wt. % SiO2) with the ratio of B2O3:SiO2 at 0.5. It is also noted that the addition of B2O3 reduces the grain growth which occurs with SiO2. Further research needs to be carried out to understand how B2O3 facilitates densification at a lower temperature while inhibiting grain growth. In addition, one of the limitations of the sintering method for producing transparent polycrystalline ceramics is the cost of sintering. As YAG sintering requires high temperatures (∼1750°C) and/or high pressures (∼90 MPa), the costs and availability of equipment are a concern; thus, improved solutions are desired. These works [Citation22,Citation81] show that the sintering temperature can be decreased from 1750°C to 1600°C, which allows the application of a significantly less expensive furnace and reduces the energy consumption. The 1600°C furnace is more suitable for large-scale and high-throughput productions compared to higher temperature furnaces, vacuum furnaces, or pressure-assisted furnaces (i.e. SPS and HIP).
5. Conclusion
Fissionable and fissile isotopes doped YAG is a promising scintillation material to measure neutron flux over time. Transparent polycrystalline YAG-based ceramic, made by powder synthesis and sintering methods, is a more cost-efficient material than the single crystal of YAG made by the crystal growth method. It not only improves the dopant concentration but also requires less time and cost of fabrication. Through reviewing the past work of polycrystalline YAG fabrication, we found that most of the work is focused on YAG and lanthanide-doped YAG, while fissionable and fissile isotopes doped YAG is rare. This paper reviewed all the powder synthesis and pressureless sintering methods which have been used to make transparent polycrystalline YAG-based ceramics, and found the relationship between processing parameters and their effects on resultant microstructure and light transmittance. This review work provides insights on the fabrication of fissionable and fissile isotopes doped YAG. The method of powder synthesis, co-precipitation or solid-state reaction, has no effect on material densification. Therefore, either method is feasible. Particle morphology and crystallization have significant effects on material densification. A change of approximately 20% in-line transmittance could be observed as a result of particle processing. Calcination has an important effect on sample densification in both co-precipitation and SSR powders. Optimal fabrication of new materials, in particular the fissionable and fissile isotopes doped YAG, will require refinement of the calcination procedure. Green body formation assists in obtaining high-density materials though it is highly dependent on the particle morphology. Refinement of particle processing will result in higher density green bodies. Sintering of green body to high density requires the thorough removal of pores in the material. Removal of these pores is assisted by using a vacuum atmosphere during sintering or alternatively using a gaseous atmosphere (e.g. oxygen) which is able to diffuse out of the material. It should be noted that argon, nitrogen, and air atmosphere cannot achieve transparent YAG as the gas can hardly diffuse out of pores. Sintering temperature and time are predominately affected by the choice of sintering aids (SiO2, MgO, and B2O3). For a transparent YAG, only a small amount of sintering aid is allowed. Silicon oxide significantly reduces sintering time and temperature; however, grain growth must be controlled through the use of MgO. Boron oxide may reduce the sintering dwell temperature as required by SiO2 and/or MgO, from > 1700°C to 1600°C, being an effective factor to lower the fabrication cost.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1 The reverse strike co-precipitation method adds the cation solution to precipitant solution.
References
- Xiao Z, Yu S, Li Y, et al. Materials development and potential applications of transparent ceramics: a review. Mater Sci Eng R Rep. 2020;139:100518. doi:10.1016/J.MSER.2019.100518.
- Moszyński M, Ludziejewski T, Wolski D, et al. Properties of the YAG:Ce scintillator. Nucl Instrum Methods Phys Res A Accel Spectrom Detect Assoc Equip. 1994;345:461–467. doi:10.1016/0168-9002(94)90500-2.
- Zhang Q, Lu T, Lu T, et al. Synthesis of pure-phase uranium-doped YAG powder via co-precipitation method. Mater Lett. 2017;188:396–398. doi:10.1016/j.matlet.2016.11.093.
- Zeng Q, Zhang Q, Qi J, et al. Fabrication and luminescence properties of U:YAG transparent ceramic. Opt Mater 2018;82:56–59. doi:10.1016/j.optmat.2018.05.004.
- Gong C, Chen J, Huang Q, et al. Synthesis and characterization of structural and optical properties of Ce, U codoped YAG transparent ceramics. Opt Mater Express. 2018;8:1274. doi:10.1364/ome.8.001274.
- Ikesue A, Kinoshita T, Kamata K, et al. Fabrication and optical properties of high-performance polycrystalline Nd:YAG ceramics for solid-state lasers. J Am Ceram Soc. 1995;78:1033–1040. doi:10.1111/j.1151-2916.1995.tb08433.x.
- Wagner A, Ratzker B, Kalabukhov S, et al. Photoluminescence of doped YAG transparent ceramics fabricated by spark plasma sintering. Isr J Chem. 2020;60:550–556. doi:10.1002/ijch.201900131.
- Shawley CR. Optical and defect studies of wide band gap materials [dissertation]. 2008.
- Burke JE. Lucalox alumina: the ceramic that revolutionized outdoor lighting. MRS Bull. 1996;21:61–68. doi:10.1557/S0883769400046133.
- Ikesue A, Aung YL. Synthesis and performance of advanced ceramic lasers. J Am Ceram Soc. 2006;89:1936–1944. doi:10.1111/j.1551-2916.2006.01043.x.
- Lupei V, Lupei A, Georgescu S, et al. The effect of Nd concentration on the spectroscopic and emission decay properties of highly doped Nd:YAG ceramics. Phys Rev B. 2001;64:92102. doi:10.1103/PhysRevB.64.092102.
- Apetz R, Van Bruggen MPB. Transparent alumina: a light-scattering model. J Am Ceram Soc. 2003;86:480–486. doi:10.1111/j.1151-2916.2003.tb03325.x.
- Tsabit AM, Yoon D-H. Review on transparent polycrystalline ceramics. J Korean Ceram Soc. 2021;59:1–24. doi:10.1007/s43207-021-00140-6.
- Kochawattana S. Phase formation and sintering of YAG ceramics [dissertation]. 2007.
- Singh G, Anand AS, Selvamani R, et al. Effect of yttrium variation on phase, transparency, and micro-structure of neodymium doped yttrium aluminum garnet ceramic. Scr Mater. 2019;167:61–65. doi:10.1016/j.scriptamat.2019.03.046.
- Patel AP, Levy MR, Grimes RW, et al. Mechanisms of nonstoichiometry in Y3Al5O12. Appl Phys Lett. 2008;93:191902. doi:10.1063/1.3002303.
- Villars P, Okamoto H, editors. Al-O-Y vertical section of ternary phase diagram: datasheet from ‘PAULING FILE multinaries edition—2012’. Springer Materials; n.d. Available from: https://materials.springer.com/isp/phase-diagram/docs/c_0204409.
- Kuklja MM, Pandey R. Atomistic modeling of native point defects in yttrium aluminum garnet crystals. J Am Ceram Soc. 1999;82:2881–2886. doi:10.1111/j.1151-2916.1999.tb02172.x.
- Jain A, Ong SP, Hautier G, et al. The materials project: a materials genome approach to accelerating materials innovation. APL Mater. 2013;1:11002. doi:10.1063/1.4812323.
- Ong SP, Richards WD, Jain A, et al. Python materials genomics (pymatgen): a robust, open-source python library for materials analysis. Comput Mater Sci. 2013;68:314–319. doi:10.1016/j.commatsci.2012.10.028.
- Gates-Rector S, Blanton T. The powder diffraction file: a quality materials characterization database. Powder Diffr. 2019;34:352–360. doi:10.1017/S0885715619000812.
- Stevenson AJ, Kupp ER, Messing GL. Low temperature, transient liquid phase sintering of B2O3-SiO2-doped Nd:YAG transparent ceramics. J Mater Res. 2011;26:1151–1158. doi:10.1557/jmr.2011.45.
- Zhou T, Zhang L, Selim FA, et al. Annealing induced discoloration of transparent YAG ceramics using divalent additives in solid-state reaction sintering. J Eur Ceram Soc. 2017;37:4123–4128. doi:10.1016/J.JEURCERAMSOC.2017.05.030.
- Vorona I, Balabanov A, Dobrotvorska M, et al. Effect of MgO doping on the structure and optical properties of YAG transparent ceramics. J Eur Ceram Soc. 2020; 40:861–866. doi:10.1016/j.jeurceramsoc.2019.10.048.
- Zhou T, Zhang L, Li Z, et al. Toward vacuum sintering of YAG transparent ceramic using divalent dopant as sintering aids: investigation of microstructural evolution and optical property. Ceram Int. 2017;43:3140–3146. doi:10.1016/j.ceramint.2016.11.131.
- Fang C, Jing Z, Qin X, et al. Effect of heat treatment of green bodies on the sintering and optical properties of large-size and thick transparent YAG ceramics. Ceram Int. 2021;47:9606–9612. doi:10.1016/j.ceramint.2020.12.097.
- Mohammadi F, Mirzaee O, Tajally M. The effects of sintering atmosphere on the fabrication of transparent polycrystalline YAG ceramics. Adv Mater Res. 2019;1152:53–63. doi:10.4028/www.scientific.net/amr.1152.53.
- Sang Y, Liu H, Sun X, et al. Formation and calcination temperature-dependent sintering activity of YAG precursor synthesized via reverse titration method. J Alloys Compd. 2011;509:2407–2413. doi:10.1016/j.jallcom.2010.11.031.
- Jiang W, Cheng X, Xiong Z, et al. Static and dynamic mechanical properties of yttrium aluminum garnet (YAG). Ceram Int. 2019;45:12256–12263. doi:10.1016/j.ceramint.2019.03.136.
- Yang H, Qin X, Zhang J, et al. The effect of MgO and SiO2 codoping on the properties of Nd:YAG transparent ceramic. Opt Mater. 2012;34:940–943. doi:10.1016/j.optmat.2011.05.029.
- Li Y, Zhou S, Lin H, et al. Fabrication of Nd:YAG transparent ceramics with TEOS, MgO and compound additives as sintering aids. J Alloys Compd. 2010;502:225–230. doi:10.1016/j.jallcom.2010.04.151.
- Boulesteix R, Bonnet L, Maître A, et al. Silica reactivity during reaction-sintering of Nd:YAG transparent ceramics. J Am Ceram Soc. 2017;100:945–953. doi:10.1111/jace.14680.
- Li J, Wu Y, Pan Y, et al. Fabrication, microstructure and properties of highly transparent Nd:YAG laser ceramics. Opt Mater. 2008;31:6–17. doi:10.1016/J.OPTMAT.2007.12.014.
- Ikesue A, Yoshida K, Yamamoto T, et al. Optical scattering centers in polycrystalline Nd:YAG laser. J Am Ceram Soc. 1997;80:1517–1522. doi:10.1111/j.1151-2916.1997.tb03011.x.
- Bagayev SN, Osipov VV, Solomonov VI, et al. Fabrication of Nd3+:YAG laser ceramics with various approaches. Opt Mater. 2012;34:1482–1487. doi:10.1016/j.optmat.2012.03.004.
- Stevenson AJ, Li X, Martinez MA, et al. Effect of SiO2 on densification and microstructure development in Nd:YAG transparent ceramics. J Am Ceram Soc. 2011;94:1380–1387. doi:10.1111/j.1551-2916.2010.04260.x.
- Pan Y, Liu W, Zhang W, et al. Influence of pH values on (Nd + Y):Al molar ratio of Nd:YAG nanopowders and preparation of transparent ceramics. J Alloys Compd. 2010;503:525–528. doi:10.1016/j.jallcom.2010.05.048.
- Yagi H, Yanagitani T, Takaichi K, et al. Characterizations and laser performances of highly transparent Nd3+:Y3Al5O12 laser ceramics. Opt Mater. 2007;29:1258–1262. doi:10.1016/j.optmat.2006.01.033.
- Lu J, Ueda KI, Yagi H, et al. Neodymium doped yttrium aluminum garnet (Y3Al5O12) nanocrystalline ceramics—a new generation of solid state laser and optical materials. J Alloys Compd. 2002;341:220–225. doi:10.1016/S0925-8388(02)00083-X.
- Chaika MA, Mancardi G, Vovk OM. Influence of CaO and SiO2 additives on the sintering behavior of Cr,Ca:YAG ceramics prepared by solid-state reaction sintering. Ceram Int. 2020;46:22781–22786. doi:10.1016/j.ceramint.2020.06.045.
- Zhang G, Carloni D, Wu Y. 3D printing of transparent YAG ceramics using copolymer-assisted slurry. Ceram Int. 2020;46:17130–17134. doi:10.1016/J.CERAMINT.2020.03.247.
- Zhang W, Lu TC, Wei N, et al. Co-precipitation synthesis and vacuum sintering of Nd:YAG powders for transparent ceramics. Mater Res Bull. 2015;70:365–372. doi:10.1016/j.materresbull.2015.04.063.
- Ma B, Wang B, Zhang W, et al. Promotion of powder crystallinity and its influence on the properties of Nd:YAG transparent ceramics. Opt Mater. 2017;64:384–390. doi:10.1016/j.optmat.2017.01.006.
- Zhang W, Lu T, Ma B, et al. Improvement of optical properties of Nd:YAG transparent ceramics by post-annealing and post hot isostatic pressing. Opt Mater. 2013;35:2405–2410. doi:10.1016/j.optmat.2013.06.042.
- Zhang W, Lu T, Wei N, et al. Effect of annealing on the optical properties of Nd:YAG transparent ceramics. Opt Mater. 2012;34:685–690. doi:10.1016/j.optmat.2011.10.001.
- Huang Y, Jiang D, Zhang J, et al. Sintering of transparent Nd:YAG ceramics in oxygen atmosphere. J Rare Earths. 2013;31:153–157. doi:10.1016/S1002-0721(12)60250-6.
- German RM. Chapter nine—sintering with a liquid phase. In: RM German, editor. Sintering: from empirical observations to scientific principles. Boston (MA): Butterworth-Heinemann; 2014. p. 247–303. doi:10.1016/B978-0-12-401682-8.00009-4.
- Francis LF. Chapter 5—powder processes. In: LF Francis, editor. Materials processing. Boston (MA): Academic Press; 2016. p. 343–414. doi:10.1016/B978-0-12-385132-1.00005-7.
- Veith M, Mathur S, Kareiva A, et al. Low temperature synthesis of nanocrystalline Y3Al5O12 (YAG) and Ce-doped Y3Al5O12 via different sol–gel methods. J Mater Chem. 1999;9:3069–3079. doi:10.1039/A903664D.
- Zhydachevskii Y, Syvorotka II, Vasylechko L, et al. Crystal structure and luminescent properties of nanocrystalline YAG and YAG:Nd synthesized by sol-gel method. Opt Mater. 2012;34:1984–1989. doi:10.1016/j.optmat.2011.12.023.
- Qiu F, Pu X, Li J, et al. Thermal behavior of the YAG precursor prepared by sol-gel combustion process. Ceram Int. 2005;31:663–665. doi:10.1016/j.ceramint.2004.08.004.
- Sakar N, Gergeroglu H, Akalin SA, et al. Synthesis, structural and optical characterization of Nd:YAG powders via flame spray pyrolysis. Opt Mater. 2020;103:109819. doi:10.1016/j.optmat.2020.109819.
- Kinsman KM, McKittrick J, Sluzky E, et al. Phase development and luminescence in chromium-doped yttrium aluminum garnet (YAG:Cr) phosphors. J Am Ceram Soc. 1994;77:2866–2872. doi:10.1111/j.1151-2916.1994.tb04516.x.
- Li JG, Ikegami T, Lee JH, et al. Characterization of yttrium aluminate garnet precursors synthesized via precipitation using ammonium bicarbonate as the precipitant. J Mater Res. 2000;15:2375–2386. doi:10.1557/JMR.2000.0342.
- Tong S, Lu T, Guo W. Synthesis of YAG powder by alcohol-water co-precipitation method. Mater Lett. 2007;61:4287–4289. doi:10.1016/j.matlet.2007.01.087.
- Wang L, Kou H, Zeng Y, et al. The effect of precipitant concentration on the formation procedure of yttrium aluminum garnet (YAG) phase. Ceram Int. 2012;38:3763–3771. doi:10.1016/j.ceramint.2012.01.022.
- Chen X, Lu T, Wei N, et al. Systematic optimization of ball milling for highly transparent Yb:YAG ceramic using co-precipitated raw powders. J Alloys Compd. 2015;653:552–560. doi:10.1016/j.jallcom.2015.09.026.
- Tong SH, Lu TC, Guo W, et al. Sinterability of Nd:YAG powder prepared by alcohol-water co-precipitation method. Key Eng Mater. 2008;368–372:423–425. doi:10.4028/www.scientific.net/kem.368-372.423.
- Uematsu K. Processing defects in ceramic powders and powder compacts. Adv Powder Technol. 2014;25:154–162. doi:10.1016/j.apt.2014.01.009.
- Crouch IG, Franks GV, Tallon C, et al. 7—Glasses and ceramics. In: IG Crouch, editor. The science of armour materials. Oxford: Woodhead Publishing; 2017. p. 331–393. doi:10.1016/B978-0-08-100704-4.00007-4.
- Rahaman MN. Ceramic processing and sintering. 2nd ed. Boca Raton (FL): CPC Press; 2017. doi:10.1201/9781315274126.
- Chaim R, Marder-Jaeckel R, Shen JZ. Transparent YAG ceramics by surface softening of nanoparticles in spark plasma sintering. Mater Sci Eng A. 2006;429:74–78. doi:10.1016/j.msea.2006.04.072.
- Chaim R, Kalina M, Shen JZ. Transparent yttrium aluminum garnet (YAG) ceramics by spark plasma sintering. J Eur Ceram Soc. 2007;27:3331–3337. doi:10.1016/j.jeurceramsoc.2007.02.193.
- Frage N, Kalabukhov S, Sverdlov N, et al. Densification of transparent yttrium aluminum garnet (YAG) by SPS processing. J Eur Ceram Soc. 2010;30:3331–3337. doi:10.1016/j.jeurceramsoc.2010.08.006.
- Frage N, Kalabukhov S, Sverdlov N, et al. Effect of the spark plasma sintering (SPS) parameters and LiF doping on the mechanical properties and the transparency of polycrystalline Nd-YAG. Ceram Int. 2012;38:5513–5519. doi:10.1016/j.ceramint.2012.03.066.
- Golovkina LS, Orlova AI, Nokhrin V, et al. Spark plasma sintering of fine-grain ceramic-metal composites based on garnet-structure oxide Y2.5Nd0.5Al5O12 for inert matrix fuel. Mater Chem Phys. 2018;214:516–526. doi:10.1016/j.matchemphys.2018.03.091.
- Kosyanov DY, Vornovskikh AA, Zakharenko AM, et al. Influence of sintering parameters on transparency of reactive SPSed Nd3+:YAG ceramics. Opt Mater. 2021;112:110760. doi:10.1016/j.optmat.2020.110760.
- Rahmani M, Mirzaee O, Tajally M, et al. A comparative study of synthesis and spark plasma sintering of YAG nano powders by different co-precipitation methods. Ceram Int. 2018;44:10035–10046. doi:10.1016/j.ceramint.2018.02.148.
- Sokol M, Kalabukhov S, Kasiyan V, et al. Mechanical, thermal and optical properties of the SPS-processed polycrystalline Nd:YAG. Opt Mater. 2014;38:204–210. doi:10.1016/j.optmat.2014.10.030.
- Spina G, Bonnefont G, Palmero P, et al. Transparent YAG obtained by spark plasma sintering of co-precipitated powder. Influence of dispersion route and sintering parameters on optical and microstructural characteristics. J Eur Ceram Soc. 2012;32:2957–2964. doi:10.1016/j.jeurceramsoc.2012.02.052.
- Suárez M, Fernández A, Menéndez JL, et al. Transparent yttrium aluminium garnet obtained by spark plasma sintering of lyophilized gels. J Nanomater. 2009;2009:138490. doi:10.1155/2009/138490.
- Wagner A, Ratzker B, Kalabukhov S, et al. Highly-doped Nd:YAG ceramics fabricated by conventional and high pressure SPS. Ceram Int. 2019;45:12279–12284. doi:10.1016/j.ceramint.2019.03.141.
- Wagner A, Ratzker B, Kalabukhov S, et al. Residual porosity and optical properties of spark plasma sintered transparent polycrystalline cerium-doped YAG. J Eur Ceram Soc. 2019;39:1436–1442. doi:10.1016/j.jeurceramsoc.2018.11.006.
- Wagner A, Meshorer Y, Ratzker B, et al. Pressure-assisted sintering and characterization of Nd:YAG ceramic lasers. Sci Rep. 2021;11:1–12. doi:10.1038/s41598-021-81194-8.
- Wang R, Liu J, Ji W, et al. Effects of ball-milling on fabrication of YAG ceramics by a phase transformation assisted spark plasma sintering. J Alloys Compd. 2017;701:279–287. doi:10.1016/j.jallcom.2017.01.128.
- Zhang G, Carloni D, Wu Y. Ultraviolet emission transparent Gd:YAG ceramics processed by solid-state reaction spark plasma sintering. J Am Ceram Soc. 2020;103:839–848. doi:10.1111/jace.16785.
- Ikesue A, Kamata K. Microstructure and optical properties of hot isostatically pressed Nd:YAG ceramics. J Am Ceram Soc. 1996;79:1927–1933. doi:10.1111/j.1151-2916.1996.tb08015.x.
- Lee SH, Kupp ER, Stevenson AJ, et al. Hot isostatic pressing of transparent Nd:Yag ceramics. J Am Ceram Soc. 2009;92:1456–1463. doi:10.1111/j.1551-2916.2009.03029.x.
- Esposito L, Piancastelli A, Bykov Y, et al. Microwave sintering of Yb:YAG transparent laser ceramics. Opt Mater 2013;35:761–765. doi:10.1016/j.optmat.2012.07.014.
- Chen Z, Li J, Xu J, et al. Fabrication of YAG transparent ceramics by two-step sintering. Ceram Int. 2008;34:1709–1712. doi:10.1016/j.ceramint.2007.05.015.
- Stevenson AJ. The effects of sintering aids on defects, densification, and single crystal conversion of transparent Nd:YAG ceramics [dissertation]. Pennsylvania State University; 2010.
- Coble RL. Sintering crystalline solids. I. Intermediate and final state diffusion models. J Appl Phys. 1961;32:787–792. doi:10.1063/1.1736107.
- German RM. Chapter seven—thermodynamic and kinetic treatments. In: RM German, editor. Sintering: from empirical observations to scientific principles. Boston (MA): Butterworth-Heinemann; 2014. p. 183–226. doi:10.1016/B978-0-12-401682-8.00007-0.
- Coble RL. Sintering alumina: effect of atmospheres. J Am Ceram Soc. 1962;45:123–127. doi:10.1111/j.1151-2916.1962.tb11099.x.
- Huang Y, Jiang D, Zhang J, et al. Sintering of transparent yttria ceramics in oxygen atmosphere. J Am Ceram Soc. 2010;93:2964–2967. doi:10.1111/j.1551-2916.2010.03940.x.
- Gupta RK, Anil Kumar V, Khanra GP. Reactive and liquid-phase sintering techniques. In: R Mitra, editor. Intermetallic matrix composites. Cambridge: Elsevier; 2018. p. 303–318. doi:10.1016/b978-0-85709-346-2.00011-x.
- Zhang L, Zhou T, Selim FA, et al. Single CaO accelerated densification and microstructure control of highly transparent YAG ceramic. J Am Ceram Soc. 2018;101:703–712. doi:10.1111/jace.15233.
- Kochawattana S, Stevenson A, Lee S-H, et al. Sintering and grain growth in SiO2 doped Nd:YAG. J Eur Ceram Soc. 2008;28:1527–1534. doi:10.1016/j.jeurceramsoc.2007.12.006.