 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A longstanding challenge with high Mg content Al alloys is the trade-off between corrosion resistance and strength, because the stabilization temperature is lower than the recrystallization temperature corresponding to a no stabilization temperature range. Herein, a stabilization temperature range is found with an excellent match of strength and corrosion resistance of high Mg content Al alloy via pulsed electric current. The stimulated atomic diffusion rate resulted in discontinuous grain boundary precipitates, along with a lower electric wind force development rate to maintain dislocation strengthening. These results provide insights into developing high-performance Al-Mg alloys by the pulsed electric field treatment.
GRAPHICAL ABSTRACT

IMPACT STATEMENT
The pulsed electric current treatment provides a new measure to develop high-performance Al alloys with high Mg content, improving the corrosion resistance while ensuring dislocation strengthening.
1. Introduction
5xxx series aluminum alloy has become a pillar material in marine engineering due to its excellent strength-to-weight ratio, outstanding weldability, and advantageous corrosion resistance [Citation1–3]. The alloys are primarily strengthened by strain hardening and solution strengthening of Mg atoms [Citation4,Citation5]. However, when the alloy is used for a long time in an environment above 50 °C, sensitization will occur for the alloy with Mg contents above 3.5 wt.%. The β-Al3Mg2 phase will preferentially and continuously precipitate along the grain boundary (GB), which will significantly reduce the corrosion resistance of the alloy and seriously threaten the safety of ships in service [Citation6–8]. By annealing above the stabilization treatment temperature, usually above 230 °C, the corrosion resistance is improved in long-term service due to the discontinuous distribution of grain boundary precipitates (GBPs) [Citation9]. The alloy can guarantee high dislocation strengthening and excellent corrosion resistance if the stabilization treatment temperature is below the recrystallization starting temperature. Such a temperature range is called the stabilization temperature range [Citation2]. However, the stabilization treatment temperature is higher than the recrystallization starting temperature in high-strength 5xxx series alloys with Mg contents above 6.0 wt.%, resulting in a region below the stabilization treatment temperature and above the recrystallization starting temperature, which is sensitive to intergranular corrosion (IGC) and excessive loss of dislocation strengthening due to recrystallization [Citation6,Citation10,Citation11].
Micro-alloying is typically utilized to improve the dislocation strengthening and corrosion resistance of the alloy simultaneously. The synergistic addition of Er-Zr [Citation1,Citation12,Citation13] and Sc-Zr [Citation14,Citation15] elements dramatically increase the recrystallization starting temperature of Al-Mg alloy, making it possible to stabilize a high Mg content alloy, ensuring the discontinuous distribution of GBPs and the formation of a large number of dispersed phases within the grain. The addition of Y [Citation16], Nb [Citation17], Sr [Citation18–20], B [Citation21], Zn [Citation22,Citation23] elements to Al-Mg alloy can create novel precipitated phases in the grain thereby inhibiting continuous precipitation on the GB. Thermo-mechanical treatment based on GB engineering and the heat treatment repair process for corroded and failed plates are also used to guarantee the dislocation strengthening and corrosion resistance of the alloy [Citation24,Citation25]. However, excessive reliance on micro-alloying resources and complex thermo-mechanical processes raise the cost and hamper the sustainable development for alloy production [Citation26]. Hence, a new development path is required to overcome this hurdle. Therefore, it is of great significance to produce sustainable ‘plain materials' to significantly improve the overall performance of Al-Mg alloys [Citation27,Citation28].
Pulsed electric current treatment (PEC), as a form of electromagnetic field, has become an essential means of metal material modification, which is a method that has been widely used in many metallurgical engineering fields such as solidification structure control [Citation29,Citation30], solid-state phase transformation [Citation30], the electro-plastic effect [Citation31], and crack healing [Citation32]. It has advantages of high efficiency, convenience, greenness, energy-saving, and safety, of which conventional heat treatment (CHT) processes cannot achieve [Citation33]. A previous study showed that PEC can regulate the diffusion rate of GB elements and form discontinuously distributed GBPs, thus improving the corrosion resistance of materials based on the physical properties of the precipitated phase and the differences in electrical properties between the second phase and the matrix [Citation34]. It can also promote the dislocation movement and improve the elongation of the alloy without reducing the strength [Citation35–37].
In addition, the electric wind force is the result of momentum transfer between electrons and metal ion cores [Citation38,Citation39]. The generation of electric wind force is mainly due to the momentum transfer of electrons in the grain boundary region [Citation38,Citation39]. Furthermore, the semi-classical model of the electric wind force assumes ballistic transport, exhibiting minimal disturbance from the lattice [Citation40]. D. Waryoba et al. [Citation41] believe that electric wind force is the interaction of electronic defects and is very effective in two-dimensional defects such as grain boundaries.
This study is a comprehensive comparison of PEC and CHT on alloys relative to the corrosion resistance improvement rate and strength reduction rate.
2. Results and discussion
A schematic diagram of the pulsing device is provided in Fig. S1. The materials and methods are detailed in the Supplementary Material.
Fig. S2 is the microstructure of the cold rolled alloy. From the Electron Backscatter Diffraction (EBSD) results in Fig. S2(a)-(c), it can be seen that after the cold deformation of the alloy, the grains are elongated in the form of bands and there is high stress between the grains. As seen in Fig. S2(e)-(f), the alloy in the cold rolled state has a very low concentration of GBPs at the grain boundaries, indicating that the solid solution of the alloy is high [Citation1–4]. When the alloy is left at room temperature, the precipitation of the Al3Mg2 phase causes the alloy to lose strength. At the same time, stress corrosion and intergranular corrosion occur due to the continuous distribution of the precipitated phase at the grain boundaries [Citation5–9].
Figure shows the hardness variation and EBSD recrystallization fraction of different alloys under PEC and CHT. In general, the microhardness of the alloy decreases with an increase in annealing temperature. Combined with the statistical results of the recrystallization fraction of the alloys after CHT in EBSD, the recrystallization starting temperature is determined when the recrystallization fraction is above 5% [Citation12]. The boundary between 2° and 15° was set as a low angle grain boundary, and above 15° was selected as a high angle grain boundary [Citation42–45]. Under CHT, by compiling the results of the recrystallization fraction and hardness under CHT of the Al-3.97Mg annealed at 220 °C, the Al-4.82Mg annealed at 240 °C, the Al-5.84Mg annealed at 250 °C, and the Al-6.98Mg annealed at 260 °C, it was determined that the recrystallization starting hardness (RSH) values of different alloys were 99.3 ± 3.0 HV, 102.5 ± 3.0 HV, 105.0 ± 3.0 HV, 115.8 ± 3.0 HV, respectively, as shown by the four dashed lines in Figure (a). Under PEC, the dashed line intersects the hardness curves of Al-3.97Mg at 220 °C, Al-4.82Mg at 230 °C, Al-5.84Mg at 230 °C, Al-6.98Mg at 240 °C, respectively, which are taken as the recrystallization starting temperatures under PEC. As shown in Figure (a), at the same microhardness, the temperature of PEC is lower than that of CHT.
Figure 1. (a) Hardness changes of different alloys under pulsed electric current treatment (PEC) and conventional heat treatment (CHT); Under CHT, statistical chart of EBSD recrystallization fractions of (b) Al-3.97Mg alloy annealed at 220°C for 1 h, (c) Al-4.82Mg alloy annealed at 240°C for 1 h, (d) Al-5.84Mg alloy annealed at 250°C for 1 h, (e) Al-6.98Mg alloy annealed at 220°C for 1 h.
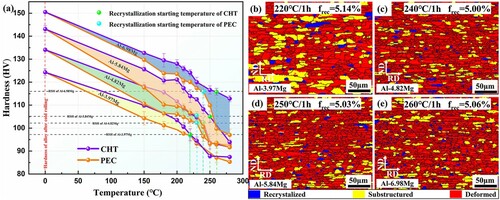
The IGC resistance of alloys treated by different processes is shown in Figure (a). When the Mg content is less than 3.97 wt.%, the alloy has excellent IGC resistance regardless of the treatment method. With an increase in Mg content, the IGC resistance of the alloy is closely related to the treatment process and temperature. Specifically, with increasing temperature, the mass loss of alloys shows a decreasing trend, showing that the alloy's IGC resistance has been gradually improved. The temperature at which the corrosion mass loss of the alloy is below 15 mg/cm2 for the first time is the stabilization temperature of the alloy [Citation12]. It can be found that the stabilization temperatures of Al-4.82Mg, Al-5.84Mg, and Al-6.98Mg under CHT are 230, 260, and 270 °C, respectively, and 210, 230, and 240 °C, respectively, under PEC. XRD tests the dislocation densities of Al-6.98Mg alloys under different processes, and the values are shown in Figure (b). Combining the results in Figures and , the alloy can obtain good corrosion resistance at 270 °C after CHT, and the dislocation density is 3.77×1012 m−2. After PEC, the alloy can obtain good corrosion resistance at 240 °C, and the dislocation density is 1.67×1013 m−2, which is 4.4-fold increase compared to CHT.
Figure 2. (a) Mass loss of different alloys under pulsed electric current treatment (PEC) and conventional heat treatment (CHT), (b) Dislocation densities of Al-6.98Mg under PEC and CHT, (c) The engineering stress - strain curves for Al-6.98Mg under PEC and CHT, (d) The data for the average yield stresses, ultimate tensile strengths and total elongations of Al-6.98Mg under PEC and CHT.
As seen from the tensile test results in Figure (c)-(d), the yield strengths of the alloys are above 300 MPa when the alloy stabilization treatment temperature is lower than the recrystallization starting temperature. Combined with Figure (a), it can be seen that the corrosion resistance of the alloy under PEC is much higher than that of the alloy under CHT while ensuring strength. Moreover, when the corrosion performance of the alloy under CHT is enhanced, the strength of the alloy decreases significantly. When the alloy shows maximum corrosion resistance, the yield strength of the alloy under CHT is reduced by 30 MPa compared to PEC.
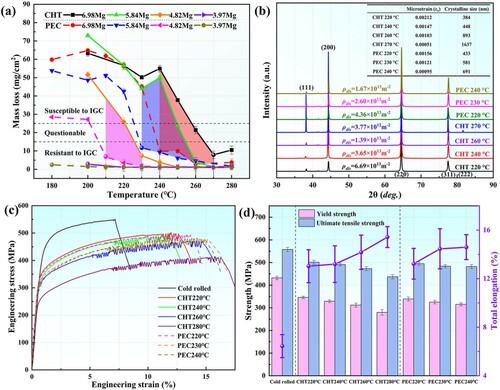
The evolution of GBPs in Al-6.98Mg alloy under different processes is observed, as shown in Figure . As seen from the distribution of GBPs after phosphoric acid erosion, the change in number of GBPs is consistent under CHT and PEC, both of which show the evolution law of GBPs becoming intermittent with increasing temperature. When the treatment temperature is higher than 220 °C, the rate of change of GBPs of the alloy after PEC is faster than that of CHT.
Figure 3. Distribution of grain boundary precipitates (GBPs) in Al-6.98Mg alloy observed by optical microscope (a) (b) (c) (d) and TEM (i) (j) under conventional heat treatment (CHT), by optical microscope (e) (f) (g) (h) and TEM (k) (l) under pulsed electric current treatment (PEC).
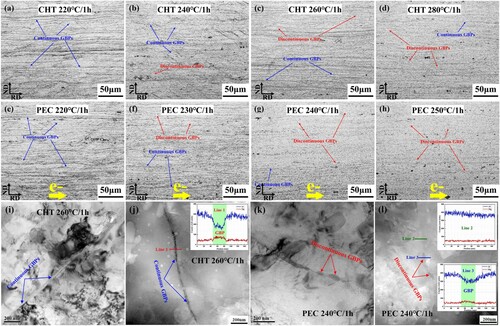
By comparing the distribution of GBPs in Figure (c) and (g), the GBPs with more intermittent distribution are processed by PEC. Meanwhile, Figure (a) shows that the Al-6.98Mg alloy has a comparable hardness under CHT at 260 °C for 1 h and PEC at 240 °C for 1 h. By analyzing the TEM structure in these two states, as shown in Figure (i) and (j), CHT has a narrower and more continuous GBP morphology on the GB. The GBP distribution observed by scanning transmission electron microscopy is shown in Figure (k) and (l). By analyzing the line scanning results at specific positions, it can be observed that the alloy after PEC presents an intermittent distribution of GBPs and reduces the enrichment degree of the Mg element in GBPs.
In Fig. S3, the continuous precipitate is prolate, while the discontinuous precipitate is stubby. Combining the results of Fig. S3 and S4, it can be found that with increasing temperature, the distribution of precipitates becomes more intermittent, the aspect ratio of the precipitates becomes smaller, and the discontinuous precipitate becomes coarser and larger. Comparing CHT and PEC, it can be seen that the GBPs can be distributed intermittently under PEC at a lower treatment temperature, and the coarsening rate of the precipitated phase under PEC is significantly coarser than that of CHT.
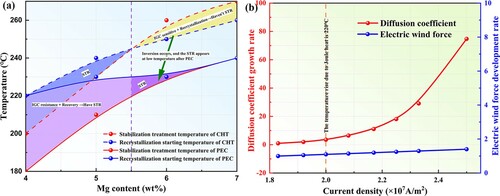
The above results indicate that dislocation strengthening of the Al-6.98Mg alloy occurred, and there is a superior IGC resistance in Al-6.98Mg alloy treated by PEC, which corresponds to the experimental results in Figure (a). With increasing temperature, the temperature drops of the stabilization treatment temperature and recrystallization starting temperature of the alloy treated by PEC are more significant than those treated by CHT. By careful comparison, a fascinating phenomenon can be seen. The temperature drop of the stabilization treatment temperature after PEC is greater than that of the recrystallization starting temperature, which changes the sensitization-stabilization transition temperature range of the PEC in contrast to CHT. When the Mg content is greater than 5.5 wt.%, a low-temperature inversion occurs, and the alloy has a stabilization temperature range after PEC, which does not exist in CHT. Moreover, when the Mg content is less than 5.5 wt.%, the alloy treated by PEC has a broader stabilization temperature range than the alloy treated by CHT.
Figure 4. (a) Stabilization temperature range (STR) after pulsed electric current treatment (PEC) and conventional heat treatment (CHT), (b) Rate change curves of the Mg element diffusion coefficient growth and electric wind force development under PEC.
The electric wind force development rate and diffusion coefficient growth rate of the alloy treated under PEC are calculated. When PEC is applied to the metal material, it applies additional energy, , which is closely related to the electric resistance of the material [Citation46]. The deformed metals own higher electric resistance than those in the recovery and recrystallization states. When PEC passes through the deformed metal, the driving force for recrystallization can be described as [Citation46]
(1)
(1)
where
is the deformation storage energy of deformed material and
is the variation in free energy due to the influence of PEC expressed as
(2)
(2)
where
is the magnetic permeability,
is a positive constant related to the material,
is the volume of the recrystallized nucleus,
is the electrical conductivity of deformed metal,
is the electrical conductivity of the recrystallized metal, and
is the current density.
It is known that deformed metals contain a considerable quantity of dislocations. After recovery and recrystallization, dislocation slip, and annihilation, dislocation density decreases accordingly, which explains that is less than
. Hence,
is a negative value, which shows that the free energy of the alloy in the deformation state is higher than that in the recrystallization state under the action of PEC.
In the recovery and recrystallization process, the free energy decreases due to the dislocation migration, and the dislocation will be subjected to the pressure of migration. H. Conrad et al [Citation31,Citation41] quantitatively calculated the force exerted by the current on unit dislocation length after applying pulse processing, which showed the electronic wind force to be:
(3)
(3)
where
is the electric resistivity (related to the state of the material and the values are shown in Table and Fig. S5),
is the electron charge, and
is the valence electron concentration, the value is 2 [Citation47]. The electronic wind force is the interaction of drifting electrons and dislocations generated when current passes through the metal material, which can promote the annihilation of dislocations, reduce the dislocation density, and promote the blocked dislocation clusters [Citation35,Citation36].
Table 1. The detailed parameters of pulsed electric current treatment.
Then, the electric wind force development rate can be calculated under the PEC
(4)
(4)
where
is the electronic wind force under the PEC at a specific temperature related to current density, where the current density corresponding to temperature can be obtained from , and
is the electronic wind force under the PEC at 200 °C, and current density is shown to be
.
On the other hand, the additional electrical free energy introduced by the PEC based on the current density distribution reduces the atomic transition energy barrier and the atomic diffusion activation energy, accelerating the interface diffusion velocity of elemental Mg [Citation34]. Due to the similar particle radii of Al and Mg atoms, the vacancy diffusion mechanism is dominant in the Al-Mg alloy. The diffusion coefficient of the Mg atom under PEC can be expressed as [Citation34]
(5)
(5)
where
is the diffusion constant,
is the diffusion coefficient of Mg atom under the action of PEC,
is the diffusion activation energy,
is the effective valence, the sum of the wind and direct valence related to the electric resistivity and the values are shown in [Citation41,Citation48],
is Avogadro's constant,
is the molar gas constant, and
is the Kelvin temperature. The parameters used in the calculation of Al-6.98Mg alloy are as follows:
[Citation49],
[Citation37],
,
,
, and
[Citation34]. And, Fig. S6 shows the anisotropic diffusion of atoms under PEC.
At the same time, we mainly consider the influence of the elemental diffusion coefficient under a pulsed electric field, and the detailed calculation formulas are as follows
(6)
(6)
where
is the change in diffusion coefficient under PEC at a specific temperature,
is the diffusion coefficient growth rate, and
is the change in diffusion coefficient under PEC at 200 °C. The calculation results are shown in Figure (b).
When the current density is greater than 2×107 A/m2, the electric wind force development rate increases linearly, while the diffusion coefficient growth rate increases exponentially. The Joule heat temperature caused by PEC at a current density of 2×107 A/m2 increases to 220 °C. This temperature corresponds precisely to the low-temperature inversion interval under the action of PEC in Figure (a). This infers that the effect of PEC on the size of GBPs is more significant than the loss of hardness in the alloy recovery stage, especially when the current density is higher than 2×107 A/m2. It is the main reason for the low-temperature inversion interval caused by the effect of the electric field.
High Mg content Al-Mg alloys under CHT show that the stabilization treatment temperature is higher than the recrystallization starting temperature. There is no stabilization temperature range, which means that the alloy cannot show good corrosion resistance and dislocation strengthening properties simultaneously. However, after PEC, the alloy has a stabilization temperature range at low temperatures, which can have excellent corrosion resistance and dislocation strengthening ability. The results are innovative and have not been reported before.
3. Conclusions
In summary, this study proposes an innovative stabilization treatment method for Al-Mg alloy with high Mg content using PEC.
The stabilization treatment temperature and recrystallization starting temperature were simultaneously reduced by using PEC.
The stabilization treatment temperature was significantly reduced compared with the recrystallization starting temperature due to the higher Mg atom diffusion rate and slower wind force development rate under PEC.
The stabilization temperature range under PEC was reversed at low temperature compared with CHT to achieve excellent corrosion resistance and relatively high dislocation strengthening simultaneously for high Mg content aluminum alloys.
Supplemental Material
Download PDF (1.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ding Y, Gao K, Huang H, et al. Nucleation and evolution of β phase and corresponding intergranular corrosion transition at 100–230°C in 5083 alloy containing Er and Zr. Mater Des. 2019;174:107778.
- Kramer L, Phillippi M, Tack WT, et al. Locally reversing sensitization in 5xxx aluminum plate. J Mater Eng Perform. 2012;21(6):1025–1029.
- Nikulin I, Kipelova A, Malopheyev S, et al. Effect of second phase particles on grain refinement during equal-channel angular pressing of an Al-Mg-Mn alloy. Acta Mater. 2012;60(2):487–497.
- Li X, Xia W, Chen J, et al. Bimodal-structured Al-Mg alloy with high strength and ductility processed by high strain rate rolling at medium temperature. Met Mater-Int. 2021;27(12):5191–5198.
- Nagarajan S, Gurao NP, Parameswaran V. On the kinetics of texture development in Al-Mg alloy under high strain rate tension. Mater Charact. 2020;163:110303.
- Dix Jr EH, Anderson WA, Shumaker MB. Influence of service temperature on the resistance of wrought aluminum-magnesium alloys to corrosion. Corrosion. 1959;15(2):19–26.
- D’Antuono DS, Gaies J, Golumbfskie W, et al. Grain boundary misorientation dependence of β phase precipitation in an Al-Mg alloy. Scr Mater. 2014;76:81–84.
- Steiner MA, Agnew SR. Modeling sensitization of Al-Mg alloys via β-phase precipitation kinetics. Scr Mater. 2015;102:55–58.
- Yang YK, Allen T. Direct visualization of β phase causing intergranular forms of corrosion in Al-Mg alloys. Mater Charact. 2013;80:76–85.
- Zhang R, Steiner MA, Agnew SR, et al. Ror2 signaling regulates Golgi structure and transport through IFT20 for tumor invasiveness. Sci Rep. 2017;7(1):1–14.
- Oguocha INA, Adigun OJ, Yannacopoulos S. Effect of sensitization heat treatment on properties of Al-Mg alloy AA5083-H116. J Mater Sci. 2008;43(12):4208–4214.
- Ding Y, Gao K, Xiong X, et al. High corrosion resistance and strain hardening of high Mg Al-alloy with Er and Zr by using a new reverse stabilization process. Scr Mater. 2019;171:26–30.
- Ding Y, Wu X, Gao K, et al. The influence of stabilization treatment on long-term corrosion resistance and microstructure in Er and Zr containing 5083 aluminum alloy. Mater Charact. 2020;161:110143.
- Wang Z, Lin X, Kang N, et al. Directed energy deposition additive manufacturing of a Sc/Zr-modified Al-Mg alloy: effect of thermal history on microstructural evolution and mechanical properties. Mater Sci Eng A. 2021;802:140606.
- Zhao Y, Polyakov MN, Mecklenburg M, et al. The role of grain boundary plane orientation in the β phase precipitation of an Al–Mg alloy. Scr Mater. 2014;89:49–52.
- Li X, Xia W, Yan H, et al. Enhancing the intergranular corrosion resistance of high Mg-alloyed Al–Mg alloy by Y addition. Mater Corros. 2020;71(11):1802–1811.
- Wang Y, Gupta RK, Sukiman NL, et al. Influence of alloyed Nd content on the corrosion of an Al-5Mg alloy. Corros Sci. 2013;73:181–187.
- Zhang P, Xia W, Yan H, et al. Mechanical properties, corrosion behavior, and microstructure of Sr modified Al-9.2Mg-0.7Mn alloys. Mater Corros. 2019;70(10):1798–1807.
- Sukiman NL, Gupta RK, Buchheit RG, et al. Influence of microalloying additions on Al-Mg alloy. Part 1: corrosion and electrochemical response. Corros Eng Sci Technol. 2014;49(4):254–262.
- Sukiman NL, Gupta RK, Zhang R, et al. Influence of microalloying additions on Al-Mg alloy. Part 2: phase analysis and sensitisation behaviour. Corros Eng Sci Technol. 2014;49(4):263–268.
- Goswami R, Qadri SB. Suppression of Samson phase formation in Al-Mg alloys by boron addition. Mater Lett. 2017;200:21–23.
- Meng C, Zhang D, Cui H, et al. Mechanical properties, intergranular corrosion behavior and microstructure of Zn modified Al-Mg alloys. J Alloys Compd. 2014;617:925–932.
- Meng C, Zhang D, Zhuang L, et al. Correlations between stress corrosion cracking, grain boundary precipitates and Zn content of Al-Mg-Zn alloys. J Alloys Compd. 2016;655:178–187.
- Tan L, Allen TR. Effect of thermomechanical treatment on the corrosion of AA5083. Corros Sci. 2010;52(2):548–554.
- Halap A, Radetić T, Popović M, et al. Influence of the thermomechanical treatment on the intergranular corrosion susceptibility of Zn-modified Al-5.1 Wt Pct Mg-0.7 Wt Pct Mn alloy sheet. Metall Mater Trans A. 2014;45(10):4572–4579.
- Reck BK, Graedel TE. Challenges in metal recycling. Science. 2012;337(6095):690–695.
- Lu K. Stabilizing nanostructures in metals using grain and twin boundary architectures. Nat Rev Mater. 2016;1(5):1–13.
- Li X, Lu K. Playing with defects in metals. Nat Mater. 2017;16(7):700–701.
- Liao X, Zhai Q, Luo J, et al. Refining mechanism of the electric current pulse on the solidification structure of pure aluminum. Acta Mater. 2007;55(9):3103–3109.
- Sheng Y, Hua Y, Wang X, et al. Application of high-density electropulsing to improve the performance of metallic materials: mechanisms, microstructure and properties. Materials (Basel). 2018;11(2):185.
- Conrad H. Electroplasticity in metals and ceramics. Mater Sci Eng A. 2000;287(2):276–287.
- Yu T, Deng D, Wang G, et al. Crack healing in SUS304 stainless steel by electropulsing treatment. J Clean Prod. 2016;113:989–994.
- Youssef KMS, Koch CC, Fedkiw PS. Improved corrosion behavior of nanocrystalline zinc produced by pulse-current electrodeposition. Corros Sci. 2004;46(1):51–64.
- Liu X, Zhang D, Wang H, et al. Regulating solute partitioning utilized to decorate grain boundary for improving corrosion resistance in a model Al-Cu-Mg alloy. Corros Sci. 2021;181:109219.
- Krishnaswamy H, Kim MJ, Hong ST, et al. Electroplastic behaviour in an aluminium alloy and dislocation density based modelling. Mater Des. 2017;124:131–142.
- Kapoor R, Sunil S, Reddy GB, et al. Electric current induced precipitation in maraging steel. Scr Mater. 2018;154:16–19.
- Zhang D, Zhang Z, Pan Y, et al. Current-driving intergranular corrosion performance regeneration below the precipitates solvus temperature in Al-Mg alloy. J Mater Sci Technol. 2020;53:132–139.
- Basaran C, Lin M. Damage mechanics of electromigration induced failure. Mech Mater. 2008;40(1-2):66–79.
- Asoka-Kumar P, O’brien K, Lynn KG, et al. Detection of current-induced vacancies in thin aluminum-copper lines using positrons. Appl Phys Lett. 1996;68(3):406–408.
- Huntington HB, Grone AR. Current-induced marker motion in gold wires. J Phys Chem Solids. 1961;20(1-2):76–87.
- Waryoba D, Islam Z, Wang B, et al. Low temperature annealing of metals with electrical wind force effects. J Mater Sci Technol. 2019;35(4):465–472.
- Huang Y, Humphreys FJ. Measurements of subgrain growth in a single-phase aluminum alloy by high-resolution EBSD. Mater Charact. 2001;47(3-4):235–240.
- Yan J, Heckman NM, Velasco L, et al. Ultrastructural characterization of the lower motor system in a mouse model of krabbe disease. Sci Rep. 2016;6(1):1–10.
- Zhao X, Li S, Yan F, et al. Microstructure evolution and mechanical properties of AZ80 Mg alloy during annular channel angular extrusion process and heat treatment. Materials (Basel). 2019;12(24):4223.
- Mohtadi-Bonab MA, Eskandari M, Szpunar JA. Texture, local misorientation, grain boundary and recrystallization fraction in pipeline steels related to hydrogen induced cracking. Mater Sci Eng A. 2015;620:97–106.
- Jin W, Fan J, Zhang H, et al. Microstructure, mechanical properties and static recrystallization behavior of the rolled ZK60 magnesium alloy sheets processed by electropulsing treatment. J Alloy Compd. 2015;646:1–9.
- Shahri SMG, Idris MH, Jafari H, et al. Effect of solution treatment on corrosion characteristics of biodegradable Mg-6Zn alloy. Trans Nonferrous Met Soc China. 2015;25(5):1490–1499.
- Dekker JP, Lodder A, Van Ek J. Theory for the electromigration wind force in dilute alloys. Phys Rev B. 1997;56(19):12167.
- Goswami R, Spanos G, Pao PS, et al. Precipitation behavior of the ß phase in Al-5083. Mater Sci Eng A. 2010;527(4-5):1089–1095.
