Abstract
We recently reported a 59 year old female, designated WHIM-09, who was born with the rare immunodeficiency disease WHIM syndrome but underwent spontaneous phenotypic reversion as an adult. The causative WHIM mutation CXCR4R334X was absent in her myeloid and erythroid lineage, but present in her lymphoid lineage and in epithelial cells, defining her as a somatic genetic mosaic. Genomic and hematologic analysis revealed chromothripsis (chromosome shattering) on one copy of chromosome 2, which deleted 164 genes including CXCR4R334X in a single haematopoietic stem cell (HSC) (). Experiments in mice indicated that deleting one copy of Cxcr4 is sufficient to confer a selective advantage for engraftment of transplanted HSCs, suggesting a mechanism for clinical cure in WHIM-09. Genome editing may allow autologous transplantation of HSCs lacking one copy of CXCR4 without bone marrow conditioning as a general cure strategy in WHIM syndrome, safely recapitulating the outcome in patient WHIM-09.
Abbreviations
| WHIM | = | Warts, Hypogammaglobulinemia, Infections, and Myelokathexis |
| CXCR4 | = | CXC Chemokine Receptor 4 |
| CXCL12 | = | CXC Chemokine Ligand 12 |
| CCR5 | = | CC Chemokine Receptor 5 |
| HSC | = | haematopoietic stem cell |
| OMIM | = | Online Mendelian Inheritance in Man, www.omim.org |
| BMT | = | bone marrow transplantation |
| GVHD | = | Graft versus Host Disease. |
Warts, Hypogammaglobulinemia, Infections, and Myelokathexis syndrome (WHIM) (OMIM # 193670) is a rare, primary immunodeficiency that was first discovered as a non-cyclic blood neutropenia with increased numbers of neutrophils in the bone marrow (myelokathexis).Citation1,2 The 2 original publications from 1964 were both single case studies of our patient WHIM-09.Citation3 Details of the syndrome, which include severe cutaneous, oral, and anogenital human papillomavirus (HPV) disease, mild and variable hypogamm aglobulinemia and frequent infections, became more evident as additional patients were discovered.Citation4 WHIM syndrome is caused in almost all cases by mutations in the gene for the chemokine receptor CXCR4.Citation5 The syndrome is autosomal dominant and all known mutations are in the carboxy-terminus of the receptor in a region that encompasses negative regulatory elements.Citation6-9 Thus, the mutations increase and prolong signaling when the receptor is bound to its ligand, CXCL12.Citation10 Previous Phase 1 studies have demonstrated that an FDA-approved small molecule inhibitor of CXCR4 named plerixafor (Mozobil™ or AMD3100) appeared to ameliorate warts and infection frequency in a small number of patients,Citation11-13 and an ongoing trial is comparing this treatment to the standard treatment of severe congenital neutropenia (SCN) using infection frequency as the primary endpoint (www.clinicaltrials.gov, Identifier NCT02231879). Should plerixafor be safe and effective, it would likely need to be injected frequently for life, much like insulin for Type I diabetics. Moreover, it is quite expensive. Thus, a cure strategy is needed; however, WHIM syndrome is typically not sufficiently life-threatening to justify bone marrow transplantation, and to date this approach has only been reported once.Citation14 Targeted gene therapy would be ideal, and our patient WHIM-09, who was cured spontaneously by a genetic accident in one HSC, suggests that WHIM syndrome may be an excellent target disease for this approach.
CXCR4 is an HIV coreceptor especially important in late stage disease (AIDS).Citation9 It is also believed to be important in the metastasis and growth of many hematologic and other cancers.Citation15 Lack of CXCR4 or its ligand CXCL12 is perinatal lethal in mice, which die with a complex multisystem phenotype, including abnormalities in hematopoiesis.Citation16-20 CXCR4's role in HSC biology has been known for over 15 years and in fact the reason that plerixafor has been licensed is that it promotes release of CD34+ HSC to the blood from the bone marrow facilitating harvesting of these cells by apheresis for bone marrow transplantation (BMT) in cancer.Citation21,22 However, the role of CXCR4 gene copy number in HSC biology had not been previously fully explored or understood. Patient WHIM-09 provides an experiment of nature showing how CXCR4 might be manipulated in order to enhance BMT, much as transplantation of homozygous CCR5Δ32 bone marrow to an HIV+ leukemia patient (aka the Berlin patient) resulting in functional cure of HIV demonstrated the importance of CCR5 in maintenance of HIV disease.Citation23
Chromothripsis is thought to occur all at once in a single cell that either dies or acquires a selective advantage.Citation24 Thus, we postulate that chromothripsis affecting one copy of chromosome 2 in patient WHIM-09 occurred in a single primitive HSC that subsequently possessed a strong selective advantage that eventually allowed that cell to engraft in the bone marrow replacing most if not all of her previous HSC.Citation3 This is quite unprecedented. Even in BMT, where large numbers of HSCs are transplanted at the same time, engraftment requires pretreatment with a cytoreductive conditioning regimen.Citation25 Conditioning often consists of non-specific chemotherapy and/or irradiation which directly or indirectly results in much of the toxicity of BMT either by initiating graft vs. host disease (GVHD) or damaging the host immune response creating lethal infections. Thus if BMT could be achieved by successfully engrafting autologous gene-corrected HSC that would replace the patient's HSC without the need for conditioning, much of the morbidity, mortality and expense of BMT would be avoided.
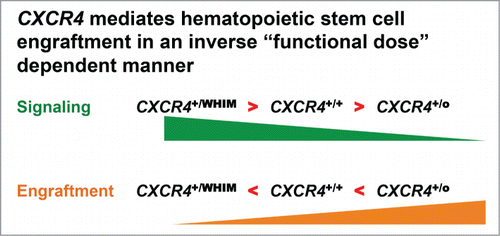
However, our patient was actually haploinsufficient for at least 163 other genes that might also have contributed to efficient engraftment of her seminal chromothriptic HSC.Citation3 Therefore, we turned to murine models of WHIM (Cxcr4+/S338X) and Cxcr4 haploinsufficiency (Cxcr4+/o), in which none of the other 163 genes was disrupted, and used competitive bone marrow repopulation experiments to define whether the competitive advantage apparent for chromothriptic Cxcr4+/o HSC in WHIM-09 could be phenocopied by non-chromothriptic mouse Cxcr4+/o HSC. We found that Cxcr4+/o bone marrow cells are able to outcompete both wild-type (Cxcr4+/+) and WHIM (Cxcr4+/S338X) bone marrow cells after irradiation of the recipient mouse.Citation3 Since CXCR4 mutations in WHIM patients increase signaling by the receptor, this suggested that increased CXCR4 signaling leads to decreased bone marrow engraftment (). We obtained the same result whether whole bone marrow cells or lineage-depleted bone marrow cells were used for transplantation. Together, the results are consistent with the notion that most if not all of ability of HSC from WHIM-09 to engraft can be attributed to the loss of one CXCR4 allele in that cell. We are now following up these findings with additional tests of the hypothesis using more stringent models of HSC transplantation.
Figure 2. Based on the patient described in this article, WHIM-09, and subsequent mechanistic murine bone marrow transplantation experiments, we have demonstrated an inverse correlation between CXCR4 signaling and bone marrow engraftment potential.
New techniques of genome editing have steadily been developed in the past 10 years to allow precise enzymatic cleavage of DNA (so called molecular scissors).Citation26 Adaptation of these techniques to surgically target and inactivate the mutant CXCR4 allele in HSC from WHIM patients might recapitulate and phenocopy outcome of chromothripsis in WHIM-09 with little risk since fully edited CXCR4−/− cells would not be expected to engraft or survive and since conditioning might be obviated.Citation17-20 This could allow WHIM syndrome to be cured as it was in WHIM-09, but by selectively targeting only CXCR4 using edited autologous HSC. Our murine experiments suggest this would be safe and efficacious. Of course, restricting editing to the mutant CXCR4 allele would be key to safety. Ultimately, as gene editing techniques develop further, it may be possible to delete one copy of CXCR4 while simultaneously correcting the underlying genetic defect with autologous HSC from patients with other haematopoietic diseases. This might work best with myeloid or erythroid genetic disorders such as chronic granulomatous disease (CGD) or sickle cell disease. Further murine experiments will clarify the safety and efficacy of this concept. It may also be possible to replicate the apparent engraftment enhancing effect of CXCR4 haploinsufficiency by using drugs, antibodies, or other blocking reagents to temporarily knockdown or inhibit CXCR4 function and then withdraw these engraftment stimulators. This could further enhance safety and broaden the application to nearly all diseases that BMT is currently used to treat. The safety of this approach is still a concern. However, WHIM-09 has been healthy with CXCR4 haploinsufficient HSC for at least 20 years.
Disclosure of Potential Conflicts of Interest
A provisional patent on CXCR4 knockdown as a method to enhance HSC engraftment has been filed by the US government with D.H.M, J.G. and P.M.M. as inventors.
Acknowledgments
We thank the research subjects for their participation in this study.
Funding
This work was supported by the Division of Intramural Research (DIR) of the National Institute of Allergy and Infectious Diseases (NIAID), NIH.
References
- Krill CE, Jr., Smith HD, Mauer AM. Chronic Idiopathic Granulocytopenia. N Engl J Med 1964; 270:973-9; PMID:14122792; http://dx.doi.org/10.1056/NEJM196405072701902
- Zuelzer WW. “Myelokathexis”–a New Form of Chronic Granulocytopenia. Report of a Case. N Engl J Med 1964; 270:699-704; PMID:14101065; http://dx.doi.org/10.1056/NEJM196404022701402
- McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, et al. Chromothriptic cure of WHIM syndrome. Cell 2015; 160:686-99; PMID:25662009; http://dx.doi.org/10.1016/j.cell.2015.01.014
- Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, Kurzrock R. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. Am J Med 1990; 89:663-72; PMID:2239986; http://dx.doi.org/10.1016/0002-9343(90)90187-I
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet 2003; 34:70-4; PMID:12692554; http://dx.doi.org/10.1038/ng1149
- Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H, Snyderman R. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J Biol Chem 1997; 272:28726-31; PMID:9353342; http://dx.doi.org/10.1074/jbc.272.45.28726
- Signoret N, Rosenkilde MM, Klasse PJ, Schwartz TW, Malim MH, Hoxie JA, Marsh M. Differential regulation of CXCR4 and CCR5 endocytosis. J Cell Sci 1998; 111 (Pt 18):2819-30; PMID:9718374
- Venkatesan S, Rose JJ, Lodge R, Murphy PM, Foley JF. Distinct mechanisms of agonist-induced endocytosis for human chemokine receptors CCR5 and CXCR4. Mol Biol Cell 2003; 14:3305-24; PMID:12925765; http://dx.doi.org/10.1091/mbc.E02-11-0714
- Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Ann Rev Immunol 1999; 17:657-700; PMID:10358771; http://dx.doi.org/10.1146/annurev.immunol.17.1.657
- McDermott DH, Lopez J, Deng F, Liu Q, Ojode T, Chen H, Ulrick J, Kwatemaa N, Kelly C, Anaya-O'Brien S, et al. AMD3100 is a Potent Antagonist at CXCR4(R334X), a Hyperfunctional Mutant Chemokine Receptor and Cause of WHIM Syndrome. J Cell Mol Med 2011; 15:2071-81; PMID:21070597; http://dx.doi.org/10.1111/j.1582-4934.2010.01210.x
- Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, Wood B, Hsu FJ. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood 2011; 118:4963-6; PMID:21835955; http://dx.doi.org/10.1182/blood-2011-06-360586
- McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O'Brien S, Penzak SR, Filho JO, Priel DA, Kelly C, Garofalo M, et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood 2011; 118:4957-62; PMID:21890643; http://dx.doi.org/10.1182/blood-2011-07-368084
- McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, Kwatemaa N, Starling J, Fleisher TA, Priel DA, et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood 2014; 123:2308-16; PMID:24523241; http://dx.doi.org/10.1182/blood-2013-09-527226
- Krivan G, Erdos M, Kallay K, Benyo G, Toth A, Sinko J, Goda V, Toth B, Marodi L. Successful umbilical cord blood stem cell transplantation in a child with WHIM syndrome. Eur J Haematol 2010; 84:274-5; PMID:19878273; http://dx.doi.org/10.1111/j.1600-0609.2009.01368.x
- Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int 2010; 60:497-505; PMID:20594270; http://dx.doi.org/10.1111/j.1440-1827.2010.02548.x
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med 1996; 184:1101-9; PMID:9064327; http://dx.doi.org/10.1084/jem.184.3.1101
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998; 393:595-9; PMID:9634238; http://dx.doi.org/10.1038/31269
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A 1998; 95:9448-53; PMID:9689100; http://dx.doi.org/10.1073/pnas.95.16.9448
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996; 382:635-8; PMID:8757135; http://dx.doi.org/10.1038/382635a0
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998; 393:591-4; PMID:9634237; http://dx.doi.org/10.1038/31261
- DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol 2009; 27:4767-73; PMID:19720922; http://dx.doi.org/10.1200/JCO.2008.20.7209
- Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 1999; 283:845-8; PMID:9933168; http://dx.doi.org/10.1126/science.283.5403.845
- Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360:692-8; PMID:19213682; http://dx.doi.org/10.1056/NEJMoa0802905
- Stephens PJ, Greenman CD, Fu B, Yang F, Bignell GR, Mudie LJ, Pleasance ED, Lau KW, Beare D, Stebbings LA, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011; 144:27-40; PMID:21215367; http://dx.doi.org/10.1016/j.cell.2010.11.055
- Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, Burns LJ, Chaudhri N, Davies S, Okamoto S, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Bone Marrow Trans 2012; 47:337-41; PMID:22395764; http://dx.doi.org/10.1038/bmt.2012.5
- Cheng JK, Alper HS. The genome editing toolbox: a spectrum of approaches for targeted modification. Curr Opin Biotechnol 2014; 30C:87-94; http://dx.doi.org/10.1016/j.copbio.2014.06.005

