Abstract
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease with an unknown cause and invariably fatal outcome. We sought to evaluate a correlation between motor neuron disease (MND) mortality rates and residential radon levels that was previously reported for counties in the United Kingdom. We examined the relationships between age-adjusted MND mortality rates in U.S. states with residential radon levels, well water use, and other variables using structural equation modeling. We observed a significant correlation between MND mortality rates and radon levels. However, in structural equation models, radon did not have a significant, direct effect on MND mortality rates. Conversely, MND mortality rates were significantly and directly predicted by race and by the percentage of the population of each state using well water (p < 0.001 and p = 0.022). We observed similar, significant effects for well water use and MND mortality for males and females separately (p < 0.05). In conclusion, we hypothesize that the association of MND mortality rates with well water use reflects contamination of wells with Legionella, a bacterium common in well water that is known to cause neurologic disease. A Legionella hypothesis is a biologically plausible cause of ALS and suggests new avenues for etiologic research.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neuromuscular disease of adult onset characterized by loss of upper motor neurons in the primary motor cortex and loss of lower motor neurons in the brain and spinal cord. Increasing muscle atrophy and weakness progress to paralysis and death, usually from failure of respiratory muscles. The incidence of ALS in the U.S. is approximately 2/100,000 per year and the average time from diagnosis to death is about three years (Citation1). Approximately 5–10% of ALS cases have a genetic or familial origin (FALS). Most cases are sporadic (SALS) and their etiology is unknown. Confirmed risk factors for SALS include increased age, male gender, and cigarette smoking (Citation2,Citation3). A leaner Body Mass Index prior to developing ALS has been observed in multiple cohorts (Citation4). Additionally, several studies suggest that military veterans have a significantly increased risk (Citation5,Citation6). Diverse etiologic hypotheses have been proposed for SALS, including chronic traumatic encephalopathy among athletes, exposure to metals, pesticides, infections, and intense physical activity (Citation7). These hypotheses have received only limited support from epidemiologic studies. Thus, important environmental causes of SALS likely remain to be discovered.
Insights into the etiology of many chronic diseases have come from investigations of their geographic distributions. Geographic investigations in ALS have focused largely on disease clusters, e.g. the ALS-Parkinsonian dementia complex (ALS/PDC) on Guam (Citation8). Conversely, the geography of SALS has attracted comparatively little attention. However, a positive correlation between MND mortality rates and residential radon was reported for counties in the United Kingdom (Citation9). We sought to examine that association using data from U.S. states.
Radon is a radioactive gas that results from the decomposition of radioactive elements, e.g. uranium and radium, in rocks and soil. It is odorless, colorless, and tasteless and can accumulate in homes (Citation10). Although most exposure to radon occurs via inhalation, some occurs via water from private wells (Citation11). We report that, after adjusting for other variables, age-adjusted mortality rates for MND in U.S. states are not associated with residential radon levels but are significantly associated with the use of well water. We hypothesize that ALS may be caused by a common bacterial contaminant of well water, Legionella.
Methods
Age-adjusted mortality rates for MND for U.S. states for the years 2001–2010 were obtained from CDC Wonder and were adjusted to the U.S. 2000 population (Citation12). The percent of the population in each state that was white (Hispanic or non-Hispanic) was obtained from the Census Bureau. Because ALS does not have a unique ICD-10 code, we used mortality rates from motor neuron disease (MND, G12.2). More than 90% of patients with MND have ALS (Citation13). Because virtually all cases of ALS will die of their disease, MND mortality rates are widely used as surrogates for ALS incidence rates (Citation14). We used data from the 47 states and District of Columbia that reported rates to the CDC.
We examined state-wide data on race, urban/rural residence, smoking history, radon levels in homes, and the use of well water. Data on residential radon levels in U.S. states were derived from the Environmental Protection Agency. Briefly, radon measurements obtained from >15,000 radon detectors were placed in a stratified sample of 5694 homes. States received a score ranging from 0.1 to 0.6 that is proportional to the weighted average of radon measured in all of the counties of that state, as previously described (Citation15).
Data on the percentage of each state that uses self-supplied water in 2005 (non-municipal water from private wells or cisterns, henceforth called ‘well water’) were obtained from the U.S. Geological Survey (Citation16). These data were compiled from multiple sources, including government records for well permits, state agencies that regulate utility rates, and bills from waste-water treatment facilities and tax appraisers’ offices (Citation17). The urban/rural status of the state was measured by the population density in urban and rural areas and the percent of the population living in rural areas. U.S. Census Bureau criteria were used to identify urban areas (Citation18). Data on smoking history were obtained from the CDC’s Behavioral Risk Factor Surveillance System (Citation19). We used data for the percentage of the population in each state that ever smoked in the past or were presently smokers (in 2014).
Statistics
We used correlation analysis to identify potential multivariate relationships between the five independent variables with age-adjusted MND death rates. Structural equations were developed using Proc Calis in SAS v 9.4. Aggregate state data for 47 states and the District of Columbia were used to estimate covariances (i.e. relationships) between the independent variables and paths (i.e. prediction estimations) from the independent variables to the dependent variable. The full model was then reduced by removing covariances and paths that were not significant. This method was repeated for age-adjusted MND death rates for males and females separately. Reduced models were compared to full models through general fit indices.
Results
Three states, Arkansas, Minnesota, and Nevada, and the District of Columbia, were missing values for at least one of the seven variables. The average MND mortality rate in whites was 2.11 per 100,000 (SD =0.34, range 1.37–3.10) (), and was highest in Vermont and lowest in Hawaii. Age-adjusted MND mortality rates were approximately 50% higher in males than in females (2.54 vs. 1.74 per 100,000). The percent of population in the state that is white ranged from 25% (Hawaii) to 96% (Vermont) with an average of 81% (SD = .13). Radon scores varied four-fold and were highest in Iowa (0.6) and lowest in Hawaii and Louisiana (0.15). The percent of the population of each state using well water varied 15-fold, with the highest value in Maine (44%) and the lowest in Utah (3%). Well water usage in Washington, D.C. was 0%. There was a large amount of inter-correlation between the five independent variables; six of the 10 possible pairs had p-values <0.10. All five variables except for smoking were directly correlated with MND death rates (p <0.05).
Table I. Descriptive data and correlations of independent variables and age-adjusted death rates per 100,000 people.
We used the data in to build structural equation models that test how well the five variables predict MND death rates while incorporating their relationships with one another. shows the original path model with all potential significant paths. Prediction paths from the independent variables to MND deaths are shown by straight arrows. Covariances are indicated by curved double arrows. The full model found that only race (% white) significantly predicted MND death rates (p <0.001). All other predictors (radon, wells, rural, and smoking) were not significant when all paths were included. Two of the covariances (race with % wells and % rural) also were not significant when all paths were considered (p = 0.233 and p = 0.234).
Figure 1. Original (A) and reduced structural equation models (B) predicting MND death rates. Significant paths are solid lines. Prediction paths are straight arrows and covariances are curved double arrows.
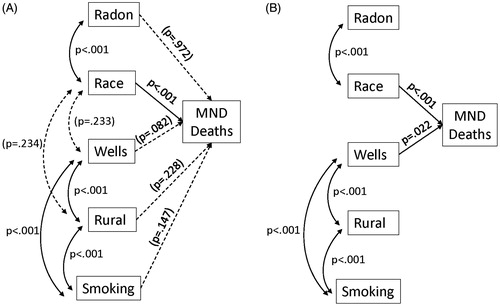
The reduced models for predicting MND death rates among males and females are shown in the lower portion of . There are no differences regarding significant paths in the three reduced models for all individuals or for males and females separately. Race (p <0.01) and well use (p <0.05) are significant predictors in all models. Differences in the strength of the parameters and the fit of the model are very minor, indicating that the model works well for males and females separately and for both genders combined. The model indicates that the MND death rate increases .0178 deaths per each one percent increase in the white population in the state. Similarly, the MND death rate increases by .00783 deaths per 100,000 people for every 1% increase in the population using well water.
Table II. Standardized coefficients from structural equation models predicting MND death rates.
Discussion
We used structural equation modeling to examine the relationships between age-adjusted mortality rates for motor neuron disease (MND) in U.S. states with geographic and demographic variables, including smoking status, rurality, residential radon levels, and use of well water. When all potentially significant relationships were entered into a path model, only race (p <0.001) and the percent of the population using well water (p = 0.022) significantly predicted MND death rates. Separate models for males and females were significant and were nearly identical. Our finding that whites had a significantly greater risk of MND mortality is consistent with previous reports (Citation20,Citation21). To our knowledge, this is the first report that mortality rates for MND in the U.S. are statistically higher in states with higher proportion of residents using well water.
Some epidemiologists have considered the frequency of ALS to be evenly distributed within the U.S. and elsewhere (with rare exceptions of ALS clusters, e.g. in the Western Pacific) (Citation22). Conversely, other groups showed that MND mortality rates in the U.S. are significantly higher in western states and display a band of high rates in the Midwest (Citation23,Citation24). However, the reasons for this uneven geographic distribution have not been identified.
We observed a significant association between MND mortality and the percent of the population of each state that uses private wells. Well water use was significantly associated with living in rural areas. However, none of the Census Bureau definitions for ‘rural’ residence significantly predicted MND mortality rates. Demographers often disagree about the precise meaning(s) of the term ‘rural’ (Citation25). Differences in the definition of rurality may result in some undercounting of the non-urban population, since, for example, individuals with ill-defined addresses (e.g. Post Office boxes) may be difficult to enumerate. We used several Census Bureau definitions of ‘rurality’; none was significantly associated with MND mortality rates. This is consistent with the majority of epidemiologic studies which do not find a rural/urban difference in ALS mortality (Citation26).
The results of our initial correlation between residential radon levels and MND mortality rates are similar to those of Neilson et al. who reported that MND mortality rates in the counties of England and Wales during 1981–1989 were positively correlated with indoor residential radon levels. However, the correlation analysis for U.K. counties was not adjusted for other factors and our findings for radon were no longer significant in more sophisticated models that contained race and well water use. Similarly, smoking was significantly associated with rural residences and with well water, but the association of smoking with MND mortality rates was not significant. Most epidemiologic studies find that smoking increases the risk for MND, but only modestly, with a recent summary Relative Risk estimate for current vs. never smokers of approximately 1.28 (Citation27).
Our finding for well water adds to a small literature on well water and ALS. In a case-control study in Italy, Vincenti et al. reported that consumption of drinking water containing high levels of inorganic selenium was associated with an increased ALS risk (Citation28). However, in that study, it appears that the ‘low selenium content’ water was municipal water and the ‘high selenium content’ water was well water. Thus, confounding between selenium content and water source makes that finding difficult to interpret. Poloni et al. described a married couple, both of whom developed ALS, with a nearly 30-year history of drinking from an artesian well. Analysis of the well water for pesticides and metals, including selenium, was negative (Citation29). In a case-control study in Pennsylvania, ALS cases were twice as likely to have a >20-year history of using water from residential wells (p > 0.03) (Citation30). Similarly, a case-control study in India found a (non-significantly) increased risk of ALS associated with water from tube wells (Citation31).
Our study has several limitations, including its ecologic design and potential biases caused by misclassification and migration. Because the latency period for ALS is unknown, our results are vulnerable to biases due to migration. However, the effects of migration are likely to be non-directional and should bias results towards the null. ALS tends to be under-reported on death certificates and misclassification of cause of death would introduce information bias (Citation32). A recent study of the accuracy of the ICD-10 death certificate coding for ALS indicated that the sensitivity of the G12.2 coding for MND was 85%, and that the positive predictive value was approximately 65% (Citation33). Similarly, a death certificate study in the Danish population found that the sensitivity of death certificates for ALS was 84.2% and the positive predictive value was 82.0% (Citation34). Thus, death certificates appear to be an adequate (albeit imperfect) tool for identifying ALS patients. Because mortality rates for ALS may be lower among Hispanics than non-Hispanics (Citation35), our use of whites, regardless of Hispanic ethnicity, may have introduced confounding. However, between 2000 and 2010, less than 10% of whites self-identified as Hispanic and the majority lived in three states (California, Texas and Florida) (Citation36,Citation37). Thus, confounding by Hispanic ethnicity is unlikely to have greatly influenced our results for 47 states. Lastly, it is well-known that associations that are observed at the level of the group may not be observed at the level of the individual. However, our findings are consistent with the results of several individual level (case-control) studies that found significant associations between well water use and ALS.
What hypotheses might the association of well water use and MND mortality suggest?
Water from private sources is not routinely monitored for contaminants or treated with disinfectants. Thus, well water may contain contaminants that have been implicated in ALS, including metals and run-off from pesticides and herbicides. Alternatively, ALS could be caused by microorganisms in well water.
We speculate that Legionella bacteria may cause ALS. Legionella are the commonest cause of waterborne outbreaks of infectious disease in the U.S. (Citation38). The best-studied species, Legionella pneumophila, causes Legionnaires’ disease, a potentially fatal pneumonia, and Pontiac fever, a milder, flu-like illness (Citation39). Exposure to Legionella typically occurs via inhalation of aerosols or aspiration of bacteria-contaminated water; it is not transmitted by person-to-person contact (Citation40). Factors that independently predict community-acquired legionellosis are cigarette smoking, recent residential plumbing repairs (which dislodge bacteria from pipes), and the use of well water (Citation41,Citation42).
Legionellosis is a multi-system disease with protean effects on the central nervous system (Citation43). Approximately 40–50% of patients with legionellosis present with encephalopathy. Neurological manifestions of Legionella infection include cerebellar dysfunction (Citation44), facial nerve palsy (Citation45), Guillain-Barré syndrome (Citation46), and transverse myelitis and acute motor sensory axonal neuropathy (Citation47,Citation48). CNS disease can occur without a preceding pneumonia (Citation49–51) and neurologic deficits may persist years after resolution of the lung infection (Citation50–52) Reactivation of Legionella infections can occur and asymptomatic infections are common (Citation53–56).
How Legionella causes neurological disease is incompletely understood. Direct invasion of the CNS and muscle has been described (Citation57–60). Other proposed mechanisms include an inflammatory response and the release of an endotoxin-like agent (Citation61,Citation62). A Legionella-ALS hypothesis is supported by similarities in the descriptive epidemiology of Legionnaires’ disease and ALS: the peak incidence of both diseases occurs after age 50 years; both show a male predominance; and the only established behavioral risk factor for ALS, cigarette smoking, is a risk factor for infection with Legionella (Citation63). Military veterans appear to have an increased risk for ALS, regardless of deployment status (Citation64). That finding is intelligible because hospitalization for pneumonia, including that caused by Legionella, is many times more common in recruit than in civilian populations (Citation65,Citation66). Lastly, the state with the highest age-adjusted-MND mortality rate in these data, Vermont, has historically had a high prevalence of legionellosis and was the site of two epidemics of Legionnaires’ disease in 1980 (Citation67,68).
A Legionella-ALS hypothesis makes numerous predictions. For example, Legionella should be found more frequently in the residential water supply of ALS cases than of controls (Citation69). Similarly, ALS cases may report more frequent use of whirlpools/heated spas, which are a reservoir for Legionella, and may have a higher pre-morbid rate of pneumonia (Citation70). Additionally, evidence of Legionella infection should be higher in biologic specimens from ALS patients than from non-neurologic controls. Importantly, if some cases of ALS are caused by persistent Legionella infection, therapy with antibiotics effective against Legionella may limit disease progression (Citation71).
Conclusion
Our ecologic analyses indicate that state-wide mortality rates for MND in the U.S. are significantly correlated with the percentage of the states’ population that uses well water. That MND mortality rates are correlated with the use of well water, a known reservoir for Legionella, and that individuals with legionellosis commonly develop central nervous system disease, suggests that some cases of ALS may be caused by Legionella.
Lastly, our study informs a debate about epidemiologic methods. Some epidemiologists have disputed the ability of ecologic studies to generate hypotheses claiming that, since the data in such studies were pre-selected, the hypothesis under discussion must have pre-existed; thus ecologic studies are not hypothesis generating (Citation72). Here we examined ecologic data on well water in order to examine an extant hypothesis about ALS and radon. Examination of the data stimulated another, novel hypothesis: that ALS is caused by Legionella. This demonstrates that ecologic studies indeed can be hypothesis generating.
Declaration of interest
The authors declare that they have no conflict of interest regarding the ideas or data presented in this paper.
References
- Salameh JS, Brown RH Jr, Berry D. Amyotrophic lateral sclerosis: review. Semin Neurol. 2015;35:469–76.
- Armon C. Smoking may be considered an established risk factor for sporadic ALS. Neurology. 2009;17:1693–8.
- Huisman MHB, de Jong SW, van Doormaal TC, Weinreich SWW, Schelhaas HJ, van der Kooi AJ, et al. Population based epidemiology of amyotrophic lateral sclerosis using capture-recapture methodology. J Neurol Neurosurg Psychiatry. 2011;82:1165–70.
- O’Reilly ÉJ, Wang H, Weisskopf MG, Fitzergerald KC, Falcon G, McCullough ML, et al. Premorbid body mass index and risk of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:205–11.
- Weisskopf MG, O’Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, et al. Prospective study of military service and mortality from ALS. Neurology. 2005;11:32–7.
- Seals RM, Kioumourtzoglou M-A, Gredal O, Hansen J, Weisskopf MG. ALS and the military: a population-based study in the Danish registries. Epidemiology. 2016;27:188–93.
- Ingre C, Roos PM, Piehl F, Kamel F, Fang F. Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol. 2015;7:181–93.
- Lee SE. Guam Dementia Syndrome revisited in 2011. Curr Opin Neurol. 2011;24:517–24.
- Neilson S, Robinson I, Clifford Rose F. Ecological correlates of motor neuron disease mortality: a hypothesis concerning an epidemiological association with radon gas and gamma radiation. J Neurol. 1996;243:329–36.
- Environmental Protection Agency. National Residential Radon Survey. Summary Report.
- Capello MA, Ferraro A, Mendelsohn AB, Prehn AW. Radon-contaminated drinking water from private wells: an environmental assessment examining a rural Colorado mountain community experience. J Environ Health. 2013;76:18–24.
- http://wonder.cdc.gov/ucd-icd10.html. Accessed January 31, 2016.
- Armon C. Amyotrophic Lateral Sclerosis. In: Nelson LM, Tanner CM, van den Eeden SK, McGuire VM, editors. Neuroepidemiology: Oxford, UK Oxford University Press: From Principles to Practice; 2004. pp 162–87.
- Marin B, Couratier P, Preux P-M, Logroscino G. Can mortality data be used to estimate amyotrophic lateral sclerosis incidence? Neuroepidemiol. 2011;36:29–38.
- Schwartz GG, Klug MG. Incidence rates of chronic lymphocytic leukemia in US states are associated with residential radon levels. Future Oncol. 2016;12:165–74.
- Kenny JF, Barber NL, Hutson SS, Linsey KS, Lovelace JK, Maupin MA. Estimated use of water in the United States in 2005. Circular 1344, U.S. Department of the Interior, U.S. Geological Survey, Reston, VA; 2009.
- Hutson SS. Guidelines for preparation of state water use estimates for 2005. U.S. Department of the Interior, U.S. Geological Survey. Available at: http://pubs.usgs.gov/tm/2007/tm4e1/pdf/tm4-e1.pdf. Accessed February 10, 2015.
- https://www.census.gov/. Accessed January 29, 2016.
- http://www.cdc.gov/brfss/. Accessed January 29, 2016.
- Noonan CW, White MC, Thurman D, Wong L-Y. Temporal and geographic variation in United States motor neuron disease mortality, 1969–1998. Neurology. 2005;64:1215–21.
- Rechtman L, Jordan H, Wagner L, Horton DK, Kaye W. Racial and ethnic differences among amyotrophic lateral sclerosis cases in the United States. Amyotroph Lateral Scler Frontal Degener. 2015;16:65–71.
- Kurtzke JF. Epidemiology of amyotrophic lateral sclerosis. Adv Neurol. 1982;36:281–302.
- Bharucha NE, Schoenberg BS, Raven RH, Pickle LW, Byar DP, Mason TJ. Geographic distribution of motor neuron disease and correlation with possible etiologic factors. Neurology. 1983;33:911–5.
- Sejvar JJ, Holman RC, Bresee JS, Kochanek KD, Schonberger LB. Amyotrophic lateral slerosis mortality in the United States, 1979–2001. Neuroepidemiol. 2005;25:144–52.
- Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health. 2005;95:1149–55.
- Belabsis L, Bellou V, Evangelou E. Environmental risk factors and amyotrophic lateral sclerosis: a umbrella review and critical assessment of current evidence from systematic reviews and meta-analyses of observational studies. Neuroepidemiol. 2016:46:96–105.
- Alonso A, Logroscino G, Hernán MA. Smoking and the risk of amyotrophic lateral sclerosis: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2010;81:1249–52.
- Vincenti M, Bonvicini F, Rothman KJ, Vescovi L, Wang F. The relation between amyotrophic lateral sclerosis and inorganic selenium in drinking water: a population based case-control study. Environ Health. 2010;9:77.
- Poloni M, Micheli A, Facchetti D, Mai R, Ceriani F, Cattalini C. Conjugal amyotrophic lateral sclerosis: toxic clustering or chance? Int J Neurol Sci. 1987;18:109–12.
- Malek AM, Burchowsky A, Bowser R, Herman-Patterson T, Lacomis D, Rana S, et al. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neurodegener Dis. 2014;14:31–8.
- Das K, Nag C, Ghosh M. Familial, environmental, and occupational risk factors in the development of amyotrophic lateral sclerosis. N Am J Med Sci. 2012;4:350–5.
- Ludolph AC, Knirsch U. Problems and pitfalls in the diagnosis of ALS. Neurol Sci. 1999;165 (Suppl): S14–20.
- Stickler DE, Royer JA, Hardin JW. Accuracy and usefulness of ICD-10 death certificate coding for the identification of patients with ALS: results from the South Carolina ALS Surveillance Pilot Project. Amyotroph Lateral Scler. 2012;13:69–73.
- Kiourmourtzoglou MA, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:224–9.
- Cronin S, Hardiman O, Traymor BJ. Ethnic variation in the incidence of ALS. A systematic review. Neurology. 2007;68:1002–7.
- Hixon L, Hepler BB, Kim MO. The White population: 2010. 2010 Census Briefs. September 2011.
- Ennis SR, Rios-Vargas M, Albert NG. The Hispanic population: 2010. 2010 Census Briefs, May 2011.
- Yoder J, Roberts V, Craun FG, Hill V, Hicks LA, Alexander NT, et al. Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking – United States 2005–2006. MMWR Surveill Summ. 2008;57:39–62.
- Steinert M, Hentschel U, Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Micrbiol Rev. 2002;26:149–62.
- Falkinham JO III, Hilborn ED, Arduino MJ, Pruden A, Edwards MA. Epidemiology and ecology of opportunistic plumbing pathogens: Legionella pneumophila, Mycobacterium avium, and Pseudomonas aeruginosa. Environ Health Perspect. 2015;123:749–58.
- Strauss WL, Plouffe JF, File JM Jr, Lipman HB, Hackman BH, Salstrom SJ,. Ohio Legionnaires Disease Group. Risk factors for domestic acquisition of Legionnaires’ disease. Arch Intern Med. 1996;156:1685–92.
- Stout JE, Yu VL, Muraca P, Joly J, Troup N, Tompkins LS. Potable water as a cause of sporadic cases of community-acquired Legionnaires’ disease. N Eng J Med. 1992;326:15–155.
- Gregory DW, Schaffner W, Alford RH, Kaiser AB, McGee ZA. Sporadic cases of Legionnaires’ disease: the expanding clinical spectrum. Ann Intern Med. 1979;90:518–21.
- Baker PCH, Price TRP, Allen CD. Brain stem and cerebellar dysfunction with Legionnaires’ disease. J Neurol Neurosurg Psychiat. 1981;44:1054–6.
- Basani S, Ahmed M, Habte-Gabr E. Legionnaires’ disease with facial nerve palsy. Case Rep Medicine. 2011;10:1–4.
- Vigneri S, Spadaro S, Faronelli I, Ragazzi R, Bolta CA, Capone JG, et al. Acute respiratory failure onset in a patient with Guillain-Barré syndrome after Legionella-associated pneumonia: a case report. J Clin Neuromusc Dis. 2014;16:74–8.
- Canpolat M, Kumandas S, Yikilmaz A, Gumus H, Koseoglu E, Poyrazoğlu HG, et al. Transverse myelitis and acute motor sensory axonal neuropathy due to Legionella pneumophila: a case report. Ped International. 2013;55:778–82.
- Vigneri S, Spadaro S, Faronelli I, Ragazzi R, Bolta CA, Capone JG, et al. Acute respiratory failure onset in a patient with Guillain-Barré syndrome after Legionella-associated pneumonia: a case report. J Clin Neuromusc Dis. 2014;16:74–8.
- Sakamoto R, Ohno A, Nakahara T, Satomura K, Iwanaga S, Saito M, et al. A patient with Legionnaires’ disease transferred after a traffic accident. Br Med J case report 2009;doi:10.1136/bcr.09.2008.0893.c.
- Shelburne SA, Kielhofner MA, Tiwary PS. Cerebeller involvement in legionellosis. South Med J. 2004;97:61–4.
- Calza L, Briganti E, Casolari S, Manfredi R, d’Orsi G, Chiodo F, et al. Severe peripheral neuropathy with arreflexia and flaccid quadriplegia complicating Legionnaires’ disease in an adult patient. Infect Dis Clin Pract. 2004;12:110–3.
- Harris LF. Legionnaires’ disease associated with acute encephalomyelitis. Arch Neurol. 1981;38:462–3.
- Lattimer GL, Rhodes LV III, Salventi JS, Galgon JP, Stonebraker V, Boley S, et al. The Philadelphia epidemic of Legionnaire’s Disease. Clinical, pulmonary, and serologic findings two years later. Ann Intern Med. 1970;90:522–36.
- Naot Y, Brown A, Elder EM, Shonnard J. IgM and IgG antibody response in two immunosuppressed patients with Legionnaires’ disease. Evidence of reactivation of latent infection. Am J Med. 1982;73:791–4.
- Gläser S, Weitzel T, Schiller R, Suttorp N, Lück PC. Persistent culture-positive Legionella infection in an immunocompetent adult. Clin Infect Dis. 2995;41:765–6.
- Nagelkerke NJD, Boshuizen HC, de Melker HE, Schellekens JFP, Peeters MF, Conyn-van Spaendonck M. Estimating the incidence of subclinical infections with Legionella pneumonia using data augmentation: analysis of an outbreak in the Netherlands. Stat Med. 2003;22:3713–24.
- Warner CL, Fayad PB, Heffner RR Jr. Legionella myositis. Neurology. 1991;41:750–2.
- Monforte R, Marco F, Estruch R, Campo E. Multiple organ involvement by Legionella pneumophila in a fatal case of Legionnaires’ disease. J Infect Dis. 1989;159:809.
- Perpoint T, Jamilloux Y, Descloux E, Ferry T, Chidiac C, Lina G, et al. PCR-confirmed Legionella non-pneumophila meningoencephalitis. Med Maladies Infect. 2013;43:32–4.
- Charles M, Johnson E, Macyk-Davey A, Henry M, Nilsson J-E, Miedzinski L, et al. Legionella micdadei brain abscess. J Clin Microbiol. 2013;51:701–2.
- Weir AI, Bone I, Kennedy DH. Neurological involvement in legionellosis. J Neurol Neurosurg Psychiatry. 1982;45:603–8.
- Wong KH, Moss CW, Hochstein DH, Arko RJ, Schalla WO. ‘Endotoxicity’ of the Legionnaires’ disease bacterium. Ann Intern Med. 1979;90:624–7.
- European Center for Disease Prevention and Control. Legionnaires’ disease in Europe 2012. Stockholm:ECDC; 2014.
- Beard JD, Kamel F. Military service, deployments, and exposures in relation to amyotrophic lateral sclerosis. Epi Rev. 2015;37:55–70.
- Amundson DE, Weiss PJ. Pneumonia in military recruits. Mil Med. 1994;159:629–31.
- McDonough EA, Metzgar D, Hansen CJ, Myers CA, Russell KL. A cluster of Legionella-acquired pneumonia cases in a population of military recruits. J Clin Microbiol. 2007;45:20127–75.
- Broome CV, Goings SA, Thacker SB, Vogt RL, Beaty HN, Fraser DW. The Vermont epidemic of Legionnaires’ disease. Ann Intern Med. 1979;90:573–7.
- Klaucke DN, Vogt RL, LaRue D, Witherell LE, Orciari LA, Spitalny KC, et al. Legionnaires’ disease: the epidemiology of two outbreaks in Burlington, Vermont, 1980. Am J Epidemiol. 1984;119:382–91.
- Atlas RM. Legionella: from environmental habitats to disease pathology, detection and control. Env Microbiol. 1999;1:283–93.
- Costa J, da Costa MS, Veríssimo A. Colonization of a therapeutic spa with Legionella spp: a public health issue. Res Microbiol. 2010;161:18–25.
- Pedro-Botet ML, Yu VL. Treatment strategies for Legionella infection. Expert Opin Pharmacother. 2009;10:1109–21.
- Savitz DA. A niche for ecologic studies in environmental epidemiology. Epidemiology. 2012; 23:53–4.
