Abstract
In the 24-week double-blind study of edaravone in ALS (MCI186-16), edaravone did not show a statistically significant difference versus placebo for the primary efficacy endpoint. For post-hoc analyses, two subpopulations were identified in which edaravone might be expected to show efficacy: the efficacy-expected subpopulation (EESP), defined by scores of ≥2 points on all 12 items of the ALS Functional Rating Scale-Revised (ALSFRS-R) and a percent predicted forced vital capacity (%FVC) ≥80% at baseline; and the definite/probable EESP 2 years (dpEESP2y) subpopulation which, in addition to EESP criteria, had definite or probable ALS diagnosed by El Escorial revised criteria, and disease duration of ≤2 years. In the 36-week extension study of MCI186-16, a 24-week double-blind comparison followed by 12 weeks of open-label edaravone (MCI186-17; NCT00424463), analyses of ALSFRS-R scores of the edaravone-edaravone group and edaravone-placebo group for the full analysis set (FAS) and EESP, as prospectively defined, were reported in a previous article. Here we additionally report results in patients who met dpEESP2y criteria at the baseline of MCI186-16. In the dpEESP2y, the difference in ALSFRS-R changes from 24 to 48 weeks between the edaravone-edaravone and edaravone-placebo groups was 2.79 (p = 0.0719), which was greater than the differences previously reported for the EESP and the FAS. The pattern of adverse events in the dpEESP2y did not show any additional safety findings to those from the earlier prospective study. In conclusion, this post-hoc analysis suggests a potential effect of edaravone between 24 and 48 weeks in patients meeting dpEESP2y criteria at baseline.
Introduction
In study MCI186-16, a double-blind 24-week trial of edaravone in the treatment of ALS, edaravone did not demonstrate superiority to placebo for the primary efficacy endpoint of change in the ALS Functional Rating Scale-Revised (ALSFRS-R). However, a beneficial trend favouring edaravone over placebo was observed (Citation1). Exploratory analyses were conducted to identify a subgroup in which edaravone might be expected to show efficacy and two study subpopulations were thus identified that did appear to show a significant effect of edaravone versus placebo (Citation2). The subpopulation first defined was the efficacy-expected sub-population (EESP), which comprised patients with scores of ≥2 points on all 12 items of ALSFRS-R (broad retained functionality), and with a percent forced vital capacity (%FVC) of 80% or greater (almost normal respiratory function) at baseline. The other subpopulation, defined as definite/probable EESP 2 years (dpEESP2y), comprised patients who, in addition to meeting EESP criteria, met the definition of definite or probable ALS diagnosed by El Escorial revised criteria and had a disease duration of two years or less.
Study MCI186-17 was a 36-week extension of MCI186-16. When the statistical analyses of MCI186-17 were prospectively planned, post-hoc analyses of MCI186-16 were also ongoing. For this reason, the FAS and EESP were prospectively defined and included in the MCI186-17 statistical analyses plan but the dpEESP2y was not. In this paper, we present the post-hoc analysis of the 24-week placebo-controlled portion of MCI186-17 for the dpEESP2y, i.e. patients who met dpEESP2y criteria at baseline of study MCI186-16. This report therefore adds to the prospectively planned investigation of safety and efficacy in the full analysis set (FAS) and EESP in MCI186-17 as reported in the previous article in this supplement (Citation3).
Patients and methods
Patients
From the FAS of MCI186-17, patients who met the following dpEESP2y criteria at baseline of study MCI186-16 were identified.
Scores of ≥2 points on all 12 items of the ALSFRS-R To enhance scale sensitivity to detect functional deterioration in each ALSFRS-R item
Forced vital capacity (%FVC) ≥80% of predicted To reduce variability in disease progression rates during the study period since very rapid progression in patients with respiratory insufficiency might mask any drug effect
Definite or probable ALS based on El Escorial revised criteria To detect disease progression based on changes in ALSFRS-R score within the 24 weeks of the study
Disease duration of ≤2 years after the first symptom To exclude patients who have ALS of longer duration because they are less likely to show marked disease progression within 24 weeks
Additional details of the hypotheses behind and identification of the dpEESP2y are described in a previous article in this supplement (Citation2).
Study design
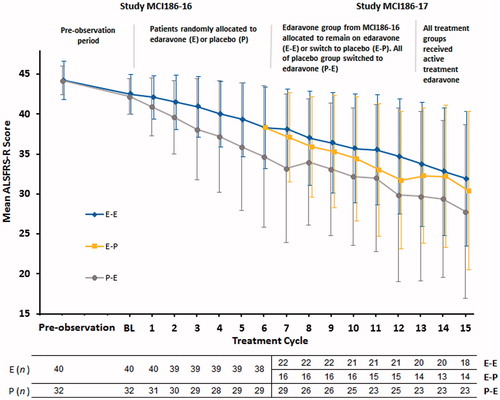
MCI186-16 was a 24-week placebo-controlled, double-blind study comparing 60 mg edaravone and placebo (Clinical trials.gov identifier NCT00330681). Its extension study, MCI186-17, consisted of a 24-week placebo-controlled, double-blind period followed by 12-week open-label active treatment period (NCT00424463) (Citation3). Patients who received edaravone in MCI186-16 were reassigned to 60 mg edaravone or to placebo and patients who received placebo in MCI186-16 were switched to edaravone. All treatment assignments (edaravone-edaravone E-E, edaravone-placebo E-P, and placebo-edaravone P-E) were established at the baseline of MCI186-16 using a dynamic randomisation of minimisation method with three factors of change in ALSFRS-R score during the pre-observation period (–1 or –2 vs. –3 or –4), initial symptom (bulbar vs. limb) and use of riluzole (yes or no). With the unblinding of MCI186-16, the assignment of P-E in MCI186-17 was revealed because these patients all came from the MCI186-16 placebo group. However, the allocation between E-E and E-P was maintained under blinding during the present study, MCI186-17. After cycle 12, patients in all treatment groups could receive an additional three cycles of active open-label edaravone treatment (and therefore could receive a total of 15 cycles in the two successive studies) (Citation3).
Efficacy evaluation
The post-hoc analysis reported here focussed on the double-blind period for comparisons between treatment groups. We analysed the changes in ALSFRS-R scores in the dpEESP2y from week 24 (start of MCI186-17) to week 48 (end of placebo-controlled treatment in MCI186-17). We also analysed the changes in ALSFRS-R scores from baseline to week 24 and from week 24 to week 48 in subpopulations that met each criterion used to define the dpEESP2y.
We applied the same statistical methods used in the prospective analyses of the FAS and EESP as previously reported (Citation3). The change from baseline of cycle 7 to the end of cycle 12 in ALSFRS-R score was compared between the E-E group and the E-P group using analysis of variance with treatment group and change in ALSFRS-R score during the pre-observation period (–1 or –2 vs. –3 or –4) as fixed effects. In MCI186-16 only, initial symptom (bulbar, limb) and use of riluzole were also fixed effects. The statistical significance level was set at 0.05 at a two-sided level without adjustment for multiplicity. The last observation carried forward (LOCF) method was applied to the patients with missing data, provided they had completed week 36 (end of cycle 9).
Additionally, a random coefficient model (Citation4) including all available patient data was applied to the ALSFRS-R to characterise the potential effect of treatment on disease progression assessed by slope between baseline in cycle 1 and the end of cycle 6 and between baseline in cycle 7 and the end of cycle 12.
We also analysed, in the dpEESP2y, percent predicted FVC, Modified Norris Scale score, the 40-item ALS Assessment Questionnaire (ALSAQ-40) score, grip strength and pinch strength.
Safety evaluation
Adverse events (AEs) and serious adverse events (SAEs) were summarised for the dpESSP2y.
Results
Patients
A total of 181 patients were enrolled in study MCI186-17. Dispositions of the FAS, EESP, and dpEESP2y populations are summarised in .
Table 1. Disposition of patients in each population.
The dpEESP2y consisted of a total of 67 patients: 22 patients in the E-E group, 16 patients in the E-P group, and 29 patients in the P-E group. Demographics of the dpEESP2y are summarised in . Percentages of male patients, patients with initial symptom of limb ALS and patients with change in ALSFRS-R score of –2, –1 during pre-observation were higher in the E-P group than in the E-E group, and median patient body weight was greater in the E-P group compared to the E-E group.
Table 2. Demographics and baseline characteristics of the dpEESP2y population.
ALSFRS-R score
Analyses of ALSFRS-R change in the dpEESP2y, and subpopulations meeting each criterion are presented in . The least square mean difference in ALSFRS-R changes between the E-E group and E-P group was 2.79 ± 1.51 in the dpEESP2y (p = 0.0719). A forest plot of ALSFRS-R changes in the FAS, EESP and dpEESP2y is shown for the double-blind periods of MCI186-16 (baseline to week 24) in and for the double-blind period of MCI186-17 (week 24 to week 48) in . Of the three study populations, the dpEESP2y showed the greatest difference between edaravone and placebo, although both subpopulations showed greater differences than in the FAS. This pattern was evident in both MCI186-16 and MCI186-17.
Figure 1. Forest plots of between-group differences in least-squares mean (Δ LS-mean) of ALSFRS-R change in the FAS, EESP, and dpEESP2y during double-blind treatment.
1a. From baseline to week 24 of study MCI186-16; 1b from week 24 to week 48 of study MCI186-17.
FAS = full analysis set; EESP = efficacy-expected sub-population of ALS patients (% forced vital capacity of ≥80% before treatment and ≥2 points for all item scores in the ALSFRS-R before treatment); dpEESP2y = subgroup of the EESP, containing patients with a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria and with disease duration of ≤2 years; ALSFRS-R = revised ALS functional rating scale; CI = confidence interval; SE = standard error.
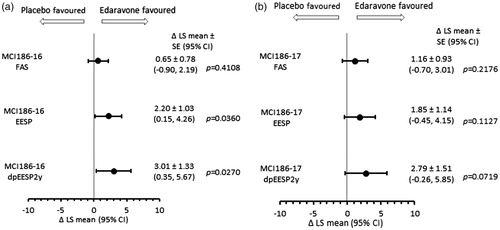
Table 3. Changes in ALSFRS-R scores in study MCI186-16 and subsequent study MCI186-17 in FAS, subpopulation of each criterion in FAS, EESP and dpEESP2y (LOCF).
ALSFRS-R score changes during the entire treatment from cycle 1 to cycle 15 are shown in . In the dpEESP2y, the slopes of the time-dependent change in ALSFRS-R (per cycle) from cycle 1 to cycle 6 in MCI186-16 were significantly different between the treatment groups (E: –0.69 ± 0.09 vs. P: –1.21 ± 0.21, p = 0.0237). The slopes between cycle 7 and cycle 12 in MCI186-17 appeared to show a separation between the E-E and E-P group, although this did not reach statistical significance (E-E: –0.67 ± 0.13 vs. E-P: –0.98 ± 0.22, p = 0.2372 or P-E: –1.11 ± 0.17).
Other efficacy endpoints
Differences between treatment groups for %FVC, Modified Norris Scale, ALSAQ40 and grip and pinch strength in the dpEESP2y are presented in . There were no statistically significant differences between the E-E group and E-P group other than a smaller decline in the Modified Norris Scale in the E-E group (p = 0.0321).
Table 4. Analysis of other efficacy endpoints in the dpEESP2y (Change from baseline of cycle 7 to the end of cycle 12) (LOCF).
Safety
AEs reported in at least two patients in any treatment group in the dpEESP2y in study MCI186-17 (cycle 7 to 15) are summarised in . The most frequently reported across the three treatment groups were gait disturbance, constipation, dysphagia, and nasopharyngitis. Many AEs were consistent with symptoms of progressing ALS. SAEs were reported in 45.5% of the E-E group, 18.8% of the E-P group, and 48.3% of the P-E group (
Table 5. Adverse events reported in at least two patients in any treatment group: cycle 7 to cycle 15.
Table 6. Serious adverse events reported in any treatment group: cycle 7 to cycle 15.
Discussion
In these post-hoc analyses of studies MCI186-16 and MCI186-17, the dpEESP2y showed a greater between-group difference in ALSFRS-R after edaravone treatment than the EESP, although the EESP showed a larger difference than the FAS. There are inherent limitations with post-hoc analysis for subpopulations without statistical multiplicity adjustment or sufficient statistical power. Due to the small size of subpopulations in each group, demographics such as gender, age, body weight and change in ALSFRS-R score during the pre-observation period were not well balanced between the E-E group and E-P group. Despite these limitations, the differences in change in ALSFRS-R were consistent in direction, with all favouring edaravone over placebo and were the most apparent in the dpEESP2y in both MCI186-16 and MCI186-17. This post-hoc analysis suggests that there may be a clinical effect of edaravone in selected ALS patients who meet the dpEESP2y criteria at baseline and that this effect may be a durable one over 24 weeks. The types of AEs were those reported in the broader study population (Citation3) and no unexpected safety findings were apparent in the dpEESP2y.
Declaration of interest
Mr. Takahashi is an employee of Mitsubishi Tanabe Pharma Corporation (MTPC). Mr. Takei and Ms. Tsuda are employees of Mitsubishi Tanabe Pharma Development America (MTDA). Dr. Palumbo is an employee of MTPC and MTDA. The edaravone (MCI-186) clinical trials were funded by MTPC. The ALSFTD supplement, Edaravone (MCI-186) in amyotrophic lateral sclerosis (ALS), was funded by MTPA.
Acknowledgements
The authors thank David Hartree, under contract with Mitsubishi Tanabe Pharma America, Inc. for medical writing assistance during manuscript revisions.
References
- Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610–7.
- The Edaravone (MCI-186) ALS 16 Study Group. A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(Suppl). Epub ahead of print. doi. 10.1080/21678421.2017.1363780.
- The Writing Group for The Edaravone (MCI-186) ALS 17 Study Group. Exploratory double-blind, parallel-group, placebo-controlled extension study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017 (Suppl). Epub ahead of print. doi:10.1080/21678421.2017.1362000
- Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74.