Abstract
Objective: To assess the efficacy of tirasemtiv, a fast skeletal muscle troponin activator, vs. placebo on respiratory function and other functional measures in patients with amyotrophic lateral sclerosis (ALS). This study was designed to confirm and extend results from a large phase IIb trial and maximize tolerability with a slower dose escalation. Methods: VITALITY-ALS (NCT02496767) was a multinational, double-blind, randomized, placebo-controlled, parallel-group study in ALS patients. Participants who tolerated two weeks of open-label tirasemtiv (125 mg twice a day) were randomized 3:2:2:2 to placebo or one of three target total daily dose levels of tirasemtiv (250, 375, or 500 mg). Participants randomized to tirasemtiv escalated their dose every two weeks to their target dose level or maximum tolerated dose. The primary outcome measure was change in slow vital capacity from baseline to 24 weeks. Secondary endpoints assessed the effect of tirasemtiv on muscle strength and certain respiratory milestones of disease progression. A four-week randomized withdrawal phase followed 48 weeks of treatment to evaluate the possibility of sustained benefit or rebound decline. Results: Data collection will be complete in the fourth quarter of 2017. Conclusions: VITALITY-ALS was a phase III trial designed to evaluate the efficacy, safety, and tolerability of tirasemtiv in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by the loss of upper and lower motor neurons that leads to progressive muscle weakness (Citation1–3). Respiratory muscle weakness often results in respiratory failure, which is the most common cause of death in ALS (Citation4,Citation5). Many patients die within 3–5 years after diagnosis (Citation6,Citation7). Riluzole and edaravone are approved in several countries as disease-modifying therapy (Citation8–10).
One approach to treating ALS is to directly target the skeletal muscles (Citation11). Tirasemtiv, a fast skeletal muscle troponin activator (FSTA), increases the contractile response of the sarcomere by selectively binding to the fast skeletal muscle troponin complex, sensitizing the sarcomere to calcium by slowing the rate of calcium ion release (Citation12,Citation13). Tirasemtiv improved physical function in the SOD1 mouse model of ALS (Citation12) and, in nerve-muscle preparations, in vivo animal models, and healthy human volunteers (Citation14), amplified the force generated during submaximal nerve stimulation.
In a large, multinational, randomized, double-blind, placebo-controlled phase IIb study (Blinded Evaluation of Neuromuscular Effects and Functional Improvement with Tirasemtiv in ALS (BENEFIT-ALS)) (Citation15), 596 participants with ALS (slow vital capacity (SVC) > 50% predicted) who tolerated open-label tirasemtiv 125 mg twice a day (BID) for one week were randomized 1:1 to placebo or tirasemtiv and received at least one dose of double-blind therapy. Participants randomized to tirasemtiv underwent dose escalation to a maximum tolerated dose of 250 mg BID in the 12-week double-blind phase of the study. Those in the tirasemtiv group taking riluzole received half their riluzole dose (their evening dose was a matched placebo), while participants in the placebo group received the full dose of riluzole. This riluzole dose reduction was included in the BENEFIT-ALS design because previous studies showed that tirasemtiv is a CYP1A2 inhibitor and when it is administered with riluzole, tirasemtiv approximately doubles riluzole exposure regardless of dose (Citation7,Citation16).
Although the primary endpoint (change from baseline in ALS Functional Rating Scale-Revised (ALSFRS-R)) did not show a significant treatment effect in the BENEFIT-ALS study, SVC and muscle strength declined significantly more slowly in patients taking tirasemtiv (Citation15). Tirasemtiv reduced the slope of decline in SVC by approximately 50% during the 12-week treatment phase (Citation17). Significant effects on the change from baseline in SVC were observed at all doses reached (least squares mean difference from placebo ± standard error: tirasemtiv 250 mg, 7.14 ± 2.10; tirasemtiv 375 mg, 5.52 ± 1.96; tirasemtiv 500 mg, 5.07 ± 1.58; p = 0.0008, 0.0054, and 0.0015, respectively) (Citation17).
Clear conclusions regarding the lack of dose dependency could not be reached because participants completed the trial on the above dose levels based on tolerability rather than randomization (Citation17). Subgroup analyses showed that tirasemtiv reduced the decline in SVC compared with placebo by a similar magnitude regardless of riluzole use and that this reduction was statistically significant at week 12 (tirasemtiv vs. placebo on riluzole: p < 0.005; tirasemtiv vs. placebo off riluzole: p < 0.0005) (Citation17).
As seen during previous studies of tirasemtiv in patients with ALS (Citation7,Citation16), dizziness was the most commonly reported adverse event (AE) in BENEFIT-ALS (Citation15). Rates of dizziness were similar in participants taking tirasemtiv regardless of whether they were also taking riluzole (tirasemtiv + riluzole: 49.5% [97/196]; tirasemtiv alone: 53.3% [56/105]) (Citation17). Furthermore, the duration of dizziness (mean and range) that began during the open-label phase when all participants were receiving tirasemtiv was similar regardless of whether the participant transitioned to placebo or remained on tirasemtiv during the double-blind randomized phase of the study (Citation18). This suggests that the one-week open-label lead-in likely helped to prevent dizziness from unblinding the double-blind treatment randomization (Citation18).
BENEFIT-ALS was the first clinical trial of its size to demonstrate a positive and potentially clinically meaningful effect on measures of respiratory and skeletal muscle function in patients with ALS, justifying the design and performance of a phase III trial to evaluate the effect of tirasemtiv over a longer duration of treatment. This trial was designed to address specific questions raised by the results of BENEFIT-ALS. As the relationship between dose and clinical response was not clear, randomization of participants to different target doses was implemented in the phase III trial. In addition, because many dropouts from tirasemtiv owing to AEs during BENEFIT-ALS occurred shortly after randomization and particularly after dose increases (Citation15), results suggested that a longer open-label phase might succeed in better selecting participants who would tolerate active treatment while a slower dose-escalation schedule would reduce the risk of study drug discontinuation. Therefore, the Ventilatory Investigation of Tirasemtiv and Assessment of Longitudinal Indices after Treatment for a Year (VITALITY-ALS; NCT02496767) was designed with modifications to address the above issues in order to further assess the effect of tirasemtiv on respiratory function in patients with ALS.
Patients and methods
Study design and participants
This multinational, double-blind, randomized, placebo-controlled, stratified, parallel-group study enrolled adults with possible, probable, or definite ALS in accordance with the revised El Escorial criteria (Citation19) from 79 sites in 11 countries (United States, Canada, and Europe).
Participants were eligible for the study if they had an upright SVC ≥70% of predicted for age, height, and sex. Eligible participants were taking riluzole at a stable dose (50 mg BID for at least 30 days before screening) or were not taking riluzole. Participants were able to swallow tablets without crushing, and in the judgement of the site investigators, would be able to swallow tablets for the duration of the study. Additionally, eligible participants did not have any illness or laboratory abnormalities that could confound the measurement of ALS progression or interfere with their ability to complete the study, and had not taken any investigational study drug within 30 days of screening or five half-lives of the prior agent before dosing. Prior exposure to tirasemtiv was exclusionary, as was pregnancy, and participants of both sexes were required to use specific contraceptive measures.
All participants in VITALITY-ALS provided written informed consent for the study, and institutional review board approvals were received at all sites before enrollment. The study was conducted in accordance with the Declaration of Helsinki. An independent data and safety monitoring board monitored safety throughout the study. This study was registered with ClinicalTrials.gov (NCT02496767) and was funded by Cytokinetics, Inc.
Study design
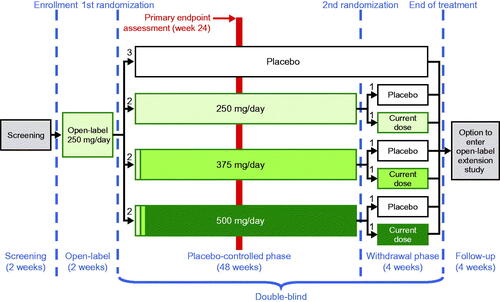
Following a screening period of no longer than 14 days, eligible participants were enrolled in the study. VITALITY-ALS included three phases of treatment with study drug (): an open-label phase, a double-blind, placebo-controlled phase, and a double-blind, placebo-controlled tirasemtiv withdrawal phase. A post-treatment follow-up period followed completion of treatment with study drug. The open-label period was extended from one week in the BENEFIT-ALS study to two weeks in this study to afford investigators and participants additional time to consider whether any AEs the participant might have experienced during the open-label phase could be tolerated during a subsequent 48 weeks of double-blind therapy, which might include an increase in the tirasemtiv dose.
Following completion of two weeks of treatment with open-label tirasemtiv 125 mg BID, participants were randomized 3:2:2:2 to placebo and three different target dose levels of tirasemtiv (250 mg, 375 mg, or 500 mg) stratified by riluzole use vs. nonuse (). Participants randomized to placebo received tirasemtiv-matched placebo, while those randomized to tirasemtiv target daily doses of 250 mg, 375 mg, and 500 mg received double-blind tirasemtiv for the next 48 weeks. All participants on riluzole took 50 mg daily from their personal supply in the morning and a blinded dose of riluzole in the evening in the same manner as in BENEFIT-ALS. All participants randomized to double-blind tirasemtiv continued at 250 mg/day for the first two weeks of double-blind treatment. Participants randomized to target daily doses of 375 mg or 500 mg tirasemtiv had their doses adjusted in 125 mg increments every two weeks until the target dose was reached or they experienced an intolerable dose. Participants did not escalate their dose if signs of intolerability were present and were returned to a previously tolerated dose level if their symptoms were believed to be due to treatment with study drug. Decisions regarding dose escalation (or not) and dose de-escalation occurred in a manner that did not compromise blinding either to treatment or to dose level. The blind was maintained by providing prepacked daily doses of tirasemtiv and matching placebo tablets during the double-blind phase of the study such that a randomized patient would take two tablets in the morning and two tablets in the evening regardless of treatment or dose level. Therefore, patients randomized to placebo would take two placebo tablets twice daily, patients taking tirasemtiv 250 mg total daily dose would take one 125 mg tirasemtiv tablet and one matching placebo tablet twice daily, patients taking tirasemtiv 375 mg total daily dose would take one 125 mg tirasemtiv tablet and one matching placebo tablet in the morning and two 125 mg tirasemtiv tablets in the evening and patients taking tirasemtiv 500 mg total daily dose would take two 125 mg tirasemtiv tablets twice daily. If the dose was increased or decreased, the patient would be dispensed a new drug supply where they would still take the same number of tablets per day but would be provided a different tirasemtiv dose level if they were randomized to treatment or they would be provided placebo if they were randomized to the placebo arm. This was done to allow for flexible dosing to tolerability while blinding treatment and dose level. Upon completion of the initial 48-week double-blind, placebo-controlled phase, participants receiving tirasemtiv were randomized a second time 1:1 to placebo or to continue their current active dose level for four weeks of additional treatment as part of the double-blind, placebo-controlled tirasemtiv withdrawal phase; participants initially randomized to placebo continued to receive placebo during this phase of the trial.
Table 1. Randomized double-blind treatment groups.
Assessments
The primary endpoint was change in percent predicted SVC from baseline to week 24 of the double-blind, placebo-controlled phase. Secondary endpoints assessed the effect of tirasemtiv on muscle strength, the respiratory subscales of the ALSFRS-R, and time to certain respiratory milestones of disease progression, such as the initiation of assisted ventilation during the 48 weeks of randomized double-blinded treatment. Safety assessments included physical examinations, clinical laboratory evaluations, vital signs, AEs, and serious AE (SAEs) monitoring. Banked samples for future biomarker analysis were collected. A schedule of events is shown in .
Table 2. Schedule of events.
Statistical methods
The primary global null hypothesis was that there is no treatment difference in the change from baseline in percent predicted SVC at week 24 between participants who had at least one post-dose SVC measure and were randomized to placebo and those randomized to tirasemtiv (pooled three target dose levels) during the placebo-controlled, double-blind treatment. It was calculated that approximately 360 participants needed to complete the 24 weeks of double-blind treatment to provide 90% power to detect a treatment difference from placebo in percent predicted SVC change from baseline to the end of the first 24-week phase of 6% for all tirasemtiv target dose groups pooled with a common standard deviation of 17% with a two-tailed alpha error of 0.05. Assuming dropout rates at 24 weeks of 16% for placebo and 25% for all tirasemtiv target dose groups combined, approximately 600 participants needed to be enrolled in the study and approximately 477 participants needed to be randomized to placebo and the three different target dose levels of tirasemtiv in an allocation ratio of 3:2:2:2 in the double-blind, placebo-controlled treatment phase. To minimize the potential for missing data, participants who discontinued study drug were encouraged to perform all remaining study visits and assessments for the duration of the study and most importantly the assessments at Week 24. Unless participants withdrew full consent for study participation, those participants who were unable to attend scheduled future study visits were contacted by phone on a monthly basis to obtain vital status and respiratory status (i.e. use of noninvasive ventilation or permanent mechanical ventilation) through 48 weeks.
Results
Baseline characteristics: open-label phase of the study
Of 866 participants screened, 744 were enrolled and 565 successfully completed the open-label phase and were randomized. Of the 744 enrolled participants, the majority were men (65.2% [485/744]), with an average age of 57.6 years and average SVC of 90.7% predicted. Time to first symptom and time to diagnosis were 20.6 months and 7.7 months, respectively (). The majority of participants (74.3% [553/744]) were taking riluzole at the start of the study. Baseline characteristics between the riluzole and non-riluzole strata were similar.
Table 3. Baseline characteristics: open-label phase.
Safety and participant disposition
In the open-label phase of the study, 82.4% (613/744) of participants experienced AEs, with dizziness (44.4% [330/744]), fatigue (25.9% [193/744]), and nausea (14.2% [106/744]) most common (). Only 1.3% (10/744) of participants experienced SAEs: three participants among those not taking riluzole and seven participants among those taking riluzole ().
Table 4. Participants with AEs: open-label phaseTable Footnotea.
Table 5. Serious AEs: open-label phase.
During the open-label phase, 24.1% (179/744) of participants discontinued of the study with similar discontinuation rates seen in the participants taking riluzole (23.3% [129/553) and participants not taking riluzole (26.2% [50/191]) (). AEs were the most common reason for discontinuation with 10.3% (77/744) discontinuing because of dizziness, 7.4% (55/744) because of fatigue, and 4.2% (31/744) because of nausea. Riluzole did not significantly affect the frequency or type of AE resulting in discontinuation.
Table 6. Participant disposition.
Discussion
In BENEFIT-ALS, a large, 12-week, randomized, placebo-controlled, double-blind phase IIb study, tirasemtiv was observed to statistically significantly reduce the slope and magnitude of SVC decline compared with placebo (Citation15). Significant effects on the change from baseline in SVC were also observed at all tirasemtiv doses reached (total daily dose of 250 mg, 375 mg, or 500 mg) (Citation17). Additionally, the results from BENEFIT-ALS demonstrated that the riluzole exposure, as measured by area under the curve, was approximately the same in participants administered with any daily dose of tirasemtiv along with half the labeled riluzole dose (i.e. 50 mg/day) compared to the participants who were randomized to placebo and taking the recommended labeled riluzole dose of 100 mg/day (Citation17). Tirasemtiv was noted to reduce SVC decline regardless of whether the participant was taking riluzole or not and regardless of other disease baseline characteristics (Citation17). Overall, the relationship between the plasma concentration and dose of tirasemtiv and its effects on SVC suggested that lower target doses of tirasemtiv than studied in BENEFIT-ALS may warrant further evaluation (Citation17), although any such conclusions are tentative based on the fact that participants were not randomized to different doses in that study.
The VITALITY-ALS study was designed to build on the findings from BENEFIT-ALS and to further evaluate and to confirm the efficacy of tirasemtiv as a treatment for patients with ALS. The design of VITALITY-ALS addresses tolerability issues by increasing the open-label lead-in from one to two weeks and by including a slower (every two weeks vs. every week) dose titration (Citation18) in participants randomized to tirasemtiv target dose levels above 250 mg/day. Participants were randomized to specific target dose levels (allowing for down titration) which is a different dosing strategy than taken in BENEFIT-ALS where the goal was to reach each patient’s maximum tolerated dose up to a maximum total daily dose of 500 mg. Randomizing to specific target dose levels in VITALITY-ALS would help better understand the relationship between each dosing strategy and the effect of tirasemtiv on SVC (Citation18).
As tirasemtiv was observed to significantly reduce SVC slope previously (Citation15), the primary endpoint in VITALITY-ALS was change in percent predicted SVC from baseline to week 24 in the double-blind, placebo-controlled phase. Key secondary endpoints included assessing the effect of tirasemtiv on muscle strength, the respiratory domain of the ALSFRS-R, and time to certain respiratory milestones of disease progression, such as the initiation of assisted ventilation. In addition, the increase from 12 to 48 weeks allows for a longer evaluation of participants with the ALSFRS-R, the measure used as the primary endpoint at 12 weeks in the BENEFIT-ALS study (Supplemental Figure 1).
Initial results from VITALITY-ALS indicate that the most commonly reported AEs were dizziness, fatigue, and nausea and were the most common reasons for study discontinuation similar to the open-label phase of BENEFIT-ALS; although dizziness, fatigue, and nausea were reported at higher percentages and discontinuation due to these AEs was also higher in VITALITY-ALS compared with BENEFIT-ALS (open-label discontinuation rates of 24.1% and 16.2%, respectively). These initial findings suggest the strategy of using a longer lead-in period in VITALITY-ALS was effective in better selecting participants who would tolerate active treatment during the randomized phase of the trial.
In conclusion, VITALITY-ALS incorporated findings from the BENEFIT-ALS trial and extended the period of active treatment to demonstrate a sustained effect of tirasemtiv in patients with ALS. VITALITY-ALS evaluated the hypotheses that tirasemtiv significantly reduces SVC decline over 24 weeks vs. placebo and has a clinically meaningful impact on other respiratory measures over a 48-week time span. Study completion is expected in the fourth quarter of 2017.
Declaration of interest
LM, AB, JL, AW, and FM are shareholders and full-time employees of Cytokinetics, Inc. JMS is a consultant to Biogen, Inc., Cytokinetics, Inc., Denali, Mitsubishi Tanabe Pharma, and Neuraltus Pharmaceuticals, Inc. MEC is a consultant/advisor to Biogen, Inc., Biohaven, Cytokinetics, Inc., Denali, Eli Lilly and Co, Karyopharm, and Mitsubishi Tanabe Pharma. OH is Editor-in-Chief of the journal, Amyotrophic Lateral Sclerosis and the Frontotemporal Degeneration. She has consulted for Biogen, Cytokinetics, Inc., Merck Serono, Mitsubishi Tanabe Pharma, and Roche. JA is a consultant to Cytokinetics, Inc. and received research grants from Neuraltus Pharmaceuticals, Inc. and Roche.
This study was funded by Cytokinetics, Inc. Cytokinetics, Inc. provided funding for writing and editorial support provided by Deb Stull, PhD, and Nicholas C. Stilwell, PhD, on behalf of Evidence Scientific Solutions, Philadelphia, PA, USA.
Supplementary material available online
IAFD_Shefner_et_al_Supplemental_Content.pdf
Download PDF (81.1 KB)References
- The EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS) – revised report of an EFNS task force. Eur J Neurol. 2012;19:360–75.
- Gordon PH. Amyotrophic lateral sclerosis: an update for 2013 clinical features, pathophysiology, management and therapeutic trials. Aging Dis. 2013;4:295–310.
- Rocha JA, Reis C, Simões F, Fonseca J, Mendes Ribeiro J. Diagnostic investigation and multidisciplinary management in motor neuron disease. J Neurol. 2005;252:1435–47.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–7.
- Goyal NA, Mozaffar T. Respiratory and nutritional support in amyotrophic lateral sclerosis. Curr Treat Options Neurol. 2014;16:270.
- Rooney J, Byrne S, Heverin M, Corr B, Elamin M, Staines A, et al. Survival analysis of Irish amyotrophic lateral sclerosis patients diagnosed from 1995–2010. PLoS One. 2013;8:e74733.
- Shefner JM, Watson ML, Meng L, Wolff AA, The Neals/Cytokinetics STUDY Team. A study to evaluate safety and tolerability of repeated doses of tirasemtiv in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14:574–81.
- Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:191–206.
- Traynor K. FDA approves edaravone for amyotrophic lateral sclerosis. Am J Health Syst Pharm. 2017;74:868.
- Yamamoto Y. Plasma marker of tissue oxidative damage and edaravone as a scavenger drug against peroxyl radicals and peroxynitrite. J Clin Biochem Nutr. 2017;60:49–54.
- Shefner JM. Muscle as a therapeutic target in amyotrophic lateral sclerosis. Exp Neurol. 2009;219:373–5.
- Hwee DT, Kennedy A, Ryans J, Russell AJ, Jia Z, Hinken AC, et al. Fast skeletal muscle troponin activator tirasemtiv increases muscle function and performance in the B6SJL-SOD1G93A ALS mouse model. PLoS One. 2014;9:e96921.
- Russell AJ, Hartman JJ, Hinken AC, Muci AR, Kawas R, Driscoll L, et al. Activation of fast skeletal muscle troponin as a potential therapeutic approach for treating neuromuscular diseases. Nat Med. 2012;18:452–5.
- Hansen R, Saikali KG, Chou W, Russell AJ, Chen MM, Vijayakumar V, et al. Tirasemtiv amplifies skeletal muscle response to nerve activation in humans. Muscle Nerve. 2014;50:925–31.
- Shefner JM, Wolff AA, Meng L, Bian A, Lee J, Barragan D, et al. A randomized, placebo-controlled, double-blind phase IIb trial evaluating the safety and efficacy of tirasemtiv in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:426–35.
- Shefner J, Cedarbaum JM, Cudkowicz ME, Maragakis N, Lee J, Jones D, et al. Safety, tolerability and pharmacodynamics of a skeletal muscle activator in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13:430–8.
- Shefner J, Andrews J, Meng L, Bian A, Wolff AA. Relationships between riluzole and tirasemtiv levels on outcomes in the BENEFITS-ALS trial. Presented at the 25th International Symposium on ALS/MND, Brussels, Belgium.
- Andrews JA, Wolff AA, Lee J, Barragan D, Meng L, Bian A, et al. Ventilatory Investigation of Tirasemtiv and Assessment of Longitudinal Indices of Treatment for a Year in ALS (VITALITY-ALS): study design of a phase III clinical trial of tirasemtiv in ALS. Presented at the MDA Clinical Conference, Arlington, VA, USA.
- Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.