Abstract
Objective: To assess masitinib in the treatment of ALS. Methods: Double-blind study, randomly assigning 394 patients (1:1:1) to receive riluzole (100 mg/d) plus placebo or masitinib at 4.5 or 3.0 mg/kg/d. Following a blinded transition from phase 2 to phase 2/3, a prospectively defined two-tiered design was implemented based on ALSFRS-R progression rate from disease-onset to baseline (ΔFS). This approach selects a more homogeneous primary efficacy population (“Normal Progressors”, ΔFS < 1.1 points/month) while concurrently permitting secondary assessment of the broader population. Primary endpoint was decline in ALSFRS-R at week-48 (ΔALSFRS-R), with the high-dose “Normal Progressor” cohort being the prospectively declared primary efficacy population. Missing data were imputed via last observation carried forward (LOCF) methodology with sensitivity analyses performed to test robustness. Results: For the primary efficacy population, masitinib (n = 99) showed significant benefit over placebo (n = 102) with a ΔALSFRS-R between-group difference (ΔLSM) of 3.4 (95% CI 0.65–6.13; p = 0.016), corresponding to a 27% slowing in rate of functional decline (LOCF methodology). Sensitivity analyses were all convergent, including the conservative multiple imputation technique of FCS-REGPMM with a ΔLSM of 3.4 (95% CI 0.53–6.33; p = 0.020). Secondary endpoints (ALSAQ-40, FVC, and time-to-event analysis) were also significant. Conversely, no significant treatment-effect according to ΔALSFRS-R was seen for the broader “Normal and Fast Progressor” masitinib 4.5 mg/kg/d cohort, or either of the low-dose (masitinib 3.0 mg/kg/d) cohorts. Rates of treatment-emergent adverse events (AEs) (regardless of causality or post-onset ΔFS) were 88% with masitinib 4.5 mg/kg/d, 85% with 3.0 mg/kg/d, and 79% with placebo. Likewise, rates of serious AE were 31, 23, and 18%, respectively. No distinct event contributed to the higher rate observed for masitinib and no deaths were related to masitinib. Conclusions: Results show that masitinib at 4.5 mg/kg/d can benefit patients with ALS. A confirmatory phase 3 study will be initiated to substantiate these data.
Introduction
Amyotrophic lateral sclerosis (ALS) is characterized by the progressive loss of motor neurons in the brain and spinal cord leading to a deterioration of muscle strength with subsequent wasting, paralysis, and loss of motor functions such as speech, swallowing, and breathing. Respiratory failure usually leads to death about 4 years after onset, although life expectancy is highly variable suggesting heterogeneity in the physiopathology of motor neuron loss (Citation1,Citation2). Worldwide prevalence of ALS is currently estimated at 235,000 with a projected 69% increase by the year 2040 (Citation1). Despite significant effort, the overwhelming majority of clinical trials have failed to demonstrate efficacy, highlighting an urgent unmet medical need (Citation3,Citation4). Riluzole, a glutamate antagonist, is the only widely available disease modifying drug for ALS patients, although its benefits are a very modest increase in survival, with no improvement in quality-of-life or slowing of functional loss. Intravenous edaravone, a free radical scavenger, recently received approval by the US Food and Drug Administration (Citation5,Citation6).
Masitinib, an oral tyrosine kinase inhibitor, has demonstrated promising preclinical activity in ALS (SOD1G93A) rat models, exerting neuroprotection via its immunomodulatory properties and in particular through targeting microglia, macrophage and mast cell activity, in both central and peripheral nervous systems (Citation7–9). Here, we report findings from study AB10015, evaluating the efficacy and safety of masitinib in ALS patients.
Methods
Study design and oversight
Study AB10015 was an international, multicenter, phase 2/3, randomized, double-blind, placebo-controlled trial over a 48-week treatment period. It commenced in April 2013 and was completed in December 2016. Patients were randomly assigned (1:1:1) to receive riluzole (100 mg/d) plus placebo or masitinib at 4.5 or 3.0 mg/kg/d (bis in die), with the high-dose cohort being predefined for primary analysis. Masitinib dose was chosen based on preclinical data (unpublished) and accumulated clinical experience for masitinib in non-oncology indications (Citation10–14). Dose reduction or treatment interruption was allowed for moderate or severe toxicity according to predefined criteria. The study protocol and amendments were approved by the institutional review board or ethics committee at each participating clinical site and was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. This trial was registered at www.clinicaltrials.gov as # NCT02588677).
Protocol amendments were implemented during the study with data remaining blinded throughout, i.e. no changes were data-driven. There were two key amendments: a non-premeditated passage from a phase 2 to a demonstrative phase 2/3 design, requiring appropriate adjustment in sample size and statistical hypothesis (first amendment dated 02 July 2013 following recruitment of 34/394 (9%) patients, of which none had completed the 48-week treatment period); and implementation of a prospectively tiered design based on aggressiveness phenotype (third amendment dated 08 October 2014 following recruitment of 142/394 (36%) patients, of which 46/394 (12%) had completed the 48-week treatment period). This latter amendment involved categorization of patients according to Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) progression rate (ΔFS) (Citation15–18), calculated from disease-onset to baseline with a dichotomizing cutoff at 1.1 points/month. Accordingly, patients receiving masitinib 4.5 mg/kg/d with post-onset ΔFS < 1.1 points/month (referred to hereafter as “Normal Progressors” and comprising an estimated 84% of the ALS population) were predefined as the primary efficacy population. All necessary information was available from patient records, meaning no retrospective data-collection was necessary, with stratification (minimization algorithm) implemented for the remaining (64%) patient recruitment to ensure balanced treatment-arms. This prospectively defined two-tiered approach defines a more homogenous target population (primary analysis), reducing variability and therefore sample size requirements, while concurrently permitting evaluation (secondary analysis) of the broader, more heterogeneous population. The rationale for this amendment (detailed in the Supplementary Information, eDiscussion Section A), assumed that heterogeneity in ALS disease aggressiveness reflects differing disease mechanisms, with dysregulated immunity being one possibly factor (Citation19), leading to an unpredictable and likely divergent treatment-effect across the overall population. Furthermore, the right-skewed (positive-skew) characteristic of ΔFS histogram distributions was a common observation in clinical practice (Citation20). This indicates that while a majority of patients fall within a fairly narrow range of post-onset ΔFS (e.g. 0.1–1.1 points/month), more rapidly progressing patients present with a far wider range and are therefore a greater source of ΔFS heterogeneity between treatment-arms.
Patients
Eligible patients were aged 18–75 years with a laboratory-supported probable, probable, or definite diagnosis of ALS (revised El Escorial criteria) (Citation21,Citation22), had less than 36 months duration of disease from the first ALS symptom (i.e. any progressive focal weakness or atrophy) and forced vital capacity (FVC) of at least 60% at baseline. Additionally, patients were receiving a stable dose of riluzole (100 mg/d) for at least 30 d prior to baseline. Patients were ineligible if presenting with gastrostomy, dementia, significant organ or system dysfunction, cancer, or uncontrolled medical condition that might interfere with trial results, and previous treatment with any investigational agent within 3 months prior to baseline.
Study measurements
The primary endpoint was decline in ALSFRS-R from baseline to week-48 (ΔALSFRS-R), with assessments performed at weeks 4, 8, 12, and every 12 weeks thereafter. Missing data were imputed via last observation carried forward (LOCF) methodology for those patients discontinuing because of toxicity or lack of efficacy before week 48. Several predefined sensitivity analyses were performed to test robustness of the primary analysis result, including full analysis dataset (non-LOCF) imputation methods and variations on LOCF via censoring on reason for discontinuation. Additional sensitivity analyses included the conservative techniques of multiple imputation, jump-to-reference, and tipping-point analysis (see Supplementary eDiscussion Section B for detailed description).
Predefined secondary analyses included assessment of the broader “Normal and Fast Progressor” masitinib 4.5 mg/kg/d cohort, and corresponding ΔFS-tiered low-dose (masitinib 3.0 mg/kg/d) cohorts. Secondary endpoints included change from baseline in ALS Assessment Questionnaire 40-item (ALSAQ-40) score (Citation23), FVC, overall survival (OS), and time-to-event analysis (an endpoint driven by both death and a fixed disease progression on the ALSFRS-R scale) (Citation24,Citation25), defined here as a deterioration of 9 points from baseline or death. Exploratory analysis included assessment in cohorts with less severe symptoms at baseline.
Patients were monitored for safety or toxicity until 28 d after discontinuing study drug. Adverse events (AE) and laboratory values (hematology, blood biochemistry, and urinalysis) were classified and graded according to Common Terminology Criteria for Adverse Events version 4 (Bethesda, MD). Safety analysis was reported regardless of causality, according to dose and patient cohort. An independent Data Safety Monitoring Committee monitored safety throughout the study.
Statistical analysis
Patients were randomized using a computerized central randomization system and minimization method according to the covariates (i.e. prognostic factors) of site of onset (spinal versus bulbar), ALSFRS-R score, age, geographical region, and post-onset ΔFS (as per the aforementioned protocol amendment). In this manner, the difference among treatment groups, with respect to prognostic factors, was minimized (). The safety dataset comprised all patients that received at least one dose of study medication, while the efficacy dataset also required patients to have received at least one intake of study medication and have at least one post-baseline efficacy assessment. Consistent with common practice, any patient dying after randomization had an ALSFRS-R score of zero imputed. All main, sensitivity, and subgroup analyses reported herein were pre-specified in the study’s statistical analysis plan prior to unblinding unless stated otherwise.
Table 1 Baseline patient characteristics according to the ITT dataset (N = 394). Treatment-arms were well balanced for all baseline parameters.
To detect a 3.3-point difference (standard deviation of 7.5 points assumed) in ΔALSFRS-R between masitinib and placebo (corresponding to an approximate 30% improvement in the rate of ALSFRS-R decline), with a two-sided 5% significance level and a power of 80% in the Normal Progressor population, a sample size of 300 patients (100 in each treatment-arm, incorporating a 5% margin) was necessary. Likewise, for the broader population analysis (i.e. Normal and Fast Progressors), an estimated 381 patients (127 per treatment-arm) were required to detect a 3.3-point difference (standard deviation of 9.0 points assumed). ΔALSFRS-R was calculated using a model of analysis of covariance (ANCOVA), adjusted on the aforementioned baseline covariates, expressing results as difference of least-squares means (ΔLSM) between treatments (masitinib versus placebo) with corresponding 95% two-sided confidence intervals (CI) and statistical test p value obtained via a re-randomization test (10,000 replicate). Secondary endpoints were similarly analyzed using ANCOVA or Kaplan–Meier methods.
One planned interim analysis was performed (cutoff date 22 February 2016) after 50% of randomized patients could have reached the 48-week timepoint. Consistent with predefined rules and justified considering the urgent unmet medical need in ALS, positive interim results led to an early regulatory submission. Because the full study population had been randomized prior to interim readout it was recommended to continue the blinded study without further amendment until final readout, thereby providing fully developed evidence and supportive follow-up analysis for the positive interim result. Consequently, all tests reported herein are performed at a significance level of 5%.
Results
Patients
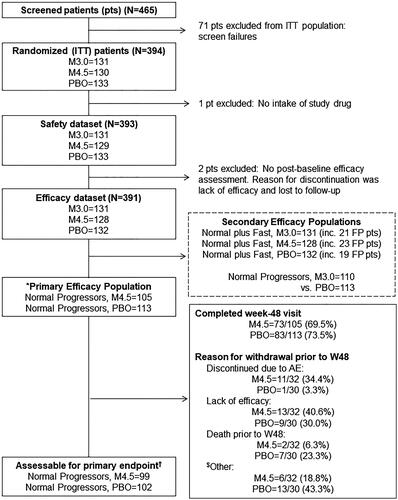
Between April 2013 and December 2015, a total of 394 patients underwent randomization from 34 sites in 9 countries. Three patients were excluded for efficacy analysis due to lack of post-baseline efficacy assessment or no drug intake, yielding a dataset of 391 patients: 132, 131, and 128 patients in the placebo, masitinib 3.0 mg/kg/d, and masitinib 4.5 mg/kg/d treatment-arms, respectively (). The primary efficacy population (i.e. “Normal Progressors” receiving masitinib 4.5 mg/kg/d versus placebo) comprised 105 and 113 patients, respectively, of which 99 and 102 patients were assessable for the primary endpoint, as determined by predefined data imputation rules (see Supplementary eDiscussion Section B). The number of patients discontinuing during the 48-week treatment period was about 34% in both treatment-arms. Randomized patients were well-balanced between treatment-arms regarding proportion of patients completing the 48-week treatment period () and on all baseline parameters (including demographics, site of onset, disease duration, ΔFS, distribution of slower progressive patients, baseline scores, and geographical region) ( and Supplementary eTable 2).
Figure 1 Patient flow diagram, detailing patient disposition for the ITT, assessable safety, assessable efficacy, primary efficacy, and secondary efficacy populations. *Primary efficacy population prospectively defined as “Normal Progressor” patients (pts) receiving masitinib at 4.5 mg/kg/d versus placebo. “Normal Progressor” dataset defined as patients with a post-onset ΔFS of less than 1.1 points/month. ΔFS = ALSFRS-R progression rate from disease-onset to baseline. ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised. ITT = intention-to-treat population. PBO = Placebo plus riluzole. M4.5 = Masitinib 4.5 mg/kg/d plus riluzole. M3.0 = Masitinib 3.0 mg/kg/d plus riluzole. Secondary efficacy populations include the “Normal and Fast Progressor” masitinib 4.5 mg/kg/d cohort and ΔFS-tiered low-dose (masitinib 3.0 mg/kg/d) cohorts. “Normal and Fast Progressor” dataset includes all patients, regardless of the post-onset ΔFS selection criterion. FP = “Fast Progressor” patients (defined as post-onset ΔFS ≥1.1 points/month). †Primary endpoint = change in ALSFRS-R from baseline to week-48. Assessable patients for primary endpoint according to rule 1 for missing data imputation, i.e. last observation carried forward methodology for those patients discontinuing because of toxicity or lack of efficacy before week 48 (see Supplementary eDiscussion B for detailed description of rules).
Primary efficacy analysis
For the primary efficacy population, ΔALSFRS-R showed a significant benefit for masitinib over placebo with a between-group difference in ΔALSFRS-R of 3.39 (−9.24 versus − 12.63); 95% CI 0.65–6.13, p = 0.016 ( and Supplementary eFigure 1). This represents a clinically meaningful 27% slowing of ALSFRS-R deterioration over the 48-week treatment period.
Table 2 Results summary for the primary efficacy population of “Normal Progressor” patients receiving masitinib 4.5 mg/kg/d versus placebo.
All predefined sensitivity analyses on the primary endpoint were statistically significant (see Supplementary eDiscussion Section B and eTable 3). Considering the most pessimistic full analysis dataset (imputation with penalty), which estimates progression for similarly clustered patients then imputes missing values using this average trend, ΔALSFRS-R for masitinib (n = 104) was −11.4 versus −14.4 for placebo (n = 111); corresponding to a ΔLSM of 3.0 and significant 26% slowing in rate of decline (p = 0.018).
Additional sensitivity analyses were performed using the recommended techniques of multiple imputation, jump-to-reference, and tipping-point. Results from these analyses were all significant (p = 0.048, p = 0.039, and a 77% penalty, respectively) and convergent with the positive primary analysis outcome (see Supplementary eDiscussion Section B). Considering the FCS-REGPMM (Fully Conditional Specification Regression Predictive Mean Matching) method, modeled on factors having maximum impact on variability of ALSFRS, ΔLSM was 3.4 (95% CI 0.53–6.33; p = 0.020).
Secondary efficacy analyses
Significance on ΔALSFRS-R was not reached for the secondary analysis populations of the “Normal and Fast Progressor” masitinib 4.5 mg/kg/d cohort (which showed a ΔLSM of 2.09 in favor of masitinib, p = 0.12), or either of the low-dose (masitinib 3.0 mg/kg/d) cohorts (Supplementary eTable 4).
The observed significant treatment-effect for “Normal Progressors” receiving masitinib 4.5 mg/kg/d (primary efficacy population) was however supported by positive benefit in various secondary endpoints ( and Supplementary eFigure 1). Patients on masitinib showed a significantly lower deterioration in quality-of-life, as measured by the ALSAQ-40 scale, with a between-group difference of 29% (ΔLSM change of 19.42 versus 27.18; p = 0.008). For respiratory function, as measured by FVC, patients on masitinib showed a significantly lower deterioration with a between-group difference of 22% (ΔLSM change of −26.45 versus − 33.99; p = 0.03). Time-to-event analysis showed patients on masitinib had a significant 25% delay in disease progression (20 versus 16 months, p = 0.016) (Supplementary eFigure 2). Conversely, there was no discernible difference in overall survival (OS) with median OS not being reached in either the masitinib (4.5 mg/kg/d) or placebo treatment-arms.
Subgroup analysis
Subgroup analyses explored whether patient response to masitinib was influenced by baseline disease severity, as determined by ALSFRS-R individual component scores, e.g. exclusion of patients with zero-point ALSFRS-R items at randomization. Results showed that initiation of masitinib (4.5 mg/kg/d) at a less severe stage of disease produced greater treatment-effect according to ΔALSFRS-R and time-to-event analysis. This included a significant benefit in the broader “Normal and Fast Progressor” cohort (Supplementary eDiscussion Section C).
Safety
A summary of safety results during the 48-week treatment period, regardless of causality, is presented in . Generally, patients receiving masitinib at 4.5 mg/kg/d (M4.5) reported higher rates of AE, severe AE and serious AE than patients receiving masitinib at 3.0 mg/kg/d (M3.0) or placebo, which were similar. Considering the overall safety dataset (n = 393), rates of discontinuation resulting from AE were 16.3, 16.0, and 9.0% in the M4.5 (n = 129), M3.0 (n = 131), and placebo (n = 133) treatment-arms, respectively. Among 33 patients who died while on-treatment or ≤28 d after discontinuing treatment, 10 (8%), 11 (8%), and 12 (9%) were from the M4.5, M3.0, and placebo treatment-arms, respectively (). No death was related to study treatment.
Table 3 Safety summary over the 48-week treatment period according to dose and patient cohort (safety dataset, regardless of causality)Table Footnotea.
Rates of AE (any grade) were 88% with M4.5, 85% with M3.0, and 79% with placebo. The only AEs occurring at least 5% more frequently for masitinib (regardless of dose) compared with placebo were maculopapular rash and peripheral edema (). Overall rates of serious AE were 31% with M4.5, 23% with M3.0, and 18% with placebo. Overall rates of severe AE (grade 3/4) were 29% with M4.5, 22% with M3.0, and 17% with placebo. One isolated case (0.8%) of autoimmune-like hepatitis was reported in the M4.5 treatment-arm, with the patient showing increased liver transaminase levels (grade 3) at week-24 (Citation26). This resolved after masitinib was discontinued and a combination of prednisone and azathioprine was started. Three other cases of increased transaminases were reported (2 patients on M4.5 and 1 patient on M3.0), all of which were non-severe cases that resolved without sequelae following temporary treatment interruption or premature discontinuation as per protocol safety rules.
Table 4 Most frequent adverse events for masitinib treatment relative to placebo over the 48-week treatment period according to dose and patient cohort (safety dataset, regardless of severity or causality, listed as per MedDRA preferred terms)Table Footnotea.
Discussion
While patients receiving masitinib at 4.5 mg/kg/d as an add-on therapy to riluzole experienced more frequent SAE and severe AE with respect to placebo, no distinct event contributed to these higher rates (see Supplementary eTables 6 and 7, respectively). No new safety concerns were identified for masitinib, findings being consistent with masitinib’s known risk profile (Citation12–14). Masitinib showed significant benefit in ΔALSFRS-R over placebo for the study’s predefined primary efficacy population, exceeding the clinically meaningful target of slowing ALSFRS-R decline by ≥20% (Citation27). Exploratory subgroup analyses indicated further improvement is possible when initiating treatment at a less severe stage of disease (e.g. exclusion of patients with zero-point ALSFRS-R items at baseline). Secondary endpoints also showed significant improvement in terms of quality-of-life (ALSAQ40), respiratory function (FVC), and time-delay in ALSFRS-R deterioration or death (time-to-event analysis).
An innovative feature of this study design was the use of ΔFS to ensure a more homogeneous primary efficacy population, thereby potentially mitigating the risk of divergent treatment-effect across the overall population and reducing required sample size. Sensitivity analyses showed that the dichotomizing cutoff of 1.1 points/month was well-judged and associated with a sizeable “buffer zone” for maintained significant treatment-effect (see Supplementary eDiscussion Section A). This highlights that it is the action of dichotomization itself, and subsequent improved sample homogeneity, that is of key importance and not optimization of a specific cutoff value.
One possible issue for interpretation of results was the use of LOCF methodology in the primary analysis. This concern was mitigated through numerous and diverse sensitivity analyses, which together corroborate the robustness of the primary analysis and provide reassurance that the observed treatment-effect was not driven by possible LOCF bias (see Supplementary eDiscussion Section B). Protocol amendments made during the study also represent a challenge for interpretation of results; in particular the transition to phase 2/3, albeit that this change occurred with data remaining blinded and prior to any of the patients having reached the 48-week timepoint.
The reduced treatment-effect observed in the “Normal and Fast Progressor” cohort possibly indicates that “Fast Progressors” (i.e. post-onset ΔFS ≥1.1 points/month) are less susceptible to masitinib at the doses tested. One speculative explanation could be that these patients are experiencing a more aggressive form of ALS with rapid loss of motor neurons requiring earlier intervention. Additional disease mechanisms offer another explanation for relative lack of efficacy in “Fast Progressor” patients, with targeted therapies expected to work on only a subset of ALS patients. Indeed, gene expression profiling and proteomic studies in ALS have successfully differentiated patients with rapid and non-rapid progressive disease (the latter defined as having an equivalent ΔFS of around 1.0 point/month) (Citation19,Citation28–31), such evidence supporting the premise that these groups represent pathophysiologically distinct forms of ALS.
Finally, the reduced treatment-effect observed for low-dose cohorts suggests dose-dependency, with the starting dose of 3.0 mg/kg/d being too low to show significant improvement in ΔALSFRS-R over placebo at the current sample size (p value was 0.0661). This suggests that patients could possibly benefit from receiving a higher masitinib dose, any risk of increased toxicities being mitigated via implementation of a dose-escalation scheme as previously described in the literature (Citation14).
In conclusion, study AB10015 represents the first successful randomized, controlled, phase 2/3 trial in ALS of a tyrosine kinase inhibitor. Results show that masitinib at 4.5 mg/kg/d can benefit patients with ALS. A confirmatory phase 3 study will be initiated to confirm these findings.
Declaration of interest
Masitinib is under clinical development by the study funder, AB Science. AM, CDM, and VA are employees and shareholders of AB Science. OH is a co-founder, shareholder, and the President of the Scientific Committee of AB Science. PD, CA, and J-PK are consultants and shareholders of AB Science. JSM and AG have received research funding from AB Science. All remaining authors have no competing interests.
Supplemental Material
Download PDF (506.8 KB)Acknowledgments
We thank the study participants, their families, and caregivers. We also thank the AB10015 Study Group collaborators (Supplementary eTable 1). The Spanish ALS Research Foundation, FUNDELA, contributed to Dr. Mora’s group support.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/21678421.2023.2273111).
Additional information
Funding
References
- Arthur KC, Calvo A, Price TR, Geiger JT, Chiò A, Traynor BJ. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun. 2016;7:12408.
- van Es MA, Hardiman O, Chio A, Al-Chalabi A, Pasterkamp RJ, Veldink JH, et al. Amyotrophic lateral sclerosis. Lancet. 2017;390:2084–98.
- Beghi E, Chiò A, Couratier P, Esteban J, Hardiman O, Logroscino G, et al. The epidemiology and treatment of ALS: focus on the heterogeneity of the disease and critical appraisal of therapeutic trials. Amyotroph Lateral Scler. 2011;12:1–10.
- Petrov D, Mansfield C, Moussy A, Hermine O. ALS clinical trials review: 20 years of failure. Are we any closer to registering a new treatment? Front Aging Neurosci. 2017;9:68.
- Writing Group; Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:30115–1.
- Hardiman O, van den Berg LH. Edaravone: a new treatment for ALS on the horizon? Lancet Neurol. 2017;16:490–1.
- Trias E, Ibarburu S, Barreto-Núñez R, Babdor J, Maciel TT, Guillo M, et al. Post-paralysis tyrosine kinase inhibition with masitinib abrogates neuroinflammation and slows disease progression in inherited amyotrophic lateral sclerosis. J Neuroinflammation. 2016;13:177.
- Trias E, Ibarburu S, Barreto-Núñez R, Varela V ,Moura IC, Dubreuil P, et al. Evidence for mast cells contributing to neuromuscular pathology in an inherited model of ALS. JCI Insight. 2017;2:e95934.
- Trias E, King PH, Si Y, Kwon Y, Varela V, Ibarburu S, et al. Mast cells and neutrophils mediate peripheral motor pathway degeneration in ALS. JCI Insight. 2018;3:pii: 123249.
- Dubreuil P, Letard S, Ciufolini M, Gros L, Humbert M, Castéran N, et al. Masitinib (AB1010), a potent and selective tyrosine kinase inhibitor targeting KIT. PLoS One. 2009;4:e7258.
- Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–51.
- Vermersch P, Benrabah R, Schmidt N, Zéphir H, Clavelou P, Vongsouthi C, et al. Masitinib treatment in patients with progressive multiple sclerosis: a randomized pilot study. BMC Neurol. 2012;12:36.
- Piette F, Belmin J, Vincent H, Schmidt N, Pariel S, Verny M, et al. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimers Res Ther. 2011;3:16.
- Lortholary O, Chandesris MO, Livideanu CB, Paul C, Guillet G, Jassem E, et al. Masitinib for treatment of severely symptomatic indolent systemic mastocytosis: a randomised, placebo-controlled, phase 3 study. Lancet. 2017;389:612–20.
- Labra J, Menon P, Byth K, Morrison S, Vucic S. Rate of disease progression: a prognostic biomarker in ALS. J Neurol Neurosurg Psychiatry. 2016;87:628–32.
- Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66:265–7.
- Gordon PH, Cheung YK, Kimura F. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;67:1314–5.
- Kollewe K, Mauss U, Krampfl K, Petri S, Dengler R, Mohammadi B. ALSFRS-R score and its ratio: a useful predictor for ALS-progression. J Neurol Sci. 2008;275:69–73.
- Henkel JS, Beers DR, Wen S, Rivera AL, Toennis KM, Appel JE, et al. Regulatory T-lymphocytes mediate amyotrophic lateral sclerosis progression and survival. EMBO Mol Med. 2013;5:64–79.
- Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:414–25.
- Brooks BR. El Escorial world federation of neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor neuron diseases/Amyotrophic lateral sclerosis of the world federation of neurology research group on neuromuscular diseases and the El Escorial ’Clinical limits of amyotrophic lateral sclerosis’ workshop contributors. J Neurol Sci. 1994;124:96–107.
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- Jenkinson C, Fitzpatrick R, Brennan C, Swash M. Evidence for the validity and reliability of the ALS assessment questionnaire: the ALSAQ-40. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1:33–40.
- Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical investigation of medicinal products for the treatment of amyotrophic lateral sclerosis (ALS) EMA/CHMP/40105/2013. London: European Medicines Agency; 2015.
- Aggarwal SP, Zinman L, Simpson E, McKinley J, Jackson KE, Pinto H, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:481–8.
- Salvado M, Vargas V, Vidal M, Simon TM, Camacho J, Gamez J. Autoimmune-like hepatitis during masitinib therapy in an amyotrophic lateral sclerosis patient. World J Gastroenterol. 2015;21:10475–9.
- Castrillo-Viguera C, Grasso DL, Simpson E, Shefner J, Cudkowicz ME. Clinical significance in the change of decline in ALSFRS-R. Amyotroph Lateral Scler. 2010;11:178–80.
- Cooper-Knock J, Green C, Altschuler G, Wei W, Bury JJ, Heath PR, et al. A data-driven approach links microglia to pathology and prognosis in amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2017;5:23.
- Zhao W, Beers DR, Hooten KG, Sieglaff DH, Zhang A, Kalyana-Sundaram S, et al. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol. 2017;74:677–85.
- Beers DR, Zhao W, Wang J, Zhang X, Wen S, Neal D, et al. ALS patients’ regulatory T lymphocytes are dysfunctional, and correlate with disease progression rate and severity. JCI Insight. 2017;2:e89530.
- Murdock BJ, Zhou T, Kashlan SR, Little RJ, Goutman SA, Feldman EL. Correlation of peripheral immunity with rapid amyotrophic lateral sclerosis progression. JAMA Neurol. 2017;74:1446–54.
eTable 1: List of AB10015 Study Group collaborators (non-author investigators).
