Abstract
Background: Neuroinflammation and human endogenous retroviruses (HERV) are thought to have a role in the pathophysiology of amyotrophic lateral sclerosis (ALS). Therapy directed against endogenous retroviruses has demonstrated positive effects during in vitro and biomarker studies. Consequently, the present study was undertaken to assess the safety and tolerability of long-term antiretroviral therapy (ART), Triumeq (abacavir, lamivudine, and dolutegravir) exposure in patients with ALS, and efficacy against biomarkers of disease progression. Methods: Patients were observed during a 10-week lead-in period before receiving Triumeq treatment for 24 weeks at four specialist ALS centers. The primary outcomes were safety and tolerability. Secondary outcomes included HERV-K expression levels, urinary p75ECD levels, neurophysiological parameters, and clinical indicators. The ENCALS prediction model was applied to provide an estimate of the cohort survival. The trial was registered (NCT02868580). Findings: 40 patients with ALS received Triumeq and 35 (88%) completed treatment. There were no drug-related serious adverse events; one patient was withdrawn from the study due to a drug-associated increase in liver enzymes. A favorable response on HERV-K expression levels was observed, accompanied by a decline in ALSFRS-R progression rate of 21.8% (95% CI −4.8%–48.6%) and the amount of urinary p75ECD measured. One patient died five months after stopping treatment, while five were expected to have died during the treatment period (interquartile range 2–8). Interpretation: Long-term Triumeq exposure was safe and well tolerated in this cohort. There was suggestive indication for a possible biological response in some pharmacodynamic and clinical biomarkers. A larger international phase 3 trial will be deployed to assess the effect of Triumeq on overall survival and disease progression. Funding: Funding was provided by the FightMND Foundation; MND Research Institute of Australia; MND Association, United Kingdom, and GSK. ViiV Healthcare provided the Triumeq.
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease primarily affecting motor neurons, resulting in progressive paralysis and death, typically from respiratory failure, within 3 years. At present, there remains limited therapy available for ALS patients (Citation1), and recent trials have tended to be disappointing (Citation2,Citation3). As such, it seems critical to consider novel concepts regarding ALS pathophysiology (Citation4,Citation5) and new approaches to assess patients undertaking clinical trials (Citation6). Retroviruses have been suggested as a cause or trigger for ALS for more than 40 years (Citation7,Citation8) and there is increasing evidence that a human endogenous retrovirus (HERV), HERV-K (HML-2), is implicated in ALS. This retrovirus group integrated into the human genome in the last 5 million years (Citation9,Citation10). Even though all HERV-K proviruses are defective in at least one gene, many of them have complete open reading frames, and all HERV-K viral proteins can be expressed from different loci in the human genome (Citation11). Moreover, HERV-K can also form virus-like particles, particularly in cancer cells (Citation12).
Evidence linking retroviruses and HERV-K to ALS includes increased nonspecific reverse transcriptase activity in the blood and cerebrospinal fluid of ALS patients compared to relatives and controls (Citation13–16), and increased levels of different transcripts of HERV-K in brains of ALS patients compared to controls (Citation17), although not shown in all studies for the gag gene (Citation18). In one study, active loci of HERV-K were found in ALS and HERV-K expression was strongly correlated with TDP-43 expression (Citation19). HERV-K has been found in motor neurons of patients with ALS (Citation17), although one recent study did not confirm this finding (Citation20). An important study described in transgenic mice, in which the envelope protein of HERV-K was expressed under a neuronal promoter, which developed a progressive motor neuron disease with specific loss of neurons in the motor cortex and spinal cord (Citation21). In vitro observations show that nucleoside reverse transcriptase inhibitors (NRTIs) are, in some cases, more effective at inhibiting HERV-K (Citation22,Citation23) than HIV, and anti-retroviral therapy is therefore a promising option for the treatment of ALS.
Two early ALS clinical trials of antiretroviral monotherapies in patients with ALS proved negative with the NRTI, zidovudine (Citation24), or the protease inhibitor, indinavir (Citation25). The zidovudine study enrolled just 12 patients who were treated for 2–10 months. The indinavir trial enrolled 46 patients, although only 22 completed the trial due to disease progression and difficulty traveling to the trial site. Neither study used combination antiretroviral therapy (ART), which is very effective in treating HIV. ART has now developed to the extent that combination therapy is available in single tablet form, and tolerability and adherence issues have largely been ameliorated. In this context, we initiated a phase 2a study in patients with ALS to investigate the safety and tolerability of combination therapy. We selected one of the most widely used and well-tolerated ARTs, Triumeq, which is a combination of two NRTIs (abacavir and lamivudine) and an integrase inhibitor (dolutegravir).
Methods
Study design
This open-label phase 2a trial was conducted at four centers across Sydney and Melbourne, Australia. The relevant ethics committees at each site approved the protocol. The study was conducted in accordance with Good Clinical Practice (GCP) and the Declaration of Helsinki (2000). The trial was registered on clinicaltrials.gov (NCT02868580).
Patient selection criteria
Patients were aged 18–75 years, with El Escorial possible, probable, or definite ALS (Citation26); diagnosis within 24 months of screening, forced vital capacity (FVC) of >60% predicted, and no family history of autosomal dominant ALS. Patients with gastrostomy or noninvasive ventilation and those positive for HLA B*5701 at screening were excluded; the presence of this allele predicts potential allergy to abacavir (Citation27). All patients on riluzole, were on a stable dose for at least 30 days prior to screening. Full study inclusion and exclusion criteria are listed in Supplementary Appendix 1. All patients provided written informed consent. As this was an open-label study, there was no blinding or randomization.
Procedures
After an initial 7-day screening period, patients were enrolled by study investigators and then entered the 10-week observation pretreatment period. During this time, the next eligible patient replaced any patient who withdrew. During the pretreatment period, patients completed ALSFRS-R every four weeks, and then at the baseline visit when they received their first allocation of Triumeq. Face-to-face visits were scheduled for screening, baseline and weeks 4, 8, 16, and 24 and then an end-of-study visit a week later or if the patient withdrew from the trial. We selected the once-daily Triumeq dose regimen that is currently licensed for clinical use. Triumeq was supplied by ViiV Healthcare in bottles of 30 tablets that we relabelled according to each patient’s unique identifier and distributed to participating sites for dispensing. Patients were dispensed one month of Triumeq at baseline and week four, then two months at weeks 8 and 16. At each visit, patients had standard safety evaluation assessments by history, physical examination, and blood and urine tests. Any serious adverse events (SAEs) were reviewed by the data safety and monitoring board. Efficacy assessments, except ALSFRS-R, were conducted at specific time points (see Supplementary Appendix 2) and included FVC, neurophysiological index, sniff nasal inspiratory pressure (SNIP) test, hand dynamometry, urine collection for p75ECD, blood for determination of NfL, pNfH, and HERV-K by digital PCR, ALSSQOL-R and Columbia Suicide Severity Scale, and dynamic voice recording. The schedule of assessments is provided in Supplementary Appendix 2.
Outcomes
The primary outcome was the safety and tolerability of Triumeq during 24 weeks of treatment. Safety was determined by recording SAEs and adverse events (AEs) according to standard criteria of history, physical examination, and blood and urine analysis. Suicidal ideation was assessed with the Columbia Suicide Severity Scale (Citation28). When patients could not attend a visit, they were contacted by the study nurse who collected information on the reasons.
Secondary efficacy outcomes were also assessed. ALSFRS-R was assessed monthly, either in-person during face-to-face monitoring visits or by telephone during weeks 4 and 8 of the pretreatment period and if patients could not attend site visits. Health-related quality of life using the ALSSQOL-R was measured at screening and baseline and at end-of-study visit. Respiratory function was assessed by FVC and SNIP. FVC was measured using a portable spirometer using the best of three trials and reported as a percentage of predicted value. SNIP was measured using the Micro RPM device as a percentage of predicted value by age and sex. The highest pressure from a minimum of 10 short sniffs through each nostril was recorded. SNIP was expressed as a percentage of the predicted value by age and sex. Grip strength was assessed by a Jamar dynamometer using the average of three trials for each hand. Neurophysiological index was calculated for the ulnar nerve bilaterally, according to previously reported techniques (Citation29). Blood and urine were collected to assess biomarkers; stored specimens were selected for four-time points during the trial; screening, baseline (after 10 weeks pretreatment observation), and at 8 and 24 weeks on treatment, and stored at −80 °C. If patients withdrew early or did not have a specimen taken at that visit, the specimen taken closest to the visit was selected for analysis. Urine was aliquoted into one-ml containers and shipped frozen to Flinders University, South Australia, with samples assayed blinded by a technician who performed p75ECD measurements as previously described (Citation30). NfL and pNfH were measured at the Blizard Institute, Queen Mary University of London as previously described (Citation31) in serum samples shipped frozen. The quantitative determination of NfL and pNfH in serum was undertaken by single molecule array (Simoa) technology using a digital immunoassay Simoa HD-1 Analyzer (Quanterix, Lexington, MA).
HERV-K studies were conducted at the Section of Infections of the Nervous System, National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH) in Bethesda, Maryland. The methodology has not been previously published and is based on previous studies showing that HERV-K viral particles contain both DNA and RNA genomes (Citation11,Citation32–34). Investigators who performed the laboratory analysis of HERV-K were blinded to the samples.
Statistical analysis
For each patient, we calculated predicted survival time according to the ENCALS survival model (Citation35) conditional for being alive at screening. The linear predictor of the model (a summary variable of all predictors) was used to classify patients in one of five prognostic groups, ranging from very short to very long. In addition, clinical disease stage was determined based on the King’s staging algorithm (Citation36). Adverse events were coded to preferred terms from the MedRA library (version 16.1) and reported as frequency.
In addition, we evaluated the mean monthly change in clinical markers for disease progression. Linear mixed models were fitted with a fixed effect for time since screening and random slope for time and intercept per study participant. For sensitivity analysis, we combined the lead-in and treatment period and modeled time with a linear spline with one knot at treatment initiation in order to adjust for lead-in rate of decline. All models were based on restricted maximum likelihood (REML); 95% confidence intervals (95% CI) around estimates were obtained by means of bootstrapping (n = 1000). Finally, we compared the observed survival with the predicted conditional survival probabilities (i.e. 25th, median, and 75th percentile). Due to the exploratory nature of the analysis, we did not perform general tests for statistical significance. The only exception was the analysis of HERV-K, as it was determined by a novel bioassay, which we wanted to validate in vivo. The ratio of HERV-K DNA copies to RPP30 copies in the untreated samples (average of screening and baseline samples) and at weeks 8 and 24 of treatment were compared using the Wilcoxon matched-pairs signed rank test. The relative percentage change of the HERV-K/RPP30 ratio was calculated for each follow-up visit (W8 and W24) by comparing to the HERV-K/RPP ratio in the samples from the untreated visits (average of screening and baseline). The relative change in ratio was assessed using the Wilcoxon signed rank test. LME models were fitted using the R lmer function (lme4, version 1.1-18-1) (Citation37).
Results
Study participants
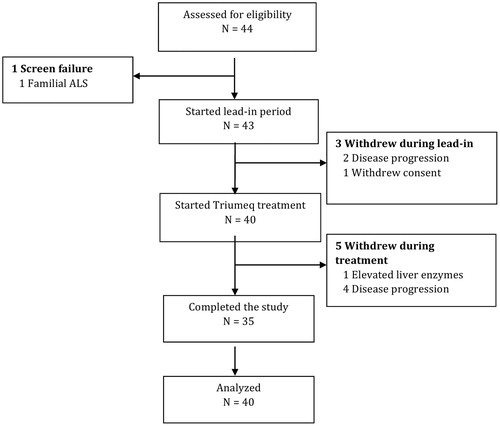
Forty-four patients were assessed for eligibility between September 2016 and April 2017. Three patients who qualified for participation withdrew during the 10 weeks pretreatment period and were excluded from the analysis. Forty patients started Triumeq treatment (). Baseline characteristics of the study participants are listed in . All patients who received at least one dose of Triumeq were included in the safety analysis.
Table 1 Baseline characteristics of the cohort.
Safety and tolerability
Tolerability was defined as the ability to complete the 24-week treatment period on study drug. In addition, we determined the number of patients who were withdrawn from the trial due to AEs and SAEs that were directly attributable to the study medication.
The trial met its primary endpoints for safety and tolerability. The most common AEs are listed in . Eighty-eight percent of the participants (n = 35) completed the 24-week treatment period. Four patients withdrew during the treatment period due to disease progression. One patient was withdrawn by the investigator due to an AE possibly associated with Triumeq. The patient had a long history of regular alcohol intake and repeatedly abnormal liver function tests during pretrial routine management. The exact amount of alcohol consumed was difficult to determine and he met the inclusion criteria for liver function tests (LFTs). However, at the clinic visit for his second monthly visit, all the LFTs were elevated and the serum ferritin was also elevated at 12,000 ng/ml with an Upper Limit of Normal of 400 ng/ml. Triumeq was ceased and further investigations included negative serology for hepatitis A/B/C and ultrasound of the liver, which demonstrated fatty changes. He was asymptomatic. As the LFTs remained elevated for several weeks and his alcohol intake was unclear, he was withdrawn from the study.
Table 2 Adverse events during study period.
There were seven AEs possibly associated with Triumeq and consistent with adverse reactions of HIV patients treated with Triumeq: six nausea and one rash. Two patients (5%) reported suicidal ideation, which was deemed unrelated to Triumeq. One patient died during the duration of the trial although they were withdrawn five months prior to death due to disease progression. Based on conditional survival probabilities of the ENCALS model, we expected five deaths during the study period (IQR: 2–8).
Clinical markers of disease progression
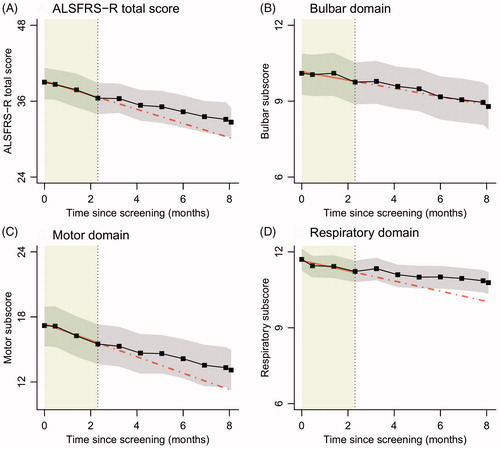
The monthly rate of change in ALSFRS-R was 1.12 (95% CI 0.63–1.60) points per month during the lead-in period and 0.76 (95% CI 0.49–1.04,) points per month during the treatment period, a 31.7% (95% CI 6.6%–56.4%) slope reduction after treatment initiation. This effect remained after adjusting for the observed pattern during the lead-in period: adjusted slope reduction of 21.8% (95% CI −4.8%–48.6%). provides the observed patterns in the ALSFRS-R and its subdomains during the treatment period. The slope reduction seems primarily driven by the motor and respiratory domains.
Figure 2 Linear mixed models with time modelled as categorical variable (black). In grey are the 95% confidence intervals; the lead-in period is highlighted in green. The red line is a linear mixed model fitted on the lead-in data (solid) and extrapolated into the treatment period (dotted).
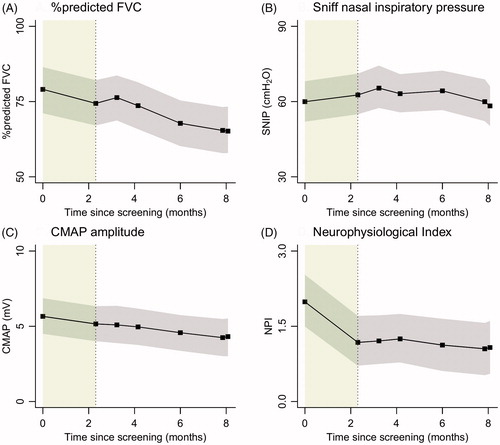
Other markers of disease progression are shown in (% predicted FVC, SNIP, CMAP amplitude, and neurophysiological index). Similar to the ALSFRS-R, none of the markers indicate an accelerating trend after Triumeq initiation. During the treatment period, the FVC declined on average by 2.22% (95% CI 1.46- 3.01), SNIP by 1.12 cmH2O (95% CI 0.20–2.07), the CMAP by 0.18 mV (95% CI 0.10–0.26), and NPI by 0.04 units (95% CI 0.00–0.08) per month.
Figure 3 Linear mixed models with time modelled as categorical variable (black). In grey are the 95% confidence intervals; the lead-in period is highlighted in green.
Biomarker results
P75ECD
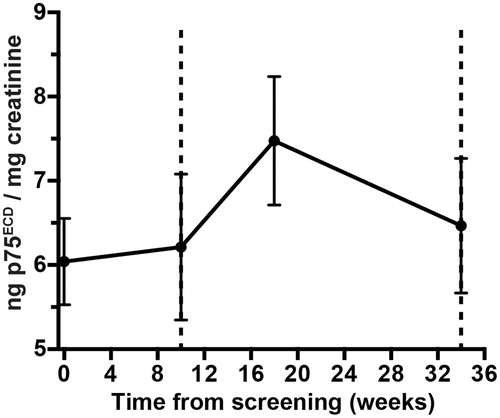
The p75ECD was measured in urine samples at these time points from 37 patients, corrected for urinary dilution using creatinine concentration. A rise in p75ECD suggests disease progression (Citation30). During the pretreatment phase, the rise in p75ECD was a mean of 0.23 ng/mg of creatinine per month; during the first phase of treatment, the rise of p75ECDincreased to 0.64 ng/mg; and during the later phase of treatment, this reversed to a monthly mean decline of −0.28 ng/mg ().
HERV-K
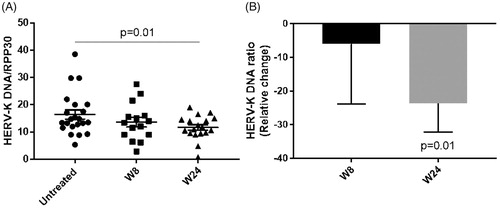
Copy numbers of HERV-K env DNA and the single copy gene RPP30 were measured in each serum sample. Since two samples were obtained before starting the treatment (screening and baseline), the average values from these two visits were used to determine the effect of treatment at weeks 8 and 24 of antiretroviral treatment. A decrease was noted in the HERV-K DNA/RPP30 ratio with treatment, achieving a statistical significance at 24 weeks of treatment compared to the untreated samples (p-value = 0.01), both in absolute value () and in relative change ().
Figure 5 HERV-K DNA and RPP30 copy numbers were measured in all samples by digital PCR and the ratio of HERV-K/RPP30 was calculated as a measure of nongenomic HERV-K. (A) Absolute value of HERV-K DNA/RPP30 in untreated samples (N = 22) and at weeks 8 (W8; N = 15) and 24 of treatment (W24; N = 18) (Wilcoxon matched-pairs signed rank test). (B) The relative percent change of HERV-K/RPP30 at each follow-up visit (W8 and W24) compared to the HERV-K/RPP30 at the untreated visits (average of screening and baseline) was calculated (Wilcoxon signed rank test).
Neurofilament light and phosphorylated heavy chains (NfL and pNfH)
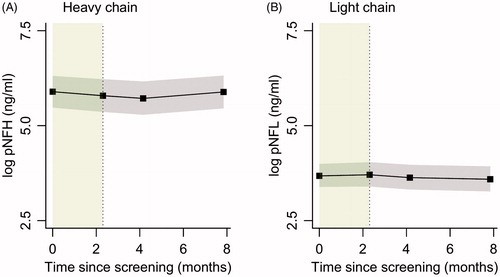
Sera concentrations of neurofilament light and phosporylated heavy chain remained stable over time (). The mean monthly rate of change (log-transformed) for NfL and pNfH was −0.01 (95% CI −0.06–0.03) and 0.0 (95% CI −0.03–0.02), respectively.
Discussion
In this phase 2a open-label study, we have shown Triumeq is safe and well tolerated. There was no observed interaction with riluzole and the biochemical safety parameters remained within normal limits. There was a biological response in laboratory and clinical biomarkers. The apparently low incidence of adverse effects of Triumeq in ALS is reassuring for potential repurposing of this combination ART and is in contrast to the two previous studies using monotherapies with poor side effect profiles. Triumeq is a combination of three ARTs in a single daily tablet. Triumeq is taken orally as one tablet once a day or crushed via a percutaneous endoscopic gastrostomy (PEG) tube. Two NRTI components have in vitro efficacy against HERV-K. The possible mechanism of HERV-K in causing ALS has been recently described in detail (Citation38), and two NRTI components of Triumeq have in vitro efficacy against HERV-K in a dose-dependent manner (Citation23,Citation22). Both abacavir and lamivudine have good penetration into the central nervous system (Citation39), compared with other NRTIs supporting their choice in a combination therapy for ALS. Dolutegravir also has good CNS penetration and achieves high clearance rates of CNS HIV, while the combination of these drugs is an effective CNS active combination (Citation40).
This trial utilized a range of standard efficacy indicators and attempted to investigate the utility of some of the available biomarkers for the first time in a clinical trial setting. It is important to note that for all efficacy variables, the trial was open label, and consequently efficacy parameters have limited value. However, we designed the study with a lead-in period to partially overcome this limitation, so the progress of each participant on Triumeq could be compared with their initial progression (Citation44). All the efficacy parameters showed trends when comparing the observation (pretreatment) period with the treatment. In addition, the decline in some other biomarkers all stabilized after initiation of treatment. This can be seen in the neurophysiological index in both right and left hands, where the pretreatment rate of decline was in keeping with the observation of de Carvalho et al. (Citation41) that the neurophysiological index declined linearly over time. This decline stabilized after commencement of Triumeq, and there was effectively no decline over the next six months. The FVC appeared to stabilize after 18 weeks on therapy and the ALSSQOL-R showed a trend to improve with therapy. The pretreatment ALSFRS-R showed a mean monthly decline of 1.12 points, indicating the study cohort progressed slightly more rapidly than the expected average decline of around 0.76 points per month. Following treatment with Triumeq, the decline slowed by around 31.7%, which may be regarded as potentially clinically interesting. While ALSFRS-R is not regarded as an optimal measure of therapeutic efficacy, it is still a widely used outcome parameter. Many of these parameters may have been influenced by patients starting open-label Triumeq as a placebo effect, and each measurement exhibited wide confidence intervals. It is not possible to determine the potentially strong psychological effect of starting treatment in this cohort, after 10 weeks of observation, and therefore any conclusion on the role of Triumeq on subjective indicators should be tempered. However, biomarkers may provide more of an objective reflection of outcome than the physical and psychological parameters.
Urinary p75ECD
It is a pharmacodynamic marker. Injured motor neurons and Schwann cells release p75ECD after ligand binding in response to injury, and it is excreted from patients within their urine and reported to increase throughout the course of ALS (Citation30). In the published ALS reference cohort, p75ECD was increased (5.6 ± 2.2 ng/mg creatinine), compared to controls (p75ECD of 3.6 ± ng/mg of creatinine) and increased at an average rate of 0.19 ng/mg creatinine per month (Citation30). In the Lighthouse cohort, the p75ECD levels were similar to those recorded in the reference cohort (6.1 ± 0.8 ng/mg creatinine). The p75ECD levels increased by a mean of 0.23 ng/mg of creatinine per month during the pretreatment observation period. Following initiation of Triumeq, urinary p75ECD increased by threefold to 0.64 ± 1.5 ng/mg of creatinine followed by a decline of −0.28 ± 0.9 ng/mg of creatinine per month between week 8 and week 24. This unique pattern of p75ECD trajectory has not been previously observed in longitudinal ALS studies. It may possibly be caused by an immune reconstitution inflammatory syndrome (IRIS), which has been documented in patients with HIV commencing on combination ART (Citation42). As p75ECD is a biomarker of neuroinflammation, it is possible that an IRIS response may have resulted in the increased trajectory of p75ECD as this is a biomarker of neuroinflammation. The subsequent reversal may indicate a biological effect of Triumeq on the progressive neuroinflammatory process that results in destruction of motor neurons in ALS. It will be important to investigate this observation further to determine the possible effect of Triumeq on the pathogenesis of ALS.
Neurofilament light and phosphorylated heavy chain
Assessments of NfL and pNfH did not show any trends, either during the pretreatment phase or the treatment phase. The lack of any trend in trajectory supports previous observations that both NfL and NfH levels remain stable over time (Citation43,Citation31) and therefore may not be useful short-term markers of disease progression or as indicators of possible ameliorating effect of disease-modifying interventions. While these biomarkers appear to be possibly good predictors of the projected rate of deterioration of ALS patients, they may have limited utility in clinical trial monitoring.
HERV-K
There was a significant decrease in HERV-K DNA in serum with treatment. While these findings may suggest a biological effectiveness of Triumeq on HERV-K gene expression in ALS, the data need to be interpreted with caution. First, not all samples were available for analysis, and second, the source of HERV-K DNA is unclear, potentially representing either chromosomal DNA or reverse-transcribed viral DNA. To account for chromosomal DNA, we simultaneously measured levels of RPP30 which is present as a single copy in the human haploid genome. Thus, a decrease in the ratio of HERV-K DNA to RPP30 may represent a decrease in reverse-transcribed viral DNA. This would be consistent with an effect of the antiretroviral drugs, specifically the two reverse transcriptase inhibitors present in Triumeq (abacavir and lamivudine).
Survival analysis
As shown in the baseline table, there were relatively more patients with a good prognosis in the trial cohort, and patients with a bad prognosis were proportionately underrepresented. This is a well-known phenomenon, where patients with a bad prognosis usually do not participate in medical research due to fatigue and rapid progression. There was one observed death which occurred five months after the patient withdrew from the study; the interquartile range of the expected number of deaths ranged from 2 to 8 with a median of 5. Nevertheless, there was still a 25% probability that there would be two or fewer deaths during this trial.
In summary, Triumeq appears to be safe and well tolerated in patients with ALS who are HLA B*5701 negative. It has considerable administration advantages as a single once-daily tablet which can be taken either whole or crushed. Moreover, it is already licensed for HIV treatment and is available in most countries. Initial results indicate Triumeq may have a biological effect, both clinically and on biomarkers in patients with ALS and all effects appear to be positive, such that an international multicenter phase 3 group sequential design placebo-controlled trial is planned to determine if Triumeq ameliorates both survival and clinical progression.
Declaration of interest
The authors have no conflict of interest with respect to the research or data presented in this study. No funder was involved in the study design or interpretation or reporting of the results.
Supplimentary_Data.docx
Download MS Word (166.7 KB)Acknowledgments
The authors would like to acknowledge the advice of Professors Edward Byrne, Pamela Shaw, Richard Bedlack and Bruce Brew. Also, the involvement of Tina Soulis, Leonid Churilov, Vyoma Modi and Drs John Pottage and Fraser Drummond. We very much appreciate the collaboration of the families and people with Motor Neurone Disease who particpated in this study and especially Neale Daniher, Dr. Ian Davis and Pat Cunningham, without whom this work would not have been possible.
Additional information
Funding
References
- Fang T, Al Khleifat A, Meurgey J-H, Jones A, Leigh PN, Bensimon G, et al. Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. 2018;17:416–22.
- Meininger V, Genge A, van den Berg LH, Robberecht W, Ludolph A, Chio A, et al. Safety and efficacy of ozanezumab in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:208–16.
- Cudkowicz ME, van den Berg LH, Shefner JM, Mitsumoto H, Mora JS, Ludolph A, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2013;12:1059–67.
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–55.
- Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639–49.
- Al-Chalabi A, Hardiman O, Kiernan MC, Chiò A, Rix-Brooks B, van den Berg LH. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 2016;15:1182–94.
- Younger DS, Rowland LP, Latov N, Hays AP, Lange DJ, Sherman W, et al. Lymphoma, motor neuron diseases, and amyotrophic lateral sclerosis. Ann Neurol. 1991;29:78–86.
- Engel W. RNA metabolism in relation to amyotrophic lateral sclerosis. In: Rowland L, ed. Amyotrophic lateral sclerosis and other motor neuron diseases. New York, NY: Raven Press; 1991: 125–53.
- Barbulescu M, Turner G, Seaman MI, Deinard AS, Kidd KK, Lenz J. Many human endogenous retrovirus K (HERV-K) proviruses are unique to humans. Curr Biol. 1999;9:861–8.
- Hughes JF, Coffin JM. Human endogenous retrovirus K solo-LTR formation and insertional polymorphisms: implications for human and viral evolution. Proc Natl Acad Sci. 2004;101:1668–72.
- Garcia-Montojo M, Doucet-O’Hare T, Henderson L, Nath A. Human endogenous retrovirus-K (HML-2): a comprehensive review. Crit Rev Microbiol. 2018;44:715–38.
- Muster T, Waltenberger A, Grassauer A, Hirschl S, Caucig P, Romirer I, et al. An endogenous retrovirus derived from human melanoma cells. Cancer Res. 2003;63:8735–41.
- Andrews WD, Tuke PW, Al-Chalabi A, Gaudin P, Ijaz S, Parton MJ, et al. Detection of reverse transcriptase activity in the serum of patients with motor neurone disease. J Med Virol. 2000;61:527–32.
- Steele AJ, Al-Chalabi A, Ferrante K, Cudkowicz ME, Brown RH, Garson JA. Detection of serum reverse transcriptase activity in patients with ALS and unaffected blood relatives. Neurology. 2005;64:454–8.
- McCormick AL, Brown RH, Cudkowicz ME, Al-Chalabi A, Garson JA. Quantification of reverse transcriptase in ALS and elimination of a novel retroviral candidate. Neurology. 2008;70:278–83.
- MacGowan DJL, Scelsa SN, Imperato TE, Liu K-N, Baron P, Polsky B. A controlled study of reverse transcriptase in serum and CSF of HIV-negative patients with ALS. Neurology. 2007;68:1944–6.
- Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol. 2011;69:141–51.
- Mayer J, Harz C, Sanchez L, Pereira GC, Maldener E, Heras SR, et al. Transcriptional profiling of HERV-K(HML-2) in amyotrophic lateral sclerosis and potential implications for expression of HML-2 proteins. Mol Neurodegener. 2018;13:39.
- Alfahad T, Nath A. Retroviruses and amyotrophic lateral sclerosis. Antiviral Res. 2013;99:180–7.
- Garson JA, Usher L, Al-chalabi A, Huggett J, Day EF, Mccormick AL. Quantitative analysis of human endogenous retrovirus-K transcripts in postmortem premotor cortex fails to confirm elevated expression of HERV-K RNA in amyotrophic lateral sclerosis. Acta Neuropathol Commun. 2019;2:1–9.
- Li W, Lee MH, Henderson L, Tyagi R, Bachani M, Steiner J, et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med. 2015;7.307ra153.
- Contreras-Galindo RA, Dube D, Fujinaga K, Kaplan MH, Markovitz DM. Susceptibility of human endogenous retrovirus type-K to reverse transcriptase inhibitors. J Virol. 2017;91:e01309–17.
- Tyagi R, Li W, Parades D, Bianchet MA, Nath A. Inhibition of human endogenous retrovirus-K by antiretroviral drugs. Retrovirology. 2017;14:1–13.
- Westarp ME, Bartmann P, Rössler J, Geiger E, Westphal K-P, Schreiber H, et al. Antiretroviral therapy in sporadic adult amyotrophic lateral sclerosis. Neuroreport. 1993;4:819–22.
- Scelsa SN, MacGowan DJL, Mitsumoto H, Imperato T, LeValley AJ, Liu MH, et al. A pilot, double-blind, placebo-controlled trial of indinavir in patients with ALS. Neurology. 2005;64:1298–300.
- Ludolph A, Drory V, Hardiman O, Nakano I, Ravits J, Robberecht W, et al. A revision of the El Escorial criteria – 2015. Amyotroph Lateral Sc Fr. 2015;16:291–2.
- Mallal S, Phillips E, Carosi G, Molina J-M, Workman C, Tomažič J, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;122:S194–S5.
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia–Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. AJP. 2011;168:1266–77.
- Swash M, de Carvalho M. The neurophysiological index in ALS. Amyotroph Lateral Sc. 2004;5:108–10.
- Shepheard SR, Wuu J, Cardoso M, Wiklendt L, Dinning PG, Chataway T, Schultz DW, Benatar M, Rogers ML. Urinary p75 (ECD): A prognostic, disease progression, and pharmacodynamic biomarker in ALS. Neurology. 2017;88:1137–1143.
- Lu C-H, Petzold A, Topping J, Allen K, Macdonald-Wallis C, Clarke J, et al. Plasma neurofilament heavy chain levels and disease progression in amyotrophic lateral sclerosis: insights from a longitudinal study. J Neurol Neurosurg Psychiatry. 2015;86:565–73.
- Laderoute MP, Giulivi A, Larocque L, Bellfoy D, Hou Y, Wu H-X, et al. The replicative activity of human endogenous retrovirus K102 (HERV-K102) with HIV viremia. Aids. 2007;21:2417–24.
- Laderoute MP, Larocque LJ, Giulivi A, Diaz-Mitoma F. Further evidence that human endogenous retrovirus K102 is a replication competent foamy virus that may antagonize HIV-1 replication. TOAIDJ. 2015;9:112–22.
- Dube D, Contreras-Galindo R, He S, King SR, Gonzalez-Hernandez MJ, Gitlin SD, et al. Genomic flexibility of human endogenous retrovirus type K. J Virol. 2014;88:9673–82.
- Westeneng H-J, Debray TPA, Visser AE, van Eijk RPA, Rooney JPK, Calvo A, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018;17:423–33.
- Balendra R, Jones A, Jivraj N, Knights C, Ellis CM, Burman R, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Sc Fr. 2014;15:279–84.
- Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48.
- Küry P, Nath A, Créange A, Dolei A, Marche P, Gold J, et al. Human endogenous retroviruses in neurological diseases. Trends Mol Med. 2018;24:379–94.
- Ene L, Duiculescu D, Ruta SM. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. 2011;4:432–9.
- Letendre SL, Mills AM, Tashima KT, Thomas DA, Min SS, Chen S, et al. ING116070: a study of the pharmacokinetics and antiviral activity of dolutegravir in cerebrospinal fluid in HIV-1-infected, antiretroviral therapy-naive subjects. Clin Infect Dis. 2014;59:1032–7.
- De Carvalho M, Pinto S, Costa J, Evangelista T, Ohana B, Pinto A. A randomized, placebo-controlled trial of memantine for functional disability in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11:456–60.
- Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. Aids. 2005;19:399–406.
- Lu C-H, Macdonald-Wallis C, Gray E, Pearce N, Petzold A, Norgren N, et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology. 2015;84:2247–57.
- Park SB, Vucic S, Cheah BC, Lin CS, Kirby A, Mann KP, et al. Flecainide in Amyotrophic Lateral Sclerosis as a Neuroprotective Strategy (FANS): A Randomized Placebo-Controlled Trial. EBioMedicine 2015;2:1916–22.