Abstract
Objective: To measure the correlation between single breath counting (SBC) and forced vital capacity (liters, FVCL) in amyotrophic lateral sclerosis (ALS) patients and to define the utility of SBC for determining when patients meet the threshold for initiation of noninvasive positive pressure ventilation (FVC < 50% predicted [FVCpred]). Methods: Both patient paced (SBCpp) or externally paced (SBCep) counting along with FVCL+pred and standard clinical data were collected. Linear regression was used to examine SBCpp and SBCep as a predictor of FVCL. Receiver operating characteristic curve analysis evaluated the sensitivity and specificity of SBC categorically predicting FVCpred of ≤50%. Results: In 30 ALS patients, SBC explained a moderate proportion of the variance in FVCL (SBCpp: R2= 0.431, p < 0.001; SBCep: R2 = 0.511, p < 0.01); this proportion improved when including covariates (SBCpp: R2= 0.635, p < 0.01; SBCep: R2= 0.657, p < 0.01). Patients with minimal speech involvement performed similarly in unadjusted (SBCpp: R2 = 0.511, p < 0.01; SBCep: R2= 0.595, p < 0.01) and adjusted (SBCpp: R2 = 0.634, p < 0.01; SBCep: R2= 0.650, p < 0.01) models. SBCpp had 100% sensitivity and 60% specificity (area under curve (AUC) = 0.696) for predicting FVCpred <50%. SBCep had 100% sensitivity and 56% specificity (AUC = 0.696). With minimal speech involvement SBCpp and SBCep both had 100% sensitivity and 76.1% specificity (SPCpp: AUC = 0.845; SBCep: AUC = 0.857). Conclusions: SBC explains a moderate proportion of variance in FVC and is an extremely sensitive marker of poor FVC. When FVC cannot be obtained, such as during the current COVID-19 pandemic, SBC is helpful in directing patient care.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive motor neuron disorder that is uniformly fatal. Decline in pulmonary function correlates closely with risk of death (Citation1). Noninvasive positive pressure ventilation (NIPPV) has demonstrated a survival benefit in ALS (Citation2). Initiation of NIPPV is commonly based upon the vital capacity (VC).
The COVID-19 pandemic has instigated an abrupt change in our approach to outpatient care. Most ALS multidisciplinary clinics are now managing people with ALS, who are considered a high-risk population, using telemedicine techniques. The inability to measure VC in ALS individuals using telemedicine is challenging and problematic. In the absence of a formal VC measurement, a method to estimate VC would help guide clinical decisions, such as the timing of initiating NIPPV.
The single breath count (SBC) is a bedside screen widely used to estimate the respiratory muscle strength. SBC is performed by inhaling maximally and counting aloud to as high a number as possible in a single breath. There is remarkably little literature regarding SBC (Citation3–5) or standardization of the counting procedure for ALS. SBC may be useful as a screening method for respiratory muscle weakness in people with ALS when formal VC measurement is not possible. We aimed to measure the strength of the relationship between forced vital capacity (FVC) and SBC in a sample of our ALS clinic patients. We also examined SBC as a screening tool for determining when patients would need to initiate NIPPV (conventionally when FVC is less than 50% of predicted value).
Methods
This study was approved by Institutional Review Boards (IRB) at the University of Massachusetts Medical School and University of Pennsylvania. All study participants provided consent prior to performing any study procedures.
Participants were included if they were over 18 years of age, carried a diagnosis of ALS meeting the El Escorial Criteria of possible, probable, probable lab-supported, or definite ALS, could provide informed consent and performing study evaluations. Participants were excluded if they had a superimposed pulmonary disorder unrelated to ALS that could affect their function, or if they had undergone a tracheostomy.
Study procedures were performed by experienced ALS clinicians, nurses, and clinical research coordinators. Evaluators were trained in performing FVC and the Revised ALS Functional Rating Scale (ALSFRS-R) (Citation6) for the purposes of multicenter trials through the Northeast ALS Consortium (NEALS).
SBC
We used two methods to evaluate SBC: (1) Patient paced (SBCpp): participants were given minimal instructions. They were told only to inhale as deeply as possible and then count in a single breath in their normal speaking voice as quickly as they can while keeping the numbers audibly distinct. The examiner provided a demonstration of the intended procedure. (2) Externally paced (SBCep): participants were shown a video of a metronome (http://www.youtube.com/watch?v=2BYT9lL-1Q0), set to 120 beats per minute and asked to count in unison with the metronome (two numbers per second). The beat per minute was selected as 120 BPM based on experience of the study staff, with the goal of eliminating pause time between number vocalizations without rushing annunciation.
Sitting FVC
Study participants performed spirometry using the EasyOne Plus Diagnostic Spirometry System (NDD, Andover, MA) in a seated position. Both the liters (FVCL) and percent predicted (FVCpred (Citation7)) were recorded. Two trials were performed and the average FVC was used for analysis. In patients in whom SBC was collected on multiple visits, their last SBC and FVC (collected the same visit) were used to allow for greater representation of SBC in patients with lower FVCs.
The ALSFRS-R and demographic data were recorded during the same visit.
Statistical methods
Descriptive statistics were used to characterize the demographic and clinical characteristics of study participants. Nonparametric test and exact tests were used to compare the full study cohort and sub-cohort with minimal speech involvement.
Linear regression was used to examine SBCpp and SBCep as a predictor of FVCL both in the total cohort and in a subcohort of patients with minimal speech involvement, defined as having an ALSFRS-R Question 1 score of ≥3. We also evaluated each of the above models adjusting for three covariates known to impact respiratory volume: sex, age, and height. An receiver operating characteristic (ROC) analysis was used to evaluate the sensitivity and specificity of SBC categorically predicting FVCpred of ≤50%, a common criteria for initiating NIPPV.
Results
Forty participants met the selection criteria and consented for the study. Four were not able to complete an FVC, five were not able to complete SBCep, and one could not complete either. Demographic and clinical data for the 30 participants who could complete the study procedures, and a subcohort of 25 participants with minimal speech involvement, are reported in . This sub-cohort was representative of the full cohort on all demographic and clinical features (all p > 0.3).
Table 1 Demographic and clinical characteristics of the full ALS patient cohort and the minimal speech cohort.a
In the full cohort, SBC was able to explain a moderate proportion of the variance in FVCL ( top, SBCpp: R2= 0.431, p < 0.001; SBCep: R2 = 0.511, p < 0.01). In the total cohort, ∼14 digits for SBCpp (β = 0.036) and ∼12 digits for SBCep (β = 0.042) were equivalent to 500mL FVC (defined as 0.5L/β). When including covariates, SBC explained even more variance in FVCL across both procedures (SBCpp: R2= 0.635, p < 0.01; SBCep: R2= 0.657, p < 0.01). Of the covariates, age appeared to have the largest impact. Older age was associated with lower FVCL, (across models β ranges from 0.21–0.23, p < 0.01), as clinically expected. We observed similar performance in the minimal speech involvement cohort for unadjusted (SBCpp: R2 = 0.511, p < 0.01; SBCep: R2= 0.595, p < 0.01; bottom) and adjusted (SBCpp: R2 = 0.634, p < 0.01; SBCep R2= 0.650, p < 0.01) models. In this subcohort, approximately 13 digits for SBCpp (β = 0.040) and approximately 11 digits for SBCep (β = 0.045) is equivalent to 500mL FVCL.
Figure 1 (A) Association between single breath counting and FVCL for patient paced and externally paced study procedures.(B) ROC curves for predicting FVCpred <50% adjusting for age, sex, and height. Top-row: full cohort (N = 30); Bottom-row: minimal speech cohort (N = 25).
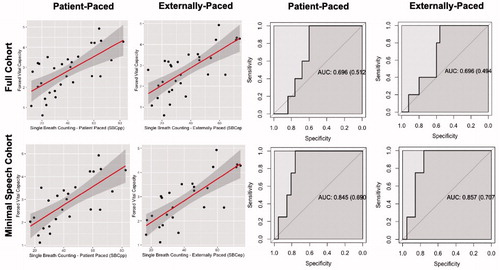
ROC adjusted for age, sex, and height revealed moderate sensitivity and specificity for predicting FVCpred of ≤50%. In the full cohort SBCpp had 100% sensitivity and 60% specificity (area under curve (AUC) = 0.696) and similarly SBCep had 100% sensitivity and 56% specificity (AUC = 0.696; top). In the minimal speech involvement cohort SBCpp and SBCep both had 100% sensitivity and 76.1% specificity (SPCpp: AUC = 0.845; SBCep: AUC = 0.857; bottom).
Conclusions
Our findings suggest that SBC explains a moderate proportion of variance in FVC and is an extremely sensitive marker of poor FVC. SBCep demonstrated slightly better predictive value, though at the cost of a higher performance failure rate.
ALS clinician’s base important clinical decisions on FVC scores, including initiation of NIPPV. When FVC cannot be obtained, such as during the current COVID-19 pandemic, an estimation of FVC is helpful in directing patient care. In participants with minimal bulbar dysfunction, SBC is reasonably predictive of FVC, demonstrating that it can be used to help guide clinical decisions when FVC cannot be obtained. As an example: our data indicate that for a 50-year old with average height (5’3” for female, 5’9” for male) an SBC of <23 for males and <10 for females predict a FVC < 50%, which would be the threshold for initiating NIPPV.
Further studies could explore SBCep in a larger group of participants and in telemedicine settings to further evaluate its real-world clinical utility. In addition, the participants in this study had a median FVC of 71.0 [interquartile range (IQR) = 55.12–85.75] with only a small subset of approximately 16% having a FVC < 50% . It will be important for future studies to include a wider distribution of FVCs including lower performance to refine the suggested threshold for initiating NIPPV. While we observe relatively similar performance across the sub-analyses of the minimal speech involvement group and full cohort it will also be important for future studies to evaluate the impact of bulbar dysfunction more equally on SBC and its correlation with FVC. However, despite these caveats, it is our hope that this article stimulates future research into the potential application of SBC as a proxy for FVC into remote clinical practices.
Author contributions
Dr. Colin Quinn: Conceptualization, design, and drafting the manuscript.
Dr. Corey McMillan: Statistical analysis and drafting the manuscript.
Dr. Margaret A. Owegi and Kelly Almasy: Collection of clinical data.
Catherine Douthwright and Diane McKenna-Yasek: Collection of clinical data and drafting the manuscript.
Namita A. Goyal, James Berry, and Robert H. Brown Jr: Conceptualization, design, and drafting the manuscript.
Declaration of interest
Colin Quinn: advisory board and consulting fees from Amylyx Pharmaceuticals, Acceleron Pharma, and Amicus Therapeutics. Research support from Amylyx Pharmaceuticals, Acceleron Pharma, Amicus Therapeutics, and Orphazyme.
Corey McMillan: receives research funding from Biogen Inc., and provides consulting services for Invicro and Axon Advisors on behalf of Translational Bioinformatics, LLC. He also receives an honorarium as Associate Editor of NeuroImage: Clinical.
Margaret A. Owegi: nothing to disclose.
Kelly Almasy: nothing to disclose.
Catherine Douthwright: nothing to disclose.
Diane McKenna-Yasek: nothing to disclose.
Namita Goyal: received research support from Brainstorm Cell Therapeutics, Cytokinetics, Fulcrum, Kezar, Novartis, Octapharma, Orion, and Orphazyme.
Dr. Goyal has served on Advisory Boards for Acceleron, Alexion, Argenx, Biogen, CSL Behring, Cytokinetics, MT Pharma, Novartis, Sanofi Genzyme, and Sarepta. In relation to these activities, she has received travel reimbursement and honoraria. She has also served on the speaker’s bureau for CSL.
James Berry: advisory board consulting fees from Biogen, Clene Nanomedicine, and Alexion. He has received research support from Biogen, MT Pharma of America, Anelixis Therapeutics, Amylyx Therapeutics, Brainstorm Cell Therapeutics, Genentech, nQ Medical, NINDS, Muscular Dystrophy Association, ALS One, and ALS Finding A Cure.
Robert H. Brown Jr: scientific co-founder and consultant ApicBio, Inc. Holds equity in Amylyx and Imstar.
Data availability statement
Anonymized data is available upon request from any qualified investigator for the purposes of replicating procedures and results.
References
- Ringel SP, Murphy JR, Alderson MK, Bryan W, England JD, Miller RG, et al. The natural history of amyotrophic lateral sclerosis. Neurology. 1993;43:1316–22.
- Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–7.
- Elsheikh B, Arnold WD, Gharibshahi S, Reynolds J, Freimer M, Kissel JT. Correlation of single-breath count test and neck flexor muscle strength with spirometry in myasthenia gravis. Muscle Nerve. 2016;53:134–6.
- Kass LE, Putnam K. Single breath counting for the evaluation of pediatric respiratory function: derivation of a "normogram". Intern Emerg Med. 2016;11:225–8.
- Ali SS, O’Connell C, Kass L, Graff G. Single-breath counting: a pilot study of a novel technique for measuring pulmonary function in children. Am J Emerg Med. 2011;29:33–6.
- Cedarbaum JM, Stambler N. Performance of the amyotrophic lateral sclerosis functional rating scale (ALSFRS) in multicenter clinical trials. J Neurol Sci. 1997;152(Suppl 1):S1–S9.
- Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127:725–34.
