Abstract
Objective: Human endogenous retroviruses (HERVs) have been gradually confirmed to be involved in the onset and progression of motor neuron disease(MND). However, noninvasive detection of HERVs in the central nervous system is lacking. The aim of this study is to verify the relationship between the level of HERV-K env in neuronal extracellular vesicles in plasma and the onset and severity of MND.
Methods: We extracted neuronal extracellular vesicles from plasma of 39 MND patients and 30 age- and sex-matched controls, and detected HERV-K env in extracellular vesicles by an enzyme-linked immunosorbent assay (ELISA).
Results: Levels of HERV-K env in neuronal extracellular vesicles positively associated with range of lower motor neurons (LMNs) involved (1.66 ± 0.37 vs. 1.35 ± 0.34, p = 0.041), ALS phenotype (1.52 ± 0.31 vs. 1.24 ± 0.37, p = 0.013) and course of disease (1.83 ± 0.35 vs. 1.42 ± 0.22, p = 0.003), and increased in advanced-phase MND (definite and probable according to revised EI Escorial criteria) compared with early-phase MND (possible and lab-supported probable), albeit without very profound significance (1.52 ± 0.34 vs. 1.29 ± 0.36, p = 0.048).
Conclusions: In conclusion, levels of HERV-K env in neuronal extracellular vesicles extracted from plasma can be used as a noninvasive biomarker of severity of MND.
Introduction
Human endogenous retroviruses (HERVs) are remnants of provirus genome that integrated into the human genome through infection over several million years (Citation1,Citation2), accounting for approximately 8% of the human genome (Citation3). As humans evolved, most HERVs lost transcriptional activity due to deletions or stop codons in the reading frames, but some HERVs have complete open reading frames (ORFs) that can express viral protein particles or proviruses. Currently, HERV-K has been confirmed as the most active, and it can express complete functional proteins. HERVs and exogenous retroviruses, such as human immunodeficiency virus (HIV), have similar genetic structures, with long terminal repeats (LTRs) and gag, pro, pol, and env regions, which encodes envelope proteins (Citation4). The env protein is structurally divided into surface unit (SU) and transmembrane (TM) moieties. Whether HERV-K Env protein is functional is a matter of debate, but some evidence suggests SU may serve as a receptor and participate in membrane fusion and immune regulation, TM may relate to intracellular signal transduction (Citation5).
HERVs participate in a number of pathophysiological processes. In the nervous system, researches on HERVs and multiple sclerosis (Citation6), schizophrenia, and motor neuron disease(MND) have gradually increased (Citation7). As early as 1973, Gardner et al. found that mice infected with type C RNA virus developed progressive motor neuron damage after several months (Citation8). In addition patients infected with type 1 human T cell leukemia virus (HTLV-1) and HIV could manifest as amyotrophic lateral sclerosis (ALS) syndrome (Citation9–11). Subsequent studies confirmed that the level of reverse transcriptase (RT) in the serum of HTLV-1 and HIV-negative ALS patients was significantly higher than that of controls, and similar trends were observed in the immediate relatives of ALS patients, suggesting that RT was endogenous and not related to exogenous virus infection (Citation12–14).
Douville et al. reported that HERV-K transcripts were increased in patients with ALS, especially in the prefrontal lobe (Citation15). Li et al. (Citation16) suggested that the expression of HERV-K was increased in autopsy brain tissue of patients with ALS compared to healthy controls and patients with Alzheimer's disease (AD). And env was increased selectively in the cytoplasm of pyramidal cells and spinal anterior horn cells but not in glial cells, white matter, hippocampal neurons and posterior cord. Cells transfected with HERV-K or the env gene all resulted in atrophy and shortened processes, which were concentration-dependent. Transgenic mice expressing the full-length or TM of env exhibited atrophy of the motor cortex and decreased protrusions of pyramidal cells. Therefore, HERVs, especially HERV-K env, may be used as a marker for the diagnosis and monitoring of disease progression in MND. There is an urgent need for a noninvasive technique to detect the expression level of HERVs derived from the central nervous system.
Extracellular vesicles are extracellular particles with a diameter of approximately 30–100 nm and a double-layered lipid membrane. Extracellular vesicles can derive from almost all type of cells, including neurons (Citation17),and can be detected in a variety of body fluids, such as blood, cerebrospinal fluid, and urine (Citation18). Tolosa et al. (Citation19) reported that the env protein syncytin-1 of HERV-W was positive in placental extracellular vesicles. Vargas et al. (Citation20) confirmed that syncytin-1/syncytin-2 in placental extracellular vesicles was associated with placental formation. Therefore, it is speculated that HERVs can be excreted through the form of extracellular vesicles, but there is no research to use extracellular vesicles for the detection of HERVs derived from the central nervous system in patients with neurodegenerative diseases.
The aim of this study is to extract neuronal extracellular vesicles from the blood of patients with MND and age-and sex-matched controls and then detect the level of HERV-K env in extracellular vesicles. By analyzing clinical data, we plan to verify the relationship between the presence of HERV-K env in extracellular vesicles and the onset and severity of MND.
Materials and methods
Study subjects
According to the revised EI Escorial diagnostic criteria (Citation21), MND patients were classified as early phase (possible and laboratory-supported probable) and advanced phase (probable and definite) (Citation22), without acute or chronic history of the central nervous system, such as acute cerebrovascular disease, brain trauma, brain tumors, encephalitis, or myelopathy; and other neurodegenerative diseases, such as AD, Parkinson’s disease (PD), dementia with Lewy bodies (DLB), or multiple system atrophy; and history of tumor. Essential clinical data included sex, age, onset site, diagnostic delay time, previous medical history, neurological signs, results of electromyography, treatment, and ALSFRS-R scores (Citation23), and ΔFS was used to assess the rate of disease progression (Citation24).
Sex-and age-matched controls were individuals without MND history or family history of MND, without acute or chronic history of the central nervous system, such as acute cerebrovascular disease, brain trauma, brain tumors, encephalitis, or myelopathy; and other neurodegenerative diseases, such as AD, PD, DLB, or multiple system atrophy; and history of tumor. This study was approved by the institutional ethics committee and all subjects signed informed consent forms.
Plasma extraction
EDTA anticoagulation tubes were used to collect 4 ml of peripheral venous blood. To extract plasma, blood samples were centrifuged at 1500g at 4 °C for 10 min to remove blood cells. The supernatant was then centrifuged at 12000g at 4 °C for 5 min to remove the platelets and cell debris. The plasma was collected and stored at −80 °C.
Extraction of neuronal extracellular vesicles
The way to extract neuronal extracellular vesicles based on previous studies (Citation25–27). About 0.5 ml plasma was incubated with 5 µl thrombin (System Biosciences, CA, USA) for 30 min. Then, 500 µl of calcium- and magnesium-free Dulbecco's Balanced Salt (DBS) solution with protease inhibitor cocktail (Roche, Mannheim, Germany) and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Rockford, USA) was added, followed by centrifugation at 6000g at 4 °C for 20 min. The supernatant was collected, followed by the addition of 1 ml polyethylene glycol and incubation at 4 °C for 1 hour. After centrifugation at 10,000g at 4 °C for 20 min and the removal of the supernatant, total extracellular vesicles were resuspended in 250 µl ddH2O containing protease inhibitor cocktail and phosphatase inhibitor cocktail and incubated at 4 °C for at least 2 hours. Then, after centrifugation at 1500 g at 4 °C for 10 min, 4 µl (2 µg) biotinylated anti-L1CAM (L1 cell adhesion molecule, also known as CD171) antibody (Thermo Fisher Scientific, CA, USA) in 46 µl of 3% bovine serum albumin (BSA, Thermo Fisher Scientific, Rockford, USA) was added to the supernatant and incubated at 4 °C for 4 hours. After the addition of 15 µl streptavidin-agarose resin (Thermo Fisher Scientific, Rockford, USA) and 25 µl of 3% BSA, the mixture was incubated at 4 °C for 4 hours. Subsequently, the supernatant was centrifuged at 200g for 10 min at 4 °C, suspended in 500 µl of 3% BSA, and centrifuged again. Then, 200 µl of 0.1 M glycine-HCl (pH = 3.0) was added, and the mixture was centrifuged at 4500g at 4 °C for 10 min; the supernatant was removed. Then, 15 µl of 1M Tris-HCl (pH = 8.0), 25 µl of 3% BSA and 360 µl of mammalian protein extraction reagent (M-PER, Thermo Fisher Scientific, Rockford, USA) were added, and the final preparation was frozen and thawed twice and stored at −80 °C. HERV-K env was quantified using the Human HERV K_7p22.1 Provirus Ancestral Env Polyprotein (ERVK6) ELISA kit (Cusabio Technology, Wuhan, China) and following the instructions. To ensure the specificity of the tests, negative control group was set up in this study. In the negative control group, the biotinylated anti-L1CAM antibody was replaced with 3% BSA. The concentration of CD81 was quantified as an internal reference using the Human CD81 Antigen ELISA kit (Cusabio Technology, Wuhan, China). The mean value for all determinations of CD81 in each assay group was set at 1.00, and the relative value for each sample were used to normalize their recovery.
Statistical methods
Statistical analysis was performed using IBM SPSS 20.0 software. In terms of descriptive statistics, continuous variables that were consistent with a normal distribution are represented by means and standard deviations (SDs), and those that did not meet the normal distribution are represented by medians and interquartile ranges (IQRs). Categorical variables were calculated as percentages. Parametric tests were performed with independent-sample T tests or t′ tests, and nonparametric tests were performed with the chi-squared, Fisher's exact test, or the Mann-Whitney U test. Pearson’s or Spearman’s correlation was used to analyze the correlation between the two variables. p < 0.05 was considered statistically significant. GraphPad Prism 5 software (GraphPad software Inc., San Diego, CA, USA) was used for plotting.
Results
Basic characteristics of the study subjects
A total of 39 MND patients were included in this study, with 25 males (64.1%). Thirty sex- and age-matched controls were also included, with 17 males (56.7%). The average age of the two groups was 50.9 (SD = 9.4) years and 50.8 (SD = 8.9) years, respectively. The median diagnostic delay time of MND patients was 13 (IQR = 17.0) months, 32 (82.1%) patients had limb onset, and 7 (18.0%) patients had bulbar onset. There were 19 (48.7%) patients in the early phase, of whom 9 were diagnosed as possible and 10 as laboratory-supported probable. Twenty patients (51.3%) were in an advanced phase, of whom 14 were diagnosed as probable and 6 as definite. Twenty-three patients (59.0%) met the diagnosis of ALS, and 16 patients (41.0%) had other phenotypes, including 5 with flail leg syndrome (FLS), 6 with flail arm syndrome (FAS), and 5 with isolated bulbar ALS (IBALS). At the initial diagnosis, the average ALSFRS-R score was 42.5 (SD = 3.7), and the median ΔFS was 0.3 (IQR = 0.4) ().
Table 1 Characteristics of MND patients and controls.
HERV-K env in neuronal extracellular vesicles
Following ISEV guidelines, we used western blot to demonstrate enrichment of exosomes by our protocol. The extracellular vesicles were positive for CD63 and Alix (Citation27) (Supplementary Figure S1). The average diameter of vesicles verified by nanoparticle tracking analysis was about 100nm (Supplementary Figure S2). Based on these data, we confirmed that exosomes were successfully collected. In the CD81 and HERV-K env test, the negative control group was at background level. The mean HERV-K env level in the neuronal extracellular vesicles of 39 MND patients was 1.41 (SD = 0.36) ng/ml, and that of the control group (n = 30) was 1.45 (SD = 0.50) ng/ml. There was no significant difference between the groups (p = 0.701) ().
Figure 1 HERV-K env level in the neuronal extracellular vesicles of MND patients and controls. The mean HERV-K env level in the neuronal extracellular vesicles of 39 MND patients was 1.41 (SD = 0.36) ng/ml, and that of the control group (n = 30) was 1.45 (SD =0.50) ng/ml. There was no significant difference between the groups (P = 0.701).
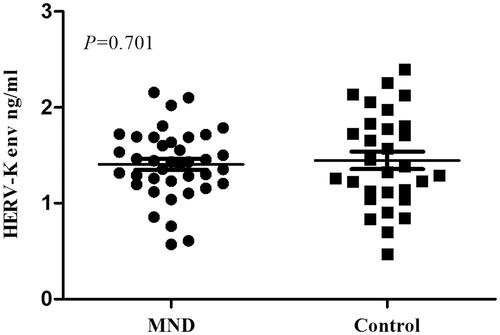
Figure 2 Analysis of HERV-K env level in the neuronal extracellular vesicles of MND patients. (A) The mean HERV-K env level in early-phase MND patients (n = 19) was lower than that in advanced-phase MND patients (n = 20) (p = 0.048). (B) Grouped by involved segments (bulbar, cervical, thoracic, lumbosacral), patients with affected motor neurons in no more than 2 segments were assigned to the LMN-1/UMN-1 groups, and those with 3–4 affected segments were assigned to the LMN-2/UMN-2 groups. The mean level of HERV-K env was positively associated with LMNs involved (p = 0.041). (C)The mean HERV-K env level in the ALS group was significantly higher than that in the other phenotype group (including 6 with FAS, 5 with FLS, and 5 with IBALS) (p = 0.013). (D)The mean level of HERV-K env in the patients with a disease course less than 2 years (MND ≤ 2) was significantly lower than that in the patients with a disease course more than 2 years (MND> 2) (p = 0.003).
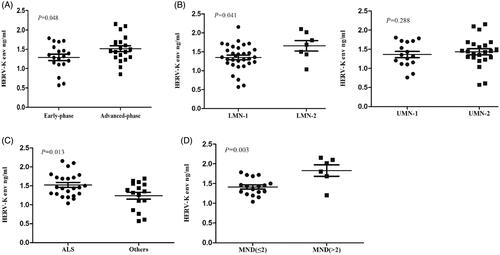
The mean HERV-K env level in neuronal extracellular vesicles in early-phase MND patients (n = 19) was 1.29 (SD = 0.36) ng/ml, and that in advanced-phase MND patients (n = 20) was 1.52 (SD = 0.34) ng/ml. The level of HERV-K env in advanced-phase patients was higher than that in early-phase patients but no profound significance was found (p = 0.048). Grouped by involved segments (bulbar, cervical, thoracic, lumbosacral), patients with affected motor neurons in no more than 2 segments were assigned to the LMN-1/UMN-1 groups, and those with 3 to 4 affected segments were assigned to the LMN-2/UMN-2 groups. The mean level of HERV-K env in LMN-1 and LMN-2 was 1.35 (SD = 0.34) and 1.66 (SD = 0.37) ng/ml, respectively (p = 0.041); and that in UMN-1 and UMN-2 was 1.35 (SD = 0.33) and 1.47 (SD = 0.35) ng/ml, respectively (p = 0.288). There were 23 patients with MND presenting with typical ALS and 16 with other phenotypes, including 6 with FAS, 5 with FLS, and 5 with IBALS. The mean HERV-K env level in the ALS group was 1.52 (SD = 0.31) ng/ml, and that in patients with other phenotypes was 1.24 (SD = 0.37) ng/ml. The HERV-K env level in the ALS group was significantly higher than that in the other phenotype group (p = 0.013). The mean level of HERV-K env in the patients with a disease course less than 2 years (MND ≤ 2) was 1.42 (SD = 0.22) ng/ml, and that in the patients with a disease course more than 2 years (MND> 2) was 1.83 (SD = 0.35) ng/ml, indicating a significant difference between the two groups (p = 0.003) ().
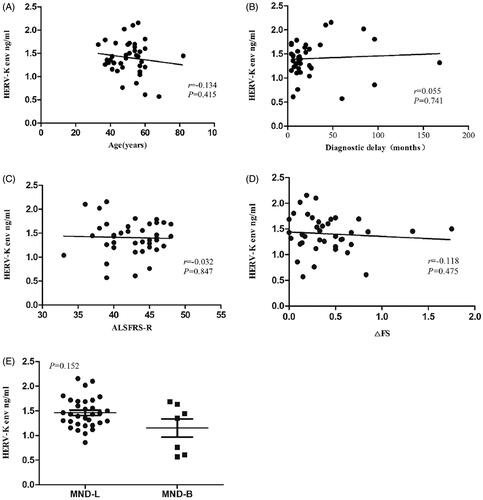
There were no significant correlations between HERV-K env and age (r=-0.134, 95% CI −0.420 to 0.156, p = 0.415), diagnostic delay time (r = 0.055, 95% CI −0.294 to 0.442, p = 0.741), ALSFRS-R score (r=−0.032, 95% CI −0.391 to 0.320, p = 0.847), ΔFS (r=−0.118, 95% CI −0.418 to 0.191, p = 0.475), and the onset site (1.15, SD = 0.49; 1.46, SD = 0.31, p = 0.152) ().
Figure 3 Correlations between HERV-K env level in the neuronal extracellular vesicles and age, diagnostic delay time, ALSFRS-R score, ΔFS and the onset site. There were no significant correlations between HERV-K env and age (r=−0.134, 95% CI −0.420 to 0.156, p = 0.415) (A), diagnostic delay time (r = 0.055, 95% CI -0.294 to 0.442, p = 0.741) (B), ALSFRS-R score (r=−0.032, 95% CI −0.391 to 0.320, p = 0.847) (C), ΔFS (r=−0.118, 95% CI −0.418 to 0.191, p = 0.475) (D), the onset site (1.15, SD = 0.49;1.46, SD = 0.31, p = 0.152) (E).
Discussion
HERVs have been found widely in different locations of the human genome, whereas, because of the occurrence of missense and nonsense mutations, most HERVs are in silence. HERV-K has been confirmed as the most active and the most common type studied in MND. Expression of HERV-K specifically elevated in MND compared with PD, AD, multiple sclerosis (MS). The env protein, a transmembrane signaling protein, has been proven to play a role in proliferation and metastasis of carcinoma, and autoimmunity in MS. A study published by Paquette et al. in 1989 suggested that retrovirus env caused MND in mice (Citation28). Li et al. (Citation16) found that both HERV-K env transgenic cells and mice showed ALS-like neurological symptoms and pathological manifestations. Arru et al. (Citation29) detected a significant increase in anti-HERV-K env-su19-37 IgG antibodies in the serum and cerebrospinal fluid of ALS patients, suggesting that a humoral immune response to HERV-K env occurred in ALS patients. It is speculated that HERV-K env plays a role in the pathogenesis and progression of MND. Therefore, this study investigated the correlation between the level of HERV-K env and the onset and severity of motor neuron disease.
Previous studies have used autopsy brain tissue, which is difficult to obtain, or detected HERV mRNA or antibodies in the blood, the origination of which cannot be confirmed. This study examined the level of HERV-K env in neuronal extracellular vesicles, suggesting that neuronal extracellular vesicles in blood can be used for noninvasive detection of HERV-K env expression in the central nervous system. Many previous studies have confirmed L1CAM is selective for extracellular vesicles derived from neurons. Fiandaca et al. (Citation25) have verified the mouse anti-human L1CAM biotinylated antibody does not bind to NK and NKT cells of the immune system and is differently distributed in the nervous system. Winston et al. (Citation26) used mouse anti-human L1CAM biotinylated antibody to extract neuron-derived exosomes in serum of AD patients. Jiang et al. (Citation30) also chose anti-L1CAM antibodies to get extracellular vesicles with characteristics of exosomes and used mass spectrometry analysis and immunoblotting confirmed the presence of neural cell adhesion molecule and L1CAM. However, it cannot be ignored that L1CAM is reported in multiple studies to also be expressed in other tissues than neurons. We look forward to a more specific and precise method to extract EVS derived from neurons in the future.
Grad et al. (Citation31) concluded that extracellular vesicles are the pathway by which neurons secrete SOD1 and that SOD1 diffuses between cells. Yohei Iguchi et al. (Citation32) used TDP43A315T transgenic mice to find that extracellular vesicles are a way for neurons to clear abnormal TDP43 deposition. Therefore, extracellular vesicles play multiple roles in neurodegenerative diseases, may be related to the removal of abnormal proteins, may also participate in the transmission of abnormal proteins between cells, leading to disease progression. Fiandaca et al. (Citation25) confirmed that tau and Aβ positive neuronal extracellular vesicles in plasma were with the sensitivity up to 96% to diagnose AD, even in preclinical patients, suggesting that neuronal extracellular vesicles in plasma can be a sensitive biomarker of the state of neurons.
The results of this study suggested that the level of HERV-K env in neuronal extracellular vesicles was related to the range of motor neuron involvement and course of disease. This is consistent with previous studies. Li et al. (Citation16) showed that the degree of atrophy of neurons and the shortening of nerve protrusion was dependent on the concentration of HERV-K env; that is, the higher the expression of HERV-K env, the more severe the damage was. Arru et al. (Citation29) showed that the concentration of serum HERV-K env antibody was negatively correlated with the ALSFRS score. Studies by Douville et al. (Citation15) and Tam et al. (Citation33) have confirmed that HERV expression is elevated in the brain tissue of autopsy patients with ALS, suggesting that HERV expression in the neurons of patients with end-stage ALS was significantly higher than that of unaffected individuals. Therefore, the increased expression of HERV-K can be used as a marker to indicate the degree of damage to motor neurons. The reason why there was no significant correlation of HERV-K and the range of upper motor neurons (UMNs) involvement may be that there are limited ways to evaluate UMNs in clinical, and UMNs signs may be masked by muscular atrophy.
In this study, the level of HERV-K env in extracellular vesicles was not significantly different between the patients and the controls. Controls in our study are sex-and age-matched individuals without MND history or family history of MND, without acute or chronic history of the central nervous system and history of tumor. Therefore, we can roughly rule out the influence of these factors on the results. An important consideration is that MND is a heterogeneous syndrome, and the clinical phenotypes may also vary. A study by Tam et al. (Citation33) classified ALS into three categories based on RNAseq analysis of the brain. One of these groups had activation of transposable elements including HERV-K that represented 20% of the samples. Then, MND patients were classified as early phase and advanced phase according to the revised EI Escorial diagnostic criteria in our study. Some patients with the phenotypes of IBALS, FAS or FLS were in the advanced group, but HERV-K env level of patients with these phenotypes was significantly lower than that in the ALS group. Manghera et al. (Citation34) suggested that binding of TDP43 to the HERV-K 5’LTR led to the disruption of autophagy pathways and the clearance of env in motor neurons, but the total expression level of HERV-K env did not increase. Therefore, the accumulation of HERV-K env in neurons of MND patients increased, especially in the participating of inflammatory factors, such as TNFα (Citation35), but HERV-K env was negative in glial cells and controls. This may be the reason why HERV-K in plasma of controls appears to be higher than the early-phase patients.
In previous studies, the results on the correlation between HERVs and age have been inconclusive. Bhat et al. (Citation36) believed that the expression level of HERV was related to age, but the results of research by Mayer et al. (Citation37) confirmed that the expression of HERV-K was independent of age. The result of this study was that the expression level of HERV-K env is independent of age. This result needs more research to confirm.
In addition, there were no significant correlations between HERV-K env and diagnostic delay time, ALSFRS-R score, ΔFS or the onset site. It is considered that different onset sites do not affect the level of HERV-K env in the blood. ΔFS is affected by many factors, such as nutritional status, medication, oxygen supply, and the correlation may be masked by other factors. The results of correlation between the diagnostic delay time, ALSFRS-R score and HERV-K env may result from the small number of study cases.
The innovation of our study is using neuronal extracellular vesicles as a tool to noninvasively detect the expression level of HERV-K in neurons. However, whether the level of HERV-K env in neuronal extracellular vesicles in plasma is consistent with that in neurons has not been confirmed. There are limitations of this study. First, the number of patients was small. Then, ERVK6 has been proven to be the full-length HERV-K env. There are different isomers of HERV-K env in vivo, so nonspecific binding is inevitable. Though the amount of CD81 protein was measured to normalize the EVs content in our study, it would be helpful to use more precise tools, like nanoparticle tracking counts, to provide more information on the concentration of HERV-K env detected in per EV or per ug of EVs.
In conclusion, this study used extracellular vesicles as a tool to confirm that HERV-K env may be used as a marker of disease progression in patients with MND, suggesting that HERV-K env may be related to the degree of damage to motor neurons.
Author contributions
YL and DF contributed to conception and design of the study. YL, YC and NZ organized the database and performed the experiment and statistical analysis. YL wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Supplemental Material
Download MS Word (362.3 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Additional information
Funding
References
- Boeke JD, Stoye JP. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin JM, Hughes SH, Varmus HE, eds. Retroviruses. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press Cold Spring Harbor Laboratory Press; 1997.
- Young GR, Stoye JP, Kassiotis G. Are human endogenous retroviruses pathogenic? An approach to testing the hypothesis. Bioessays. 2013;35:794–803.
- Griffiths DJ. Endogenous retroviruses in the human genome sequence. Genome Biol. 2001;2:1017.
- Kury P, Nath A, Creange A, Dolei A, Marche P, Gold J, et al. Human endogenous retroviruses in neurological diseases. Trends Mol Med. 2018;24:379–94.
- Dolei A, Perron H. The multiple sclerosis-associated retrovirus and its HERV-W endogenous family: a biological interface between virology, genetics, and immunology in human physiology and disease. J Neurovirol. 2009;15:4–13.
- van Horssen J, van der Pol S, Nijland P, Amor S, Perron H. Human endogenous retrovirus W in brain lesions: Rationale for targeted therapy in multiple sclerosis. Mult Scler Relat Disord. 2016;8:11–8.
- Garcia-Montojo M, Doucet-O'Hare T, Henderson L, Nath A. Human endogenous retrovirus-K (HML-2): a comprehensive review. Crit Rev Microbiol. 2018;44:715–38.
- Gardner MB, Henderson BE, Officer JE, Rongey RW, Parker JC, Oliver C, et al. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973;51:1243–54.
- Viola MV, Frazier M, White L, Brody J, Spiegelman S. RNA-instructed DNA polymerase activity in a cytoplasmic particulate fraction in brains from Guamanian patients. J Exp Med. 1975;142:483–94.
- Matsuzaki T, Nakagawa M, Nagai M, Nobuhara Y, Usuku K, Higuchi I, et al. HTLV-I-associated myelopathy (HAM)/tropical spastic paraparesis (TSP) with amyotrophic lateral sclerosis-like manifestations. J Neurovirol. 2000;6:544–8.
- MacGowan DJ, Scelsa SN, Waldron M. An ALS-like syndrome with new HIV infection and complete response to antiretroviral therapy. Neurology. 2001;57:1094–7.
- Steele AJ, Al-Chalabi A, Ferrante K, Cudkowicz ME, Brown RH, Jr., Garson JA. Detection of serum reverse transcriptase activity in patients with ALS and unaffected blood relatives. Neurology. 2005;64:454–8.
- MacGowan DJ, Scelsa SN, Imperato TE, Liu KN, Baron P, Polsky B. A controlled study of reverse transcriptase in serum and CSF of HIV-negative patients with ALS. Neurology. 2007;68:1944–6.
- McCormick AL, Brown RH, Jr., Cudkowicz ME, Al-Chalabi A, Garson JA. Quantification of reverse transcriptase in ALS and elimination of a novel retroviral candidate. Neurology. 2008;70:278–83.
- Douville R, Liu J, Rothstein J, Nath A. Identification of active loci of a human endogenous retrovirus in neurons of patients with amyotrophic lateral sclerosis. Ann Neurol. 2011;69:141–51.
- Li W, Lee MH, Henderson L, Tyagi R, Bachani M, Steiner J, et al. Human endogenous retrovirus-K contributes to motor neuron disease. Sci Transl Med. 2015;7:307ra153.
- Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–8.
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140:13–21.
- Tolosa JM, Schjenken JE, Clifton VL, Vargas A, Barbeau B, Lowry P, et al. The endogenous retroviral envelope protein syncytin-1 inhibits LPS/PHA-stimulated cytokine responses in human blood and is sorted into placental exosomes. Placenta 2012;33:933–41.
- Vargas A, Zhou S, Ethier-Chiasson M, Flipo D, Lafond J, Gilbert C, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. Faseb J. 2014;28:3703–19.
- Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- Charil A, Corbo M, Filippi M, Kesavadas C, Agosta F, Munerati E, et al. Structural and metabolic changes in the brain of patients with upper motor neuron disorders: a multiparametric MRI study. Amyotroph Lateral Scler. 2009;10:269–79.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21.
- Kimura F, Fujimura C, Ishida S, Nakajima H, Furutama D, Uehara H, et al. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology. 2006;66:265–7.
- Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimer's. Alzheimers Dement. 2015;11:600–7.e1.
- Winston CN, Goetzl EJ, Baker LD, Vitiello MV, Rissman RA. Growth hormone-releasing hormone modulation of neuronal exosome biomarkers in mild cognitive impairment. J Alzheimers Dis. 2018;66:971–81.
- Chen Y, Xia K, Chen L, Fan D. Increased interleukin-6 levels in the astrocyte-derived exosomes of sporadic amyotrophic lateral sclerosis patients. Front Neurosci. 2019;13:574.
- Paquette Y, Hanna Z, Savard P, Brousseau R, Robitaille Y, Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci USA. 1989;86:3896–900.
- Arru G, Mameli G, Deiana GA, Rassu AL, Piredda R, Sechi E, et al. Humoral immunity response to human endogenous retroviruses K/W differentiates between amyotrophic lateral sclerosis and other neurological diseases. Eur J Neurol. 2018;25:1076–e84.
- Jiang C, Hopfner F, Katsikoudi A, Hein R, Catli C, Evetts S, et al. Serum neuronal exosomes predict and differentiate Parkinson's disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry. 2020;91:720–9.
- Grad LI, Pokrishevsky E, Silverman JM, Cashman NR. Exosome-dependent and independent mechanisms are involved in prion-like transmission of propagated Cu/Zn superoxide dismutase misfolding. Prion. 2014;8:331–5.
- Iguchi Y, Eid L, Parent M, Soucy G, Bareil C, Riku Y, et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. 2016;139:3187–201.
- Tam OH, Rozhkov NV, Shaw R, Kim D, Hubbard I, Fennessey S, NYGC ALS Consortium, et al. Postmortem cortex samples identify distinct molecular subtypes of ALS: retrotransposon activation, oxidative stress, and activated glia. Cell Rep. 2019;29:1164–77.
- Manghera M, Ferguson-Parry J, Douville RN. TDP-43 regulates endogenous retrovirus-K viral protein accumulation. Neurobiol Dis. 2016;94:226–36.
- Curzio DD, Gurm M, Turnbull M, Nadeau MJ, Meek B, Rempel JD, et al. Pro-inflammatory signaling upregulates a neurotoxic conotoxin-like protein encrypted within human endogenous retrovirus-K. Cells 2020;9:1584.
- Bhat RK, Ellestad KK, Wheatley BM, Warren R, Holt RA, Power C. Age- and disease-dependent HERV-W envelope allelic variation in brain: association with neuroimmune gene expression. PLOS One. 2011;6:e19176.
- Mayer J, Harz C, Sanchez L, Pereira GC, Maldener E, Heras SR, et al. Transcriptional profiling of HERV-K(HML-2) in amyotrophic lateral sclerosis and potential implications for expression of HML-2 proteins. Mol Neurodegener. 2018;13:39.
