Abstract
Objective: Oculometric measures (OM) can be extracted from eye movements during presentation of visual stimuli. Studies have indicated the benefit of OM in assessment of neurological disorders, including Amyotrophic Lateral Sclerosis (ALS). We used a new software-based platform for the extraction of OM during patients’ assessment. Our objective was to examine the correlation between OM and clinical assessment as a part of a clinical drug trial. Methods: 32 ALS patients (mean age 60.75 ± 10.36 years, 13 females), were assessed using a validated score (ALSFRS-R), and a novel software-based oculometric platform (NeuraLight, Israel) as a part of a clinical drug trial. Correlations of ALSFRS-R with OM were calculated and compared with matched healthy subjects’ data (N = 129). Results: A moderate correlation was found between ALSFRS-R and corrective saccadic latency (R = 0.52, p = 0.002). Fixation time during smooth pursuit and peak velocity during pro-saccades were both worse in ALS patients versus healthy subjects (mean (SD)=0.34(0.06) vs. 0.3(0.07), p = 0.01, and 0.41(0.05) vs. 0.38(0.07), p = 0.04, respectively). Patients with bulbar symptoms (N = 14) had a decreased pro-saccade gain compared with patients without bulbar symptoms (mean (SD)=0.1 (0.04) vs. 0.93 (0.07), p = 0.01), and a larger error rate of anti-saccade movement (mean (SD)=0.42 (0.21) vs. 0.28 (0.16), p = 0.04). Conclusions: Oculometric measures correlated with the clinical assessment and were different from data of healthy subjects. Further studies are warranted to establish the role of oculometrics in the evaluation of patients with ALS and other neurodegenerative disorders, and its possible use in clinical trials.
Introduction
Detection of eye movement abnormalities is a significant part of a neurological examination to diagnose neurodegenerative disorders (Citation1). These abnormalities can be evaluated using oculometric measures (OM), i.e. measurements of different eye movements while performing a task in front of a visual stimulus (Citation2). The value of OM in evaluating the neurological status of patients was extensively studied during the last decades, indicating the potential benefit of using them in diagnosis and assessments of various neurological disorders, including amyotrophic lateral sclerosis (ALS) (Citation3–6). In previous studies, OM were found to be in correlation with ALS clinical symptoms (Citation7,Citation8). Furthermore, as the presence of abnormality of ocular movement is different between phenotypes of ALS, deficits in OM may shed light on different brain pathways which may be involved in different phenotypes of the disease (Citation9–11). For example, abnormal smooth pursuit, i.e. the slow eye movements designed to keep a moving stimulus on the fovea, are more prominent in ALS patients with bulbar involvement, which may be related to an impairment of the Cerebro-ponto-cerebellar pathways (Citation12). In addition, OM were suggested to be used to differentiate between ALS and healthy controls and may serve as potential biomarkers for objective evaluation (Citation12,Citation13). Finally, recent studies harnessed OM as an outcome measure for clinical drug trials, where they provided additional insights on participants’ condition (Citation14,Citation15). As a part of a phase IIb clinical trial evaluating a new drug for ALS (PrimeC, NeuroSense Therapeutics LTD., ClinicalTrials.gov Identifier: NCT05357950), we have implemented a novel software-based platform for extraction of OM, so that it can serve as an adjunct tool for clinical assessment of the participants of the study. The aim of this study was to examine the correlation between extracted OM and the assessment of ALS patients’ clinical status during a Phase IIb drug trial.
Materials and methods
A cohort of 32 ALS patients (mean age 60.75 ± 10.36 years, 13 females), all participants of a phase IIb randomized prospective double-blind placebo-controlled study, with an open label extension, conducted at Tel Aviv Sourasky Medical Center (TASMC), Israel, were enrolled. The study protocol was approved by the Tel-Aviv Sourasky Medical Center Helsinki Committee for Human Rights (trial number 0421-22) and was publicly registered with clinicaltrials.gov (Identifier NCT05407428). All participants provided written informed consent at screening. Inclusion and exclusion criteria were based on the protocol of the clinical drug trial. The main inclusion criteria were age ≤75 years, a disease duration of less than 30 months, ALSFRS-R score ≥25 and a slow vital capacity ≥60% of predicted. Further exclusion criteria for the OM study were markedly poor vision and advanced cognitive impairment, that would render the examination impossible. All patients underwent a prior neurological examination including basic eye movements testing, and an evaluation including a standard muscle testing and the Revised ALS Functional Rating Scale (ALSFRS-R) (Citation16).
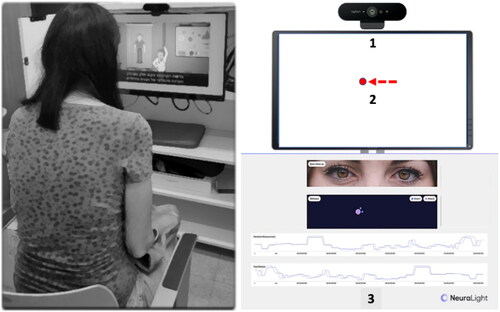
Then, all patients underwent an oculometric examination, using a novel software-based platform (NeuraLight, version 0.5, Israel) for the extraction of OM. This platform, installed on a PC and using a regular web camera, enables the capture of short videos of the patients’ eye movements, during performance of different tasks following the appearance of a visual stimulus (a red dot, ). In each task, the patients were instructed to follow the stimulus with their gaze in different ways, e.g. saccadic, anti-saccadic, or smooth pursuit movements. Data from videos were analyzed to yield various basic measurements of the physical movement of the eyes. Proprietary computer vision and deep learning algorithms were used to extract dozens of OM, e.g. latency of saccades and corrective saccades, i.e. the slow drifting eye movements performed at the end of saccadic eye movements, saccade peak velocity and saccade fixation, error rates and pro-saccade gain, i.e. the ratio of the actual saccade amplitude divided by the desired saccade amplitude. Patients were seated comfortably in an armchair or their own wheelchair and were instructed to use their far-vision glasses if required. Unlike previous studies measuring eye movements in ALS patients (Citation17,Citation18), no chin or forehead rests were needed. Following each task, patients were given a short break of 30 seconds, during which they were guided to rest and close their eyes as needed. The entire procedure lasted approximately 30 min. Oculometric data received from a larger cohort of age-matched healthy subjects (N = 129, matched age range of 39–72 years, similar means of 62.15 ± 9.32 vs. 60.75 ± 10.36 years), tested in the same conditions, were used for comparison. Trial setup is depicted in .
Figure 1 Trial setup. Patient (left image) sitting in front of a computer screen, with a standard webcam (right image, 1) placed on top. Different tasks are presented on-screen using a moving dot as a visual stimulus (2). During the tasks, oculometric measures extraction and eye movement analysis are presented in the operator’s screen (Citation3).
Statistical analysis
Since we had a small sample size, a Shapiro-Wilk test was performed and did not show evidence of a non-normal distribution (W = 0.96, p < 0.05). Correlations between captured OM and ALSFRS-R scores were calculated using Pearson correlation. Comparisons of OM between ALS patients and healthy subjects, as well as between patients with different ALS forms (with versus without bulbar symptoms) were performed using a t-test. Results were considered statistically significant if p < 0.05.
Results
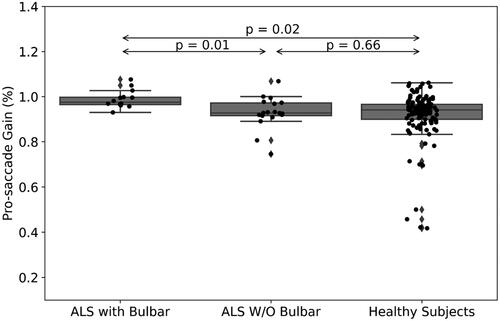
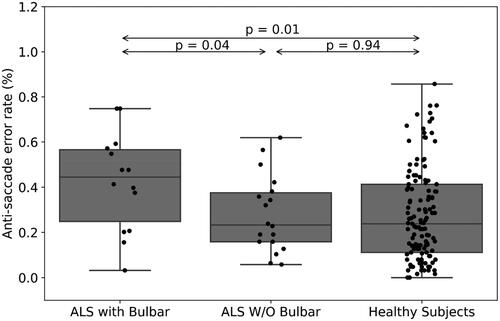
A statistically significant moderate correlation was found between ALSFRS-R scores and latency of corrective saccades, i.e. the slow drifting eye movements performed at the end of saccadic eye movements (R = 0.52, p = 0.002, ). Fixation time (ms) during smooth pursuit of ALS patients was increased compared with healthy subjects (mean (SD)=0.34 (0.06) vs. 0.3 (0.07), respectively, p = 0.01, ), however the mean peak velocity (degree/ms) in pro-saccade eye movement in ALS was increased among those patients (mean (SD)=0.4 (0.05) vs. 0.38 (0.07), respectively, p = 0.04, ). When comparing ALS patients’ subgroups (with (<12 points on the bulbar subscale of ALSFRS-R) versus without (=12 points) bulbar symptoms (N = 14, N = 18, respectively), pro-saccade gain, i.e. the ratio of the actual saccade amplitude divided by the desired saccade amplitude, was significantly different between subgroups (mean (SD)=0.99 (0.04) vs. 0.93 (0.07), respectively, p = 0.009, ). In addition, the anti-saccade error rate, i.e. the ratio of wrong movement responses to the wrong location divided by the total remaining number of trials, was also significantly different between these patients (mean (SD)=0.42 (0.21) vs. 0.28 (0.16), respectively, p = 0.04, ).
Figure 2 Correlation between ALSFRS-R scores and corrective saccadic latency (ms; R = 0.52, p = 0.002, y = 2.2x + 22.07). Each dot represents a single patient.
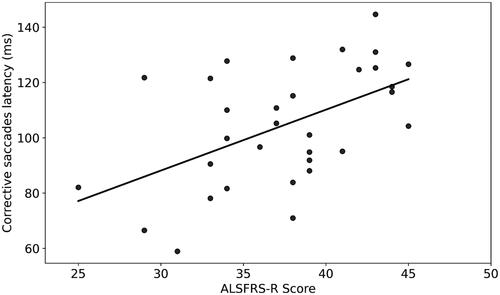
Figure 3 Fixation time (in ms) during smooth pursuit tasks of ALS patients (left) versus healthy subjects (right, p = 0.01).
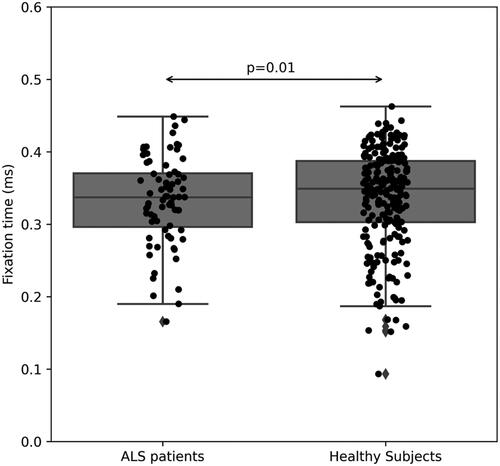
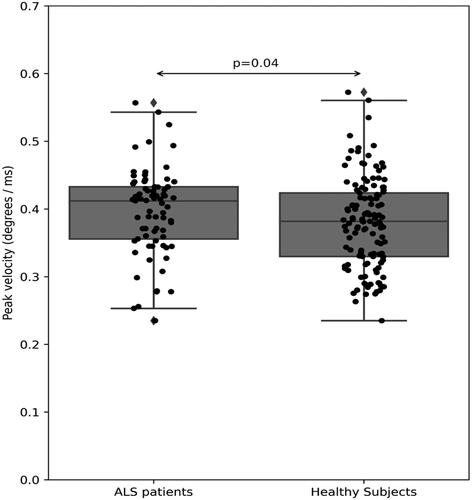
Figure 4 Peak velocity (degree/ms) in pro-saccade eye movement of ALS patients (left) versus healthy subjects (right, p = 0.04).
Discussion
As for today, the cause of ALS remains unclear, with an urgent need to find an efficient therapy (Citation19). As we move toward a better understanding of ALS underlying mechanisms, there is also an emerging need for a more personalized clinical approach, using different strategies which can enable a better diagnosis and treatment (Citation20). When aiming to assess the clinical status of ALS patients, there is still a need for objective markers of disease progression as an alternative to subjective assessment (Citation18,Citation21). In previous studies, correlations were found between oculomotor deficits and patients’ clinical presentation (Citation4,Citation17). Several OM, e.g. saccadic eye movements, corrective saccades and error rate were reported to be an objective measurement for ALS patients, as they reflect the patient’s clinical status assessed using the ALSFRS-R score (Citation18,Citation22).Our results support these previous results, as we have found a correlation between ALSFRS-R scores and several OM, e.g. corrective saccadic latency (R = 0.52, p = 0.002, ). Furthermore, OM showed a different pattern when compared with data from healthy subjects, as during a task of smooth pursuit, healthy subjects followed the presented stimulus in a smooth manner, whereas ALS patients had a significant longer fixation time, pointing out their difficulty to perform a smooth pursuit task (), as previously described (Citation3). In addition, velocity of pro-saccade eye movements of ALS patients was also slower compared with healthy subjects (0.406 (0.052) vs. 0.38 (0.066), p = 0.04, respectively, ). These results are also consistent with several previous studies, which pointed out the impairment of saccadic movement among ALS patients (Citation5,Citation6,Citation13).
With regards to bulbar symptoms, the ALSFRS-R includes a subscale which is designated to assess the bulbar function of ALS patients, e.g. impairment of speech, swallowing, and salivation. Using the data retrieved from this subscale of ALSFRS-R, we were able to divide our cohort into two subgroups: patients with (<12 points) and without (=12 points) bulbar symptoms (N = 14, N = 18, respectively). As we analyzed the oculometric data, we found out that pro-saccade gain, i.e. the ratio of the actual saccade amplitude divided by the desired saccade amplitude, was significantly higher in patients with no bulbar symptoms (mean (SD)=0.99 (0.04) vs. 0.93 (0.07), respectively, p = 0.009, ). Gorges and his colleagues compared the oculometric patterns of 68 ALS patients, 50 with spinal and 18 with bulbar onset, and found out that the only difference between the groups was the presence of higher saccade gain among the bulbar group (Citation17). In our study, we also measured an increase of error rate during anti-saccade eye movement in patients with bulbar symptoms compared with those without bulbar symptoms (0.42 (0.21) vs. 0.28 (0.16), respectively, p < 0.05, ). A similar result was found in a longitudinal study evaluating saccadic eye movements and cognitive tasks, where ALS patients with bulbar onset were found to perform more anti-saccade errors than those with limb onset (Citation18). Finally, in a recently published study, Zaino and her colleagues suggested that the difference in eye movement profiles of visually guided saccades between spinal (short saccades) and bulbar (slow saccades) ALS patients could result from a difference in involvement of cortical pathways, as the parieto-collicular-cerebellar network is more involved in spinal ALS and the fronto-brainstem circuit is more prominent in bulbar ALS (Citation23). Future studies are still needed to establish this connection; however, it holds an interesting promise with regards to ALS patients’ classification.
All ALS patients who were examined in our study participated in a clinical investigational drug trial. Recently, different types of biomarkers are included in clinical investigational drug trials for ALS, in which they can assist in better targeting of the drug, improved patient identification and as an adjunct tool to ensure drug responsiveness and safety, most of them wet biomarkers from blood or cerebrospinal fluid (Citation24,Citation25). Oculometric measures are also a part of this emerging effort, as saccadic eye movements were recorded in patients with Parkinson’s disease, as a part of a phase II clinical drug trial to assess the cognitive performance of the patients (Citation14). Since no difference was detected between the placebo arm versus the patients treated with the drug, it could be concluded that cognitive performance was not negatively affected, and the drug was safe. In another recent study, an eye tracking system was used to evaluate the efficacy of drug delivery for patients with advanced spinal muscular atrophy receiving a drug (Citation15). The results of our study can add to this trend, as they present an easy-to-use software-based platform for the extraction of OM, which was implemented as a part of the clinical assessment. Furthermore, since the oculometric data in our study were found to be in correlation with clinical assessment, as reflected by ALSFRS-R scores, future studies may involve this technology as a tool to evaluate patients’ disease progression over time. This should be done in longitudinal studies, with relevant statistical considerations to detect a change over time, as responsiveness and construct validity (Citation26). As more longitudinal data are gathered, the role of OM as a biomarker for ALS disease progression may be established.
There are several limitations to this study. First, since the patients in this study were a part of a Phase II clinical drug trial, it may be that there was a possible unknown influence of the medication given to them on their ocular performance. In addition, since they were enrolled according to the inclusion and exclusion criteria of this drug trial, therefore there may have been a selection bias, so that data are confined to a specific cohort with pre-defined terms. With regards to study design, this study provides a cross-sectional data, as patients were evaluated with a single session of an oculometric assessment. We also did not evaluate the relationship between OM and disease duration. Future longitudinal studies are needed to establish the possible use of OM as a tool for detecting change over time in the oculometric patterns of ALS patients, compared with their clinical status and duration of the disease.
Conclusions
In conclusion, oculometric measures were found to be correlated with clinical assessment scores, and different from captured data of healthy subjects. This may indicate their potential benefit as an adjunct assessment tool for ALS patients. Further studies are needed to establish the role of oculometric examination in ALS and other neurodegenerative disorders and its possible use in clinical drug trials.
Acknowledgments
The authors would like to thank Ms. Adi Asher-Megidish and the devoted team of operators for their work with the patients, and to Ms. Shani Levy for her support with data analysis and figure preparation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. Eitan Raveh, Assaf Ben-Shimon, Vova Anisimov, Rivka Kreitman and Edmund Ben-Ami are employees of NeuraLight LTD, which provided the software-based platform used for this study.
Additional information
Funding
References
- Anderson TJ, MacAskill MR. Eye movements in patients with neurodegenerative disorders. Nat Rev Neurol. 2013;9:74–85.
- Mahanama B, Jayawardana Y, Rengarajan S, Jayawardena G, Chukoskie L, Snider J, et al. Eye movement and pupil measures: a review. Front Comput Sci. 2022;3:733531.
- Proudfoot M, Menke RA, Sharma R, Berna CM, Hicks SL, Kennard C, et al. Eye-tracking in amyotrophic lateral sclerosis: a longitudinal study of saccadic and cognitive tasks. Amyotroph Lateral Scler Frontotemporal Degener. 2015;17:101–11.
- Beaufils E, Corcia P, de Toffol B, Praline J. Occurrence of eye movement disorders in motor neuron disease. Amyotroph Lateral Scler. 2012;13:84–6.
- Donaghy C, Thurtell MJ, Pioro EP, Gibson JM, Leigh RJ. Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. J Neurol Neurosurg Psychiatry. 2011;82:110–6.
- Sharma R, Hicks S, Berna CM, Kennard C, Talbot K, Turner MR. Oculomotor dysfunction in amyotrophic lateral sclerosis: a comprehensive review. Arch Neurol. 2011;68:857–61.
- Donaghy C, Pinnock R, Abrahams S, Cardwell C, Hardiman O, Patterson V, et al. Slow saccades in bulbar-onset motor neurone disease. J Neurol. 2010;257:1134–40.
- Rekik A, Mrabet S, Kacem I, Nasri A, Ben Djebara M, Gargouri A, et al. Eye movement abnormalities in amyotrophic lateral sclerosis in a Tunisian cohort. Neuroophthalmology. 2022;46:227–35.
- Takeda T, Uchihara T, Mochizuki Y, Ishihara A, Nakamura A, Sasaki S, et al. Supranuclear ophthalmoparesis and vacuolar degeneration of the cerebral white matter in amyotrophic lateral sclerosis: a clinicopathological study. Amyotroph Lateral Scler. 2012;13:74–83.
- Takeda T, Kitagawa K, Arai K. Phenotypic variability and its pathological basis in amyotrophic lateral sclerosis. Neuropathology. 2020;40:40–56.
- Rojas P, Ramírez AI, Fernández-Albarral JA, López-Cuenca I, Salobrar-García E, Cadena M, et al. Amyotrophic lateral sclerosis: a neurodegenerative motor neuron disease with ocular involvement. Front Neurosci. 2020;14:566858.
- Guo X, Liu X, Ye S, Liu X, Yang X, Fan D. Eye movement abnormalities in amyotrophic lateral sclerosis. Brain Sci. 2022;12:489.
- Coe BC, Munoz DP. Mechanisms of saccade suppression revealed in the anti-saccade task. Philos Trans Royal Soc B Biol Sci. 2017;372:20160192.
- Ellmerer P, Peball M, Carbone F, Ritter M, Heim B, Marini K, et al. Eye tracking in patients with Parkinson’s disease treated with nabilone–results of a phase II, placebo-controlled, double-blind, parallel-group pilot study. Brain Sci. 2022;12:661.
- Yae Y, Yuge K, Maeda T, Ichinose F, Matsuo M, Kobayashi O, et al. Exploratory evaluation of an eye-tracking system in patients with advanced spinal muscular atrophy type I receiving nusinersen. Front Neurol. 2022;13. DOI: 10.3389/fneur.2022.918255
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. Bdnf Als Study Group, 1A complete listing of the BDNF Study Group. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21.
- Gorges M, Müller HP, Lulé D, Del Tredici K, Brettschneider J, Keller J, et al. Eye movement deficits are consistent with a staging model of pTDP-43 pathology in amyotrophic lateral sclerosis. PLOS One. 2015;10:e0142546.
- Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:414–25.
- Barp A, Gerardi F, Lizio A, Sansone VA, Lunetta C. Emerging drugs for the treatment of amyotrophic lateral sclerosis: a focus on recent phase 2 trials. Expert Opin Emerg Drugs. 2020;25:145–64.
- Nefussy B, Drory VE. Moving toward a predictive and personalized clinical approach in amyotrophic lateral sclerosis: novel developments and future directions in diagnosis, genetics, pathogenesis and therapies. EPMA J. 2010;1:329–41.
- Gordon PH, Miller RG, Moore DH. ALSFRS ‐R Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5:90–3.
- Kang BH, Kim JI, Lim YM, Kim KK. Abnormal oculomotor functions in amyotrophic lateral sclerosis. J Clin Neurol. 2018;14:464–71.
- Zaino D, Serchi V, Giannini F, Pucci B, Veneri G, Pretegiani E, et al. Different saccadic profile in bulbar versus spinal-onset amyotrophic lateral sclerosis. Brain. 2023;146:266–77.
- Shefner JM, Bedlack R, Andrews JA, Berry JD, Bowser R, Brown R, et al. Amyotrophic lateral sclerosis clinical trials and interpretation of functional end points and fluid biomarkers: a review. JAMA Neurol. 2022;79:1312–8.
- Staats KA, Borchelt DR, Tansey MG, Wymer J. Blood-based biomarkers of inflammation in amyotrophic lateral sclerosis. Mol Neurodegener. 2022;17:11.
- Guyatt GH, Kirshner B, Jaeschke R. Measuring health status: what are the necessary measurement properties? J Clin Epidemiol. 1992;45:1341–5.