Abstract
Objective: This study assessed a national healthcare intervention launched in Sweden in 2015 to reduce the time between macroscopic haematuria, diagnosis and treatment of urinary tract cancer.
Methods: The outcome of the first 11 months was evaluated in 1697 individuals referred to a standardized care pathway for urinary tract cancer compared with 174 patients with conventionally diagnosed urothelial carcinoma.
Results: Among the referred individuals, 317 (19%) were diagnosed with cancer, 1034 (61%) had a benign diagnosis and 345 (20%) had a negative evaluation. Bladder cancer was the most common malignant diagnosis [262/317 (83%)]. Cancers were diagnosed in 23% of males and 13% of females, and showed a strong correlation with age: cancer diagnosis in 2% aged <50 years and in 44% aged ≥90 years. Results were affected by bacteriuria but not by anticoagulant medication, with 12%/22% and 19%/19% cancer detection, respectively. The standardized care pathway shortened the diagnostic delay to a median of 25 days compared to 35 days for regular referral (p = .01). However, median time to treatment was unchanged: 39 days from referral to transurethral resection, 42 days from primary resection to re-resection for stage TaG3/T1 disease and 100 days from referral to curative treatment for muscle-invasive disease.
Conclusions: Macroscopic haematuria had a cancer capture rate of 19%, with higher predictive values in men and at older age, whereas anticoagulant therapy did not influence the diagnostic yield. The demonstrated lack of effect on time to treatment underscores the need to consider the entire patient process when initiating healthcare reforms to improve outcome.
Introduction
Macroscopic haematuria is the isolated alarm symptom with the highest positive predictive value for cancer, with a malignancy detection rate of 5–34% depending on the composition of the population investigated [Citation1–4]. Sixty four per cent of patients aged ≥40 years diagnosed with bladder cancer present with macroscopic haematuria [Citation4]. Despite a well-defined alarm symptom, diagnostic delays are longer for bladder cancer than for other tumour types [Citation5]. There is ample evidence that diagnostic delays in bladder cancer are associated with decreased disease-specific and overall survival [Citation6,Citation7]. Delays reflect limited hospital resources and suboptimal efficiency, but are not always given adequate consideration by healthcare providers [Citation8,Citation9]. Furthermore, gender differences with more advanced tumours and higher cancer-specific mortality in women may be linked to referral delays and failure to adhere to diagnostic guidelines [Citation10].
In Swedish healthcare, long waiting times and failure to provide timely treatment for cancer prompted implementation of a national healthcare initiative in 2015 aimed at reducing lead times, ensuring timely treatment and increasing patient satisfaction. This initiative uses the cancer care pathway model applied in Denmark and Norway, which means that patients with symptoms and/or signs potentially signifying cancer are offered efficient diagnostic evaluation and expeditious treatment through uniform care pathways that define standard procedures and maximum lead times for uncomplicated patients. The aim here was to evaluate the impact of the Swedish initiative on the outcome in patients with symptoms of urothelial cancer. In short, a case–control study of a population of 1.3 million inhabitants was conducted that included determination of clinical findings, the positive predictive value of macroscopic haematuria in relation to confounding factors, and the impact on diagnostic lead times and time to treatment.
Materials and methods
The standardized diagnostic pathway for suspected urothelial cancer can be initiated by primary healthcare providers and by specialists. The diagnostic criteria encompass macroscopic haematuria (in patients aged ≥40 years irrespective of clinical history and in patients aged <40 if risk factors such as >20 years of smoking are present) and suspected urothelial cancer based on radiology or cystoscopy. The reason for excluding patients aged <40 years from standardized diagnostic pathway examinations was the low number of primary bladder cancers below this age in the Swedish National Bladder Cancer Registry;, however, conventional referral for a urological consultation is still advocated for these patients. The standardized diagnostics comprise computed tomography (CT) urography, urinary cytology and cystoscopy. The standardized care pathway for urinary tract cancer was accomplished by defining lead times (as described below) and by improved administration by a coordinator of all necessary measures and logistics, including prioritized or prebooked appointments for cystoscopy at the departments of urology and prebooked CT–urography examinations. Furthermore, the coordinator had access to operating capacity assigned to patients diagnosed within the standardized care pathways. The lead times are defined as follows: from suspicion to transurethral resection 9 days (12 days for patients on anticoagulant therapy) and from suspicion to radical cystectomy 32 days (35 days for patients on anticoagulant therapy). For patients not fulfilling the criteria for standardized care pathways, an ordinary urology referral, thus with lower priority, was instituted.
Study population
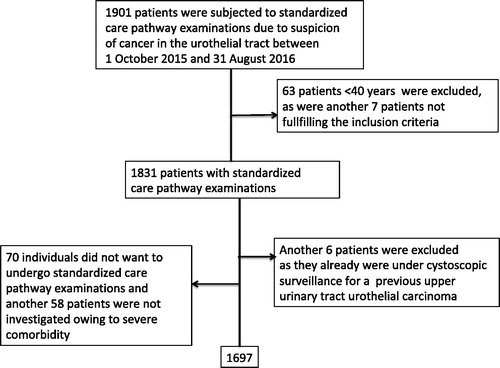
The clinical setting was population based, i.e. the Skåne County Council, which has a population of 1.3 million and six urology units in public hospitals that are responsible for the clinical evaluations. Patients eligible for the investigation were to have been included in the standardized care pathway for urothelial cancer between October 2015 and August 2016 and aged 40 years or above. In total, 1901 individuals were identified in the standardized care register compiled by Region Skåne, which is the basis for the national data collection carried out by the Swedish Association of Local Authorities and Regions. Two of the authors (JC and FL) reviewed the patient charts for all 1901 cases identified, which led to the exclusion of 63 patients aged <40 years (two of whom were diagnosed with cancer) and seven patients who did not fulfil the inclusion criteria for other reasons. Furthermore, 70 patients refused evaluation according to the standardized pathway. Also, by physicians’ choice, 58 patients were excluded from standardized pathway diagnostics owing to severe comorbidities, and six patients who were already undergoing cystoscopic surveillance for upper urinary tract urothelial carcinoma were excluded as well. Thereafter, 1697 patients remained in the study (). All patient charts were also reviewed to validate information on diagnoses during the observation period, date of haematuria, outcome of urine tests and cultures, use of anticoagulants, previous treatment with pelvic irradiation and use of bladder catheters.
Control populations
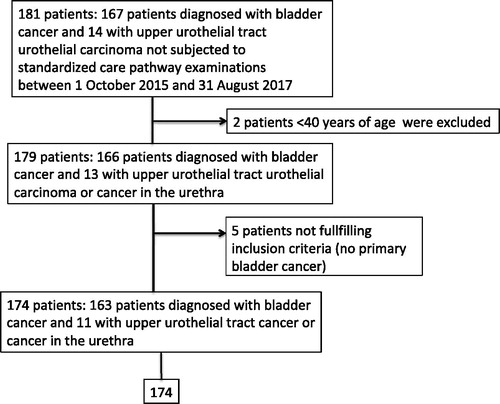
The control group was defined as all patients residing in the same geographical region (Region Skåne) who were diagnosed with urothelial cancer (cancer of the upper urinary tract, urethra or urinary bladder) during the same period. These patients were identified in the Swedish National Bladder Cancer Registry (). The same exclusion criteria were applied, which led to the exclusion of two patients <40 years of age and five without primary bladder cancer, which left 163 patients with primary bladder cancer and 11 patients with upper urinary tract urothelial carcinoma in the control group. A second control population regarding lead times from referral to transurethral resection of a bladder cancer (n = 767) and from referral to start of curative treatment for muscle-invasive disease (neoadjuvant chemotherapy or radical cystectomy; n = 65) was extracted from the Swedish National Bladder Cancer Registry for the 2 years before standardized care pathway examinations were introduced (i.e. 2013–2014) in the same geographical region (Region Skåne). For 17 patients, there were discrepancies between dates in the Swedish National Bladder Cancer Registry and Region Skåne’s standardized care registration and patient chart review, resulting in negative lead times; the lead times for 11 of those patients were adjusted according to a second review of patient charts.
Figure 2. Description of the control group, defined as all patients residing in the same geographical area (Region Skåne) who were diagnosed with urothelial cancer (in the upper urinary tract, the urethra or the urinary bladder) without being included in the standardized care pathway for urothelial cancer during the same period.
The Regional Ethical Review Board in Lund approved the study (Dnr 2016/524).
Statistics
No formal power calculation was performed before planning the analyses. Data were summarized by descriptive statistics. The Mann–Whitney test was used for comparison of continuous variables. Categorical data were assessed by the chi-squared test and logistic regression. Data were analysed using R program software (http://www.rproject.org).
Results
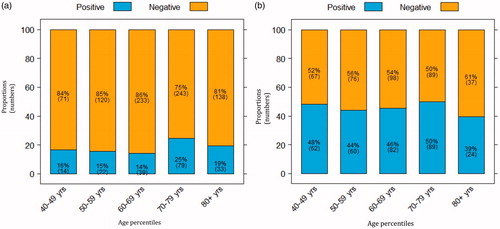
During the study period, 1697 patients with suspected urothelial cancer were referred for standardized care pathway diagnostic work-up. The median age was 69 [interquartile range (IQR) 60–77] years for the 1001 males and 63 (IQR 52–73) years for the 696 females. Considering the total cohort, 19% of the patients (n = 317) were diagnosed with cancer (), 61% (n = 1034) had a benign diagnosis () and 20% (n = 345) had a negative diagnostic examination. The median age was 72 (IQR 64–78) years in patients diagnosed with cancer and 66 (IQR 55–74) years in those with negative or benign outcomes. Among malignant diagnoses, bladder cancer was found most frequently (83%), followed by renal cell carcinoma (6%), prostate cancer (5%), upper urinary tract urothelial cancer (4%) and other cancers including gynaecological cancer (3%) (). The cancer detection for age percentiles increased from 2% at age 40–49 years to 44% at >90 years (). In relation to gender, cancer was diagnosed in 23% of males (227/1001) and 13% of females (90/696) ).
Figure 3. Proportions (numbers) of patients with cancer diagnosed after standardized care pathway examinations, by age percentiles.
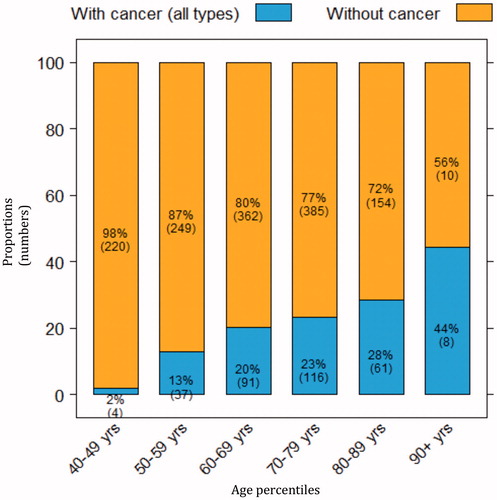
Figure 4. Proportions of (a) referred males and (b) referred females with or without cancer, stratified by age.
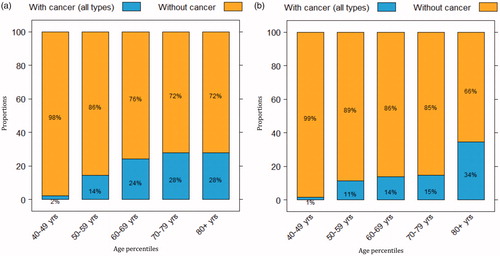
Table 1. Cancer causes underlying referral to the standardized care pathway.
Table 2. Benign causes underlying referral to the standardized care pathway.
In 504 patients, macroscopic haematuria was linked to urinary tract infection with a positive dipstick test (leucocyturia and/or nitrite test) and/or a positive urinary culture. Despite these findings, cancer was diagnosed in 61 (12%) of the 504 patients, with at least one of these tests being positive, compared to 255 (22%) of the 1172 patients with negative dipstick and urinary culture results (p < .001). Among males, the proportions diagnosed with cancer were similar for those with and those without urinary tract infection: 41/187 (22%) and 186/805 (23%), respectively. In females, cancer detection rates were lower for those with a positive dipstick test and/or urinary culture compared to those without signs of bacteriuria [20/317 (6%) vs 69/367 (19%)]. On the other hand, the age-dependent proportion of urinary tract infections was two to three times higher in females than in males ). Oral anticoagulant medication (warfarin or a new oral anticoagulant) was used by 221 patients, 42 (19%) of whom were diagnosed with cancer, and the cancer detection rate was similar in patients who did not receive these drugs [273/1471 (19%)]. Cancer detection rates were not increased among patients who had undergone previous pelvic irradiation, with cancer detection rates of 6/50 (12%) and 311/1647 (19%), respectively. Twenty-two patients with a urinary catheter or using clean intermittent catheterization were referred to the standardized care pathway, and cancer was detected in four (18%) of those individuals; by comparison, cancer was detected in 313 (19%) of the 1675 patients without a catheter.
Figure 5. Age-stratified proportions (numbers) of (a) males and (b) females with positive dipstick test and/or urinary culture who underwent standardized care pathway examinations. (No data available for nine male and 12 female patients.)
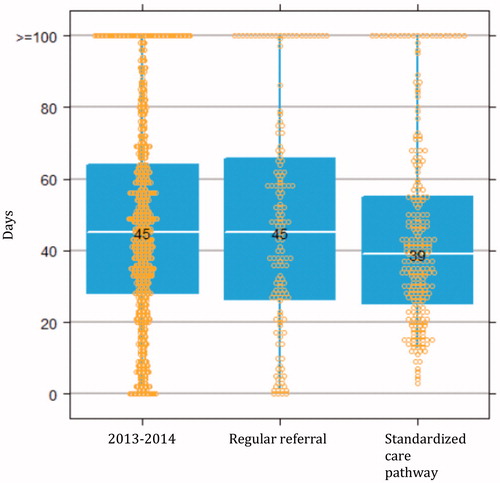
Lead-time analysis was performed on the 425 patients diagnosed with bladder cancer during the study period; 262 (62%) of these subjects were diagnosed through the standardized care pathway and 163 were evaluated through regular pathways. Tumour characteristics (grade 1, 2 or 3; tumour size 0–10 mm, 11–30 mm or >30 mm; number of tumours 1, 2, 3, 4–5 or ≥6) did not differ between the two groups (data not shown). All standardized care pathway patients who were diagnosed with bladder cancer had macroscopic haematuria, whereas four bladder cancer patients evaluated through regular pathways did not have that symptom. For patients presenting with macroscopic haematuria who were diagnosed with bladder cancer, those evaluated through the standardized pathway (n = 250) had a median of 0 (IQR 0–3) days from macroscopic haematuria to referral compared to 7 (IQR 1–55) days for those diagnosed through the regular route (n = 141) (date of referral or date of haematuria missing in 30 individuals). The corresponding lead times from haematuria to bladder cancer diagnosis were 25 (10–47) days (n = 252) and 35 (11–99) days (n = 158), respectively (p = .01). These lead times were similar irrespective of oral anticoagulation or signs of urinary tract infection (data not shown). The median lead time from diagnostic referral to transurethral resection of the bladder cancer was 39 (IQR 25–55) days for patients diagnosed within the standardized care pathway (n = 242) compared to 45 (IQR 26–66) days for those diagnosed by the regular route (n = 150) (p = .3). The lead time from primary resection to re-resection was 42 (IQR 33–60) days for the patients diagnosed with TaG3 or T1 tumours through the standardized care pathway (n = 60) versus 48 (IQR 41–55) days for those evaluated in the routine manner (n = 26) (p = .5). For patients with muscle-invasive bladder cancer who were treated with radical cystectomy or external beam radiation with curative intent, the delay from diagnostic referral to start of treatment (including start of neoadjuvant chemotherapy before radical cystectomy) was 100 (IQR 72–138) days for patients evaluated in the standardized care pathway (n = 28) and 92 (IQR 68–105) days in the other group (n = 18) (p = .5). The corresponding median lead times during 2013–2014 (i.e. before the introduction of the standardized care pathway) were 45 (IQR 28–64) days from referral to transurethral resection (n = 767) () and 103 (IQR 84–125) days from referral to initiation of curative treatment for muscle-invasive disease (n = 65).
Figure 6. Median days from referral to transurethral resection for all patients diagnosed with bladder cancer during the period 2013–2014 in the Southern healthcare region (n = 767), and for patients diagnosed with bladder cancer through regular referral (n = 150) and standardized care pathway examinations (n = 242) during the study period (October 2015 to August 2016).
Discussion
During the period 2015–2018, the standardized cancer pathway system has been introduced in Swedish healthcare to reduce diagnostic lead times, provide timely treatment and increase patient satisfaction. This report describes the first evaluation of the standardized care pathway for urothelial cancer, which reflects the first 11 months after initiation of the system. The inclusion criteria resulted in a cancer capture rate of 19%, which is comparable to a rate of 23% achieved in a recent study conducted in Denmark for patients with macroscopic haematuria [Citation11]. Of the malignancies, 83% were urothelial cancers, but several other urological and gynaecological cancers were also diagnosed through this route (). Age was an important predictor of cancer detection in the present investigation (), which agrees with data from haematuria evaluations performed in the primary care setting [Citation12]. Cancer was diagnosed in 2% of the patients aged 40–49 years, and this group constituted 13% of the total cohort. The results indicate that it may be relevant to raise the age threshold to 50 years, although such a modification should take into consideration that 1.6% of patients with urinary bladder cancer are diagnosed before the age of 40 [Citation13]. Gender also influenced capture rates, with significantly higher values for men (23%) than for women (13%). The risk of cancer in patients with macroscopic haematuria was decreased in those who had concurrent signs of bacteriuria compared to those without bacteriuria (12% vs 22%), whereas patients with and those without treatment with oral anticoagulants showed similar capture rates (19%). Thus, these factors have only a limited impact on the cancer detection rate, if used as motivation for not further evaluating the patient, and may lead to suboptimal evaluations and delayed diagnostics of an underlying urinary tract cancer [Citation14,Citation15]. Furthermore, the presence of asymptomatic bacteriuria and the risk of urothelial cancer both increase with age. In women, asymptomatic bacteriuria is found in 20–25% above the age of 65 and >50% above the age of 80 years, and in men these frequencies are 10% and 35%, respectively, which adds difficulties when estimating cancer risk in a patient with macroscopic haematuria and concomitant bacteriuria [Citation16].
International guidelines for the evaluation of patients with suspected urinary tract cancer are based on microscopic haematuria, which differs from the Swedish guidelines that require macroscopic haematuria for the initiation of cancer care pathways [Citation17,Citation18]. A study in Denmark found that cancer was diagnosed in only in 3% of 564 patients with microscopic haematuria and in 1.5% of patients without lower urinary tract symptoms or loin pain, and all diagnoses were made in patients aged >60 years [Citation11]. Hence, selective referral is recommended in the UK and Denmark for patients aged >60 years with microscopic haematuria [Citation19,Citation20]. The frequent findings of benign urological conditions (as exemplified by the data in ), many of which require active treatment, is thought provoking. For example, active treatment with a 5α-reductase inhibitor in men diagnosed with prostatic enlargement can be considered, as such medication has been shown to decrease the risk of recurring haematuria from 63% to 14%, apart from reducing the risks of acute urinary retention and prostate surgery [Citation21].
In the current investigation, the standardized care pathway shortened the median time from haematuria to bladder cancer diagnosis by 10 days (i.e. from 35 to 25 days) but did not decrease the subsequent lead times in the cohort. These results highlight the need to consider the entire patient treatment trajectory to accommodate timely procedures following initial diagnostics.
Patients with stage TaG3 and T1 tumours should be subjected to a re-resection of the previous tumour site in the bladder within 14–42 days, according to international guidelines [Citation22]. Re-resection beyond 42 days has been associated with worse recurrence-free and progression-free survival after adjusting for known confounders [Citation23]. In the present study, this ideal time interval was met for only one of every two patients subjected to healthcare pathway examinations for urothelial cancer, which implies that healthcare factors, i.e. either actual lack of capacity or organizational insufficiency, may have a negative impact on bladder cancer outcomes in the Swedish system. The median time interval between referral and curative treatment initiation was 100 days for patients with muscle-invasive bladder cancer, which exceeds stipulations in evidence-based guidelines and involves the risk of reducing cancer-specific survival [Citation7]. In muscle-invasive bladder cancer, treatment delays exceeding 84 days to radical cystectomy have been linked to an adverse outcome [Citation24]. The present evaluation demonstrates that within the first year of the introduction of standardized care pathways for urothelial cancer in Region Skåne, the treatment lead times remain unchanged for the whole bladder cancer population compared to the years before the reform, i.e. 2013–2014 (http://www.ocsyd.se/kvalitetsdata/webrapport/). Such population-based registry-derived lead times should diminish in order to rule out the possibility that resources for standardized care pathways are taken from non-fast-track individuals who consequently have even longer lead times.
It has been estimated that delays occur in two-thirds of patients with painless gross haematuria, with causes equally divided among primary care referrals, system-related factors and patient-related factors [Citation25]. Such delay occurs despite gross haematuria being a well-defined alarm symptom, a clearly defined diagnostic trajectory, and the presence of evidence that delayed treatment has a negative effect on outcome. Median lead time from bleeding to surgery was 70 days in a recent report from a one-stop haematuria clinic [Citation26], which claimed that administrative delays and resource limitations contributed to prereferral delays. In another study of patients diagnosed with muscle-invasive disease necessitating radical cystectomy at a second unit, such hospital transition was noted to contribute to further treatment delay [Citation27]. Furthermore, benign diagnoses during haematuria evaluation have been associated with delayed diagnosis of upper urinary tract urothelial carcinoma [Citation28]. From a healthcare perspective, streamlined management of patients with macroscopic haematuria has been demonstrated to be associated with reduced costs [Citation29] and fewer patient visits, and has led to the development of ‘one-stop’ haematuria clinics. From the patient’s perspective, the incentives are related to an improved likelihood of cure and reduced psychological impact from diagnostic delays.
Limitations of this investigation include potential selection bias between patients subjected to standardized care pathway examinations and those managed through regular diagnostics, although the two groups did not differ with regard to gender, age and tumour status. Thus, it is likely that the decreased delay from haematuria to diagnosis represents a true improvement for those patients diagnosed with bladder cancer through standardized care pathway examinations. The study was limited to the first 11 months of the standardized healthcare intervention in 2015–2016, which represents the initial implementation period. Nonetheless, data from the Swedish National Bladder Cancer Register confirm that the time to first treatment remained the same in 2017 (http://www.ocsyd.se/kvalitetsdata/webrapport/). Strengths of the investigation include a population-based approach comprising all patients diagnosed during the study period, and careful review of all clinical charts to verify symptoms and validate dates.
In conclusion, this report presents the outcome of the introduction of a standardized care pathway for patients with suspected urothelial cancer. Cancer capture rates were favourable at 19% and were not markedly influenced to a large extent by signs of bacteriuria or oral anticoagulation. Notably, gender and age strongly predicted the risk of an underlying urinary tract cancer. The introduction of standardized care pathways had an effect on the lead time from haematuria to bladder cancer diagnosis, which was shortened by 10 days, but has so far not decreased times to treatment, which are considerably longer than recommended in evidence-based guidelines. This observation underscores the need for full process implementation with recourse optimization throughout when new diagnostic principles are applied; otherwise, the allocation of new resources might not be meaningful.
Disclosure statement
No potential conflict of interest was reported by any of the authors.
Additional information
Funding
References
- Shapley M, Mansell G, Jordan JL, et al. Positive predictive values of ≥5% in primary care for cancer: systematic review. Br J Gen Pract. 2010;60:e366–e377.
- Sultana SR, Goodman CM, Byrne DJ, et al. Microscopic haematuria: urological investigation using a standard protocol. Br J Urol. 1996;78:691–696.
- Boman H, Hedelin H, Holmäng S. The results of routine evaluation of adult patients with haematuria analysed according to referral form information with 2-year follow-up. Scand J Urol Nephrol.2001;35:497–501.
- Schmidt-Hansen M, Berendse S, Hamilton W. The association between symptoms and bladder or renal tract cancer in primary care: a systematic review. Br J Gen Pract. 2015;65:e769–e775.
- Hansen RP, Vedsted P, Sokolowski I, et al. Time intervals from first symptom to treatment of cancer: a cohort study of 2,212 newly diagnosed cancer patients. BMC Health Serv Res. 2011;11:284–295.
- Hollenbeck BK, Dunn RL, Ye Z, et al. Delays in diagnosis and bladder cancer mortality. Cancer 2010;116:5235–5242.
- Bourgade V, Drouin SJ, Yates DR, et al. Impact of the length of time between diagnosis and surgical removal of urologic neoplasms on survival. World J Urol. 2014;32:475–479.
- Hamilton W, Stapley S, Campbell C, et al. For which cancers might patients benefit most from expedited symptomatic diagnosis? Construction of a ranking order by a modified Delphi technique. BMC Cancer. 2015;15:820–828.
- Bilimoria KY, Ko CY, Tomlinson JS, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011;253:779–785.
- Dobruch J, Daneshmand S, Fisch M, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol. 2016;69:300–310.
- Elmussareh M, Young M, Ordell Sundelin M, et al. Outcomes of haematuria referrals: two-year data from a single large university hospital in Denmark. Scand J Urol. 2017;51:282–289.
- Bruyninckx R, Buntinx F, Aertgeerts B, et al. The diagnostic value of macroscopic haematuria for the diagnosis of urological cancer in general practice. Br J Gen Pract. 2003;53:31–35.
- Lara J, Brunson A, Keegan TH, et al. Determinants of Survival for Adolescents and Young Adults with Urothelial Bladder Cancer: Results from the California Cancer Registry. J Urol. 2016;196:1378–1382.
- Vasdev N, Thorpe AC. Should the presence of a culture positive urinary tract infection exclude patients from rapid evaluation hematuria protocols? Urol Oncol. 2013;31:909–913.
- Avidor Y, Nadu A, Matzkin H. Clinical significance of gross hematuria and its evaluation in patients receiving anticoagulant and aspirin treatment. Urology 2000;55:22–24.
- Wagenlehner FM, Naber KG, Weidner W. Asymptomatic bacteriuria in elderly patients: significance and implications for treatment. Drugs Aging. 2005;22:801–807.
- Holmäng S. Mikroskopisk hematurin ingen varningsklocka för cancer. Läkartidningen 2016;113:1–3.
- Malmstrom PU. Time to abandon testing for microscopic haematuria in adults? BMJ. 2003;326:813–815.
- Danish Urological Cancer Group. Danish haematuria guideline. 2015. Available from: http://ducg.dk/dablaca-blaerecancer/kliniske-retningslinjer/
- National Institute for Health and Care Excellence. NICE guidelines NG12. Suspected cancer: recognition and referral. 2015 [cited 2018 Apr 26]. Available from: www.nice.org.uk/guidance/ng12/evidence/full-guidance-74333341
- Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag. 2009;5:535–545.
- European Association of Urology. Guidelines. Non-muscle-invasive bladder cancer. 2017. [cited 2018 Apr 26]. Available from: http://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/2017
- Baltacı S, Bozlu M, Yıldırım A, et al. Significance of the interval between first and second transurethral resection on recurrence and progression rates in patients with high-risk non-muscle-invasive bladder cancer treated with maintenance intravesical Bacillus Calmette-Guérin. BJU Int. 2015;116:721–726.
- Gore JL, Lai J, Setodji CM, et al. Urologic Diseases in America Project. Mortality increases when radical cystectomy is delayed more than 12 weeks: results from a Surveillance, Epidemiology, and End Results-Medicare analysis. Cancer 2009;115:988–996.
- Richards KA, Ruiz VL, Murphy DR, et al. Diagnostic evaluation of patients presenting with hematuria: an electronic health record-based study. Urol Oncol 2018;36:e19–88.
- McCombie SP, Bangash HK, Kuan M, et al. Delays in the diagnosis and initial treatment of bladder cancer in Western Australia. BJU Int. 2017;120(Suppl 3):28–34.
- Tomaszewski JJ, Handorf E, Corcoran AT, et al. Care transitions between hospitals are associated with treatment delay for patients with muscle invasive bladder cancer. J Urol. 2014;192:1349–1354.
- Chappidi MR, Kates M, Tosoian JJ, et al. Evaluation of gender-based disparities in time from initial haematuria presentation to upper tract urothelial carcinoma diagnosis: analysis of a nationwide insurance claims database. BJU Int. 2017;120:377–386.
- Liedberg F, Gerdtham U, Gralén K, et al. Fast-track access to urologic care for patients with macroscopic haematuria is efficient and cost-effective: results from a prospective intervention study. Br J Cancer. 2016;115:770–775.