Introduction
Acute intermittent porphyria (A.I.P.) is an autosomal dominant inherited metabolic disorder that is due to heterozygous mutations in the hydroxymethylbilane synthase enzyme of the haem biosynthesis [Citation1]. Deficiency of the enzyme alone is not sufficient to cause attacks, since most patients with such mutations are asymptomatic [Citation2]. Symptoms occur when exacerbating factors induce haem synthesis by increasing the activity of the rate limiting ALAS-1 enzyme, leading to accumulation of the porphyrin precursors porphobilinogen (P.B.G.) and aminolevulinic acid (A.L.A.) [Citation1,Citation2]. Medications are among the most important exacerbating factors and safe usage of drugs in A.I.P. patients may be a challenge [Citation3]. The most common clinical presentation of A.I.P. is acute attacks with severe abdominal pain that may be accompanied by vomiting, fatigue, dark red urine, electrolyte disturbances and/or neuropsychiatric symptoms. Attacks are highly variable, both in severity and frequency. The secretion of A.L.A. and P.B.G. in urine is increased during attacks [Citation4]. The northern parts of Sweden and Norway have the highest prevalence of A.I.P. in the world [Citation2,Citation5].
We monitored a patient with symptomatic A.I.P. who needed treatment with bicalutamide and goserelin due to metastatic adenocarcinoma of the prostate. The main concern was whether this medication could provoke attacks of porphyria due to enhanced hepatic haem synthesis. The patient was, therefore, monitored with regular urine samples to assess the biochemical disease activity [Citation4].
Case report
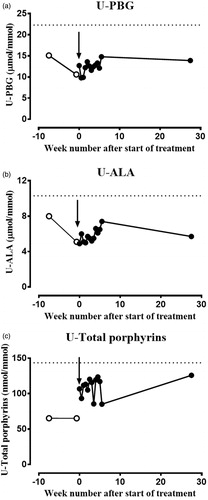
A 70-year-old man with manifest A.I.P. disease was diagnosed with metastatic highly differentiated adenocarcinoma of the prostate. The treatment plan included androgen deprivation therapy with the antiandrogen bicalutamide and the Gn.R.H. agonist goserelin (Zoladex). Documentation about the safe usage of these drugs in A.I.P. was non-equivalent in different porphyria databases. The drug database for acute porphyria at The Norwegian Porphyria Centre (www.napos.no) characterized bicalutamide and goserelin as ‘possibly porphyrinogenic’ and ‘porphyrinogenic’, respectively. The drug safety database at the American Porphyria Foundation (porphyriafoundation.com) characterized goserelin as very likely to be safe for prolonged use by individuals with an acute porphyria, based on consistent evidence. After conferring with The Norwegian Porphyria Centre, who referred the question to a Swedish specialist on medications for A.I.P. patients, it was considered that the treatment ought to be safe for this patient. In addition, goserelin had been used successfully in fertile women with symptomatic A.I.P. and recurrent attacks to reduce the frequency of attacks [Citation6]. In consent with the male A.I.P. patient (W198X mutation), treatment was started with bicalutamide, 50 mg daily, and goserelin, 10.8 mg as a subcutaneous implant, with frequent and regular urine tests to follow the biochemical disease activity. During the first treatment period, the biochemical disease activity was monitored regularly with urine samples twice a week. U-P.B.G., U-A.L.A. and U-total porphyrins expressed per mmol U-creatinine were analysed in 13 consecutive urine samples after starting the treatment. The levels of porphyrin precursors did not change significantly when assessing the values against reference change values (R.C.V.) in this period ().
Figure 1. To evaluate the influence of bicalutamide and goserelin treatment on A.I.P. disease activity, spot urine samples were collected in light-protected containers two times a week over a 5-week period in the beginning of treatment. Urine (a) P.B.G., (b) A.L.A. and (c) total porphyrins were analysed using kits from Bio-Rad (Hercules, CA). P.B.G. and A.L.A. are given as µmol/mmol creatinine, while total porphyrins are given as nmol/mmol creatinine. The reference change values of (a) U-P.B.G., (b) U-A.L.A. and (c) U-total porphyrins were calculated from the published biological coefficients of variation (C.V.B) and analytical coefficients of variation (C.V.A) and are indicated in each panel as dotted lines. The arrow indicates the start of treatment with bicalutamide and goserelin.
Results
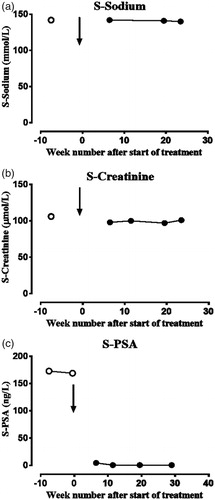
Treatment with bicalutamide and goserelin did not increase the biochemical disease activity measured as the U-P.B.G. and U-A.L.A. ratios (). The patient experienced no symptoms of porphyria or increased urine excretion of total porphyrins. To evaluate significant changes in the biochemical disease activity of acute porphyria, the R.C.V. value for each analyte, with a z-score of 1.96 for significance, was used. The analytical coefficient of variation (C.V.A) values from the laboratory and published biological coefficients of variation (C.V.B) values were applied [Citation5,Citation7]. The U-P.B.G. and U-A.L.A. were both given as μmol/mmol creatinine, and U-total porphyrins were given as nmol/mmol creatinine [Citation5]. The R.C.V. values for U-P.B.G., U-A.L.A. and U-total porphyrins were 73, 57 and 114%, respectively. The treatment efficiently reduced the serum P.S.A.-value from 169 µg/L before the treatment to 0.6 µg/L after ∼ 2 months and stayed at this level ().
Figure 2. The serum levels of (a) sodium, (b) creatinine and (c) prostate-specific antigen (P.S.A.) during the treatment with bicalutamide and goserelin. Serum sodium and creatinine were analysed on a Siemens ADVIA 1800 instrument and serum P.S.A. on Siemens Centaur X.P.T. The results are given as sodium (mmol/L), creatinine (µmol/L) and P.S.A. (ng/L). The arrow indicates the start of treatment with bicalutamide and goserelin.
Discussion
Drugs are well-established contributing factors in A.I.P. attacks. The indications for treating this male symptomatic A.I.P. case with bicalutamide and goserelin were strong and the contraindications were minimal. Relative contraindications for this androgen deprivation treatment included the possible side-effects of anorexia and nausea for both drugs, which could possibly increase the biochemical disease activity of A.I.P. Additionally, the fact that this treatment interferes with the normal sex hormone balance could contribute to enhanced disease activity [Citation8]. Bicalutamide is metabolized by hepatic C.Y.P.-enzymes, which may possibly enhance haem biosynthesis [Citation3]. The finding that U-P.B.G., U-A.L.A. and U-total porphyrins did not increase significantly after treatment with bicalutamide and goserelin may be important for other A.I.P. patients with prostate cancer, since this is the most frequent type of cancer in Norway, where the prevalence of A.I.P. is among the highest in the world. Although this case report shows that treatment with bicalutamide and goserelin was safe in a male A.I.P. patient, close follow-up of P.B.G. and A.L.A. in urine is advised in every patient with A.I.P. using these drugs. This recommendation is due to the known side-effects of nausea and anorexia and because the data on this subject are limited to this case report.
Bjugn_et_al_Caption_to_Supplementary_fig.1.docx
Download MS Word (40.4 KB)Bjugn_et_al_Suppl._Fig.1_.tiff
Download TIFF Image (681.2 KB)Disclosure statement
M.D. Mortensen and medical student F.S. Lichtwarck Bjugn declared that they have no competing financial or other interests in relation to this work.
Additional information
Funding
References
- Puy H, Gouya L, Deybach J-C. Porphyrias. Lancet. 2010;375:924–937.
- Tollali G, Nielsen EW, Brekke OL. [Acute intermittent porphyria]. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin. ny Raekke. 2002;122:1102–1105.
- Hift RJ, Thunell S, Brun A. Drugs in porphyria: from observation to a modern algorithm-based system for the prediction of porphyrogenicity. Pharmacol Ther. 2011;132:158–169.
- Andersson C, Thunell S, Floderus Y, et al. Diagnosis of acute intermittent porphyria in northern Sweden: an evaluation of mutation analysis and biochemical methods. J Intern Med. 1995;237:301–308.
- Aarsand AK, Petersen PH, Sandberg S. Estimation and application of biological variation of urinary delta-aminolevulinic acid and porphobilinogen in healthy individuals and in patients with acute intermittent porphyria. Clin Chem. 2006; 52:650–656.
- Schulenburg-Brand D, Gardiner T, Guppy S, et al. An audit of the use of gonadorelin analogues to prevent recurrent acute symptoms in patients with acute porphyria in the United Kingdom. JIMD Reports. 2017;36:99–107.
- Westgard J. Desirable Biological Variation Database specifications; 2014 [cited 2018]. Available from: https://www.westgard.com/biodatabase1.htm
- Pischik E, Kauppinen R. An update of clinical management of acute intermittent porphyria. Appl Clin Genet. 2015;8:201–214.
