Abstract
Objectives: To present a patient material of renal angiomyolipoma (AML) with focus on the risk of bleeding during active surveillance (AS).
Methods: Medical records, 1999–2014, were studied and 98 patients (80 female, 18 men) with renal AML were identified. Eleven patients had tuberous sclerosis complex (TSC). Mean age was 54 (13–89) years.
Results: Sixty patients (61%) were asymptomatic at presentation, 33 (34%) presented with flank pain and five (5%) with hematuria. Retroperitoneal bleeding or hematuria was diagnosed in 20 patients with a mean AML size of 74 mm (25–200 mm). Twenty-one patients were treated with angioembolization at time of diagnosis and 25 had surgery. Forty-five patients with sporadic AML (mean size 34 mm) and six with TSC (mean size 120 mm) were selected for AS. Only one patient with sporadic AML (46 mm) had a bleeding, whereas two of the six TSC patients had bleedings from three kidneys (AML 70–300 mm). In 25 patients (49%), the AML-size increased with 2.7 mm/year in sporadic and 5.4 mm/year in TSC-associated AML. Thirteen patients were treated with AE (including all six TSC-patients) and five with surgery in 22 kidneys due to AML-size in 16, bleeding in four and suspicion of cancer in two.
Conclusion: Bleeding occurred in 20% of AML at presentation. In patients selected for AS, we found a very low risk of bleeding in sporadic AML justifying our cut off size of 50 mm to trigger intervention. In TSC-associated AML individually tailored follow-up is needed due to a higher intervention rate.
Introduction
Angiomyolipoma (AML) is an uncommon benign tumor with a prevalence between 0.2% and 0.6% [Citation1]. It is histologically composed of fatty tissue, dysmorphic blood vessels and smooth muscle in varying proportions. The tortuous aneurysmatic vessels are prone to spontaneous rupture leading to retroperitoneal bleeding [Citation2,Citation3]. AML with small amounts of fat may radiologically mimic renal cell carcinoma (RCC). There are two major varieties of renal AML, one associated with tuberous sclerosis complex (TSC) and one sporadic form. Renal AML associated with TSC are often multiple, bilateral, symptomatic and without male or female predominance [Citation4].
Sporadic renal AML are mainly unifocal, asymptomatic and with a female predominance [Citation5]. However, cases with locally advanced AML and even tumor thrombus have been reported [Citation6].
The widespread use of abdominal imaging has increased the incidental detection of all renal masses, including AML.
Albeit its benign nature, AML presents a challenge to the clinician to decide when to intervene. Most lesions are initially followed with active surveillance. Indications for intervention have historically been bleeding, pain or suspicion of malignancy. Increased size has been proposed to be a risk of spontaneous bleeding and size >40 mm has been an accepted trigger for invention [Citation7]. Treatment options have traditionally been surgical removal (radical/partial nephrectomy), but are increasingly replaced by angioembolization (AE). Some recent reports in fact suggest that this treatment strategy also might prove valuable in the long term [Citation8,Citation9]. There have been no prospective randomized trials comparing active surveillance (AS) of AML with active treatment at diagnosis and the reported case series are relatively small. By reporting our retrospective case series, in which a substantial part was followed with active surveillance, we wanted to disclose the natural course of untreated AML with special emphasis on the increase in size and the risk of bleeding.
Material and methods
Data from 98 patients with renal AML diagnosed or treated at Sahlgrenska University Hospital, a tertiary referral center, between January 1999 and December 2014, were retrospectively reviewed. Searching for the diagnosis code D30.0 was used to identify the patients. The patients were collected consecutively, mainly originating from hospitals in our region, but also referred from other parts of Sweden. Records from the department of urology were scrutinized. CT-images were collected and re-evaluated by an experienced interventional radiologist and the tumors were measured sequentially before and after treatment. All variables were gathered in a database.
The AML was found in two patients by ultrasonography and then verified by CT. In the remaining patients CT was the primary diagnostic modality. In patients operated on due to suspicion of RCC, the diagnosis of AML was finally confirmed by postoperative histopathology. Generally, CT was performed with both native and contrast enhanced series. The native series was the best for detecting the fatty component shown by negative Hounsfield units, being the diagnostic sign for AML.
Indications for intervention were either size >50 mm, local symptoms such as flank pain, suspicion of cancer or spontaneous rupture of the AML manifested as either retroperitoneal bleeding or hematuria.
Active surveillance
Patients in AS were followed with CT or ultrasonography, annually or every second year and thereafter was follow-up individualized. Treatment was recommended when the AML was >50 mm, modified by age, kidney function, comorbidity and patient preference.
Particularly, patients with TSC had more complex cases and the treatment and/or follow-up plan was always individualized.
Angioembolization
AE was selective and mainly performed under general anesthesia (90%). Patients treated with AE were followed-up with CT after three to six months and then yearly. Patients treated with AE in one side with bilateral disease were then managed by AS on the contralateral side.
Surgery
Surgery for AML was either radical nephrectomy or partial nephrectomy. Radical nephrectomy dominated early in the study period and partial nephrectomy late in the period. Patients treated with surgery were followed postoperatively with CT after 3–6 months. Follow-up was then terminated due to the benign nature of AML. Patients with bilateral disease, operated only in one side, were subsequently managed by AS in the contralateral side.
Ethical approval was applied and granted by the regional board of ethics, application 238-17.
Results
A total of 98 patients had the diagnosis at the start or were diagnosed during the study period. For patient characteristics, see . Eleven were diagnosed with TSC.
Table 1. Characteristics of all AML-patients.
Clinical symptoms
Sixty patients (61%) were asymptomatic at presentation, whereas 33 patients (34%) presented with flank pain and five (5%) with hematuria. The mean size of the AML in the asymptomatic patients was 44 mm (5–300) and 69 mm (30–200) in the symptomatic. The diagnosis of AML was incidental in 55 of the asymptomatic patients (92%). In the remaining five asymptomatic cases (8%), AML was found in the diagnostic work-up for TSC.
Among the 33 patients presenting with flank pain, 16 had acute onset of pain, of which 15 had a radiologically demonstrable retroperitoneal bleeding and five had macroscopic hematuria. One of the 33 patients had a fatal bleeding where the diagnosis of AML was confirmed post mortem. Six (40%) patients of those with bleeding required blood transfusion. The remaining 17 cases with flank pain were diagnosed due to pain and discomfort of a non-acute nature, eventually prompting a decision to embark upon radiological evaluation.
The average size of bleeding AML was 74 mm (25–200 mm). Three (15%) of the 20 bleeding AML measured less than 40 mm and five (25%) were smaller than 50 mm.
Characteristics of TSC-patients and patients with sporadic AML
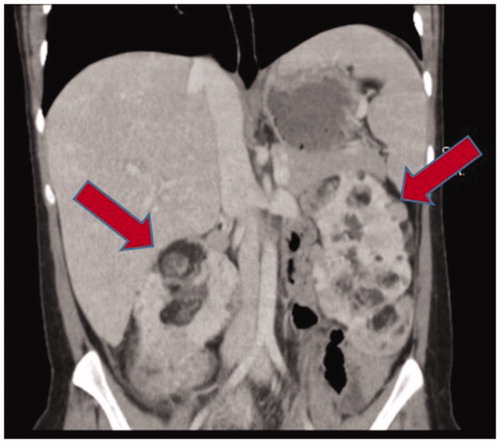
The eleven patients (11%) with TSC had a predominantly bilateral and multifocal disease with a total of 39 separate lesions as exemplified in . Mean age at presentation was 24 years and mean size of the largest lesion was 105 (50–300) mm. All patients with TSC had treatment either at diagnosis or after a period of active surveillance.
Figure 1. Twenty years old woman with tuberous sclerosis. Multifocal and bilateral angiomyolipoma with largest lesion of 60 mm. Initially followed with active surveillance but after five years intervention with angioembolization due to increasing size.
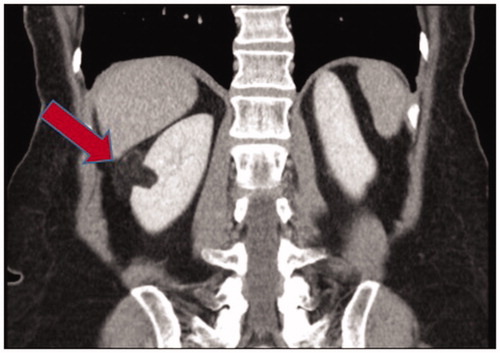
The remaining 87 patients (89%) with sporadic AML had a predominantly unifocal and unilateral disease with a total of 118 lesions as exemplified in . Their mean age was 56 years at presentation and mean size was 46 (5–200) mm.
Primary treatment
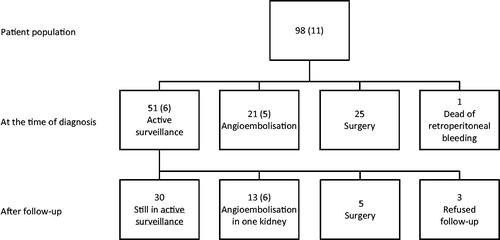
The 97 cases were either primarily selected for AE (n = 21), surgery (n = 25) or AS (n = 51) (). The indications for AE were size and bleeding in twelve and nine subjects, respectively. In the patients that underwent surgery, radical nephrectomy and partial nephrectomy was performed in eight and seventeen patients, respectively. The indications for surgery were suspicion of cancer in 15 (60%), bleeding in five (20%) and size in five (20%) patients.
Active surveillance
At diagnosis, 51 patients were recommended or chose an active surveillance (AS) strategy by their own preference. Forty-five of them (88%) had sporadic AML and six had TSC (12%).
Active surveillance for patients with sporadic AML
The mean size at diagnosis, and inclusion in AS, for the 45 patients was 34 mm (5–100 mm). Eight patients had symptoms at diagnosis, whereas 37 findings were incidental. Mean follow-up was 67 months (3–308 months) ().
Table 2. Characteristics of patients in active surveillance.
Three of the 45 patients (7%) refused follow-up but they did not appear with any complications from their AML and were alive when the study closed.
Twenty-one of the remaining 42 patients with regular follow-up (50%) had an increase in size in one or more lesions during the period of AS. Twenty-one of the 42 patients (50%) did not have any increase in size. Mean increase in size of the growing lesions was 76% (7–333%) during the follow-up period. Mean annual growth rate for the 21 cases with growth was 2.7 mm/year (0.1–11.9 mm/year).
One of 42 patients (2%) with a sporadic AML of 46 mm had a bleeding episode after 96 months of AS and was successfully treated with AE. Seven of the 42 patients (17%) with sporadic AML, initially followed by AS, were finally treated with AE. Mean time from diagnosis to first intervention with AE was 95 months (15–192). Indications for intervention with AE in the seven patients were in six cases size or progress in size and in one case bleeding.
Five patients initially followed with AS were finally treated surgically. Mean time from diagnosis to surgery was 87 months (8–188 months). Indications for surgery in the cases with delayed intervention with surgery were progress in size during active surveillance in three cases and suspicion of RCC in the remaining two.
Thirty patients were still in AS at the end of the study period.
Active surveillance for patients with TSC-associated AML
The mean size at diagnosis and inclusion in AS for the six patients was 120 mm (50–300); all of which had bilateral AML. One was diagnosed due to symptoms and the remaining five were diagnosed as part of a work-up for TSC. Mean follow up was 109 months (23–248 months) ().
Four of the six patients with TSC had an increase in size in one or more AML during the period of AS. Mean increase in size of the growing lesions was 70% (16–186%) in the follow-up period and mean annual growth-rate for the four patients with TSC was 5.4 mm/year (0.9–14 mm/year). AE was performed in seven kidneys in these four patients with growing lesions.
Two of the six patients (33%) had a bleeding AML during AS. One TSC-patient with an AML of 70 mm had a bleeding episode after 74 months and was treated with AE. The other had two separate bleeding episodes from bilateral AML of 237 and 300 mm after 145 and 96 months, respectively. AE was performed after each bleeding episode but staged bilateral nephrectomies were finally performed due to local symptoms from the giant AML.
Thus, all six subjects with TSC, initially followed with AS, were finally treated with AE. Mean time from diagnosis to first intervention with AE was 57 months (16–96). Four of the patients were treated in both kidneys with a period of AS of 8–49 months in-between. Indications for intervention with AE in the ten separate kidneys were in seven cases size, or progress in size, and in three cases bleeding.
The flowchart () illustrates both the primary treatment with AE and surgery, and that only 35% of those selected for AS had active treatment.
Discussion
The widespread use of imaging has resulted in a significant increase in incidentally diagnosed small renal masses over the last decades [Citation10]. This has led to an increase in diagnosed AML, which emphasizes the importance of guidelines to handle this benign entity. Today’s knowledge is based on rather few case series ranging from Oesterling’s pioneering work, from 1986, with a series of 13 own cases and a literature review of another 253 cases, to the largest recently published case series from Bhat et al. of 447 cases [Citation7,Citation11].
In the present paper, the majority of the patients were asymptomatic at diagnosis and incidentally found AML were smaller than the symptomatic ones. The symptomatic patients either had hematuria or flank pain due to a retroperitoneal bleeding or a more prolonged period of discomfort. Others have reported on varying numbers of symptomatic cases in their cohorts ranging from Bhat et al.’s 9% to Seyam et al.’s 50% [Citation11,Citation12]. The mean tumor size in the present study was 51 mm, which is in keeping with the paper from Seyam et al. Speculatively, these differences mirror a selection bias; the findings by us and by others reporting on larger and more often symptomatic cases, whereas smaller cases remain unrecognized in the daily practice and risk being missed in medical chart based studies like the present one. Bhat et al. [Citation11] used a radiology data-mining system that found small lesions mentioned in the radiology reports that probably were missed in this and all other so far published studies.
Tuberous sclerosis (TSC) is an autosomal dominant disorder with an estimated prevalence of 1 in 12,000 [Citation13]. Reported rates of renal AML in association with TSC vary between 55 and 90% [Citation13,Citation14]. The reported proportion of AML cases with TSC ranges from Soorkumaran 69%, Nelson and Sanda 20% to more recently Mues and Bhat both reporting 3.8% [Citation11,Citation15–17]. In the present study, eleven of 98 patients had TSC (11%). Only patients with proven TSC were counted in the present series. At least six more cases with bilateral and multifocal disease can be suspected for TSC but were either not tested or had tested negatively. Up to 25% of TSC-cases may not currently be identified with conventional genetic screening tests [Citation18]. Patients with TSC-associated AML had, in accordance with other studies, larger, multifocal and mainly bilateral lesions and were diagnosed at a younger age. All eleven TSC-patients in the present study had, at any time, an intervention compared to 61% in the group with sporadic AML. Other authors have reported on a more aggressive course of the AML-disorder in TSC-patients [Citation4]. The eleven patients in our study had in all 13 retroperitoneal bleeding episodes, two episodes of hematuria and five cases of progress in size, leading to 22 AE and three nephrectomies. 57% of all the interventions in the present report were performed on the eleven TSC-patients, hence supporting the notion that AML associated with TSC is a more severe disorder than sporadic AML, underscoring the need for a tailored follow-up of these individuals.
Oesterling proposed a 40 mm limit for intervention that for years has been referred to as a guideline [Citation7]. Some authors have supported this limit reporting that roughly 80% of AML larger than 40 mm will become symptomatic and that 50–60% will bleed spontaneously [Citation19]. Others have, however, questioned this limit reporting that tumors >40 mm conversely have a low risk of bleeding [Citation11,Citation20]. In the present series, a fifth of the patients had a bleeding episode (retroperitoneal hematoma and/or hematuria) as the initial presenting clinical sign. The mean size of the bleeding AML was 74 mm. Three patients had a bleeding AML <40 mm and five patients had a bleeding AML <50 mm.
Forty-five patients with sporadic and six with TSC-associated AML were selected to AS. During a mean follow up of 72 months only one of the patients with sporadic AML had a bleeding from a 46 mm lesion, which was successfully embolized. Two TSC-patients had in all three bleeding episodes.
Intratumoral aneurysm size might be another predictor of rupture. It has been shown that the genetic aberration in TSC predispose to aneurysm formation in the AML and that the presence of aneurysm larger than 5 mm increases the risk of rupture [Citation3]. However this was not assessed in the present paper.
A recent study of AS showed in the subgroup of AML >40 mm that only four of 38 patients (11%) had hemorrhage or hematuria at a mean follow up of 49 months [Citation20].
An interesting observation is that the only truly giant AML in the present series (bilateral 300/237 mm) was followed by AS for 96 months before the first bleeding event which is in agreement with Danforth et al. [Citation21] reporting on two patients with similar lesions managed conservatively for 20 years.
The previously stated 40 mm threshold for intervention has probably strongly influenced the management in many institutions. An increasing number of studies have demonstrated the feasibility of AS but currently none is done with a higher level of evidence [Citation17,Citation20,Citation22,Citation23]. Ouzaid et al. proposed that AS should be considered as the standard for all uncomplicated AML at presentation.
In our material of 98 patients, size of AML was the indication in 52% of the AE and in 24% of the surgical interventions. The majority had symptoms with prolonged flank pain (67%) but a minority were symptom free and could maybe have been managed with AS.
Eighteen of our 51 patients in AS (35%) had active treatment over the years. Corresponding figures for Ouzaid et al. was 13% and Mues et al. 8% [Citation17,Citation20]. Mean follow-up was in our study 72 months and in theirs 49 and 55 months, respectively. Reasons for our higher intervention rate may be a longer follow-up or larger sizes at inclusion in AS, 43 mm, compared to 17 mm in Mues study. Ouzaid et al. have not reported exact sizes but 70% were smaller than 40 mm.
Causes to end AS were size, bleeding and suspicion of cancer. Four patients with unchanged sizes >50mm were initially recommended intervention but refused due to the benign nature of the disease but changed their minds during AS.
Our finding that 25 of 48 patients (52%) had an increase in size in one or more AML during the period of AS is much higher than those previously reported. De Luca reported on three AML increasing in size in 38 lesions (8%) at a mean follow-up 60.2 months [Citation22]. Mues et al. [Citation17] did not state the percentage of growing lesions but reported an increase in size of less than 1 mm/year for their cohort at a mean follow-up of 54.8 months. They had one case with an accelerated growth rate of 7 mm/year. Bhat et al. [Citation11] concluded that >90% of their AML grew slowly or not at all but they identified a subpopulation of 9% with an accelerated growth that they named ‘growers’. Our AS-cohort had larger sizes (mean 43 mm) as compared to De Luca (97%<5 cm) and Mues (mean 17 mm) suggesting that size might be a possible indicator for growth. On the contrary, Bhat et al. could not find any differences in growth rates between lesions larger or smaller than 40 mm at a mean follow-up of 43 months. We tried to analyze differences in our ‘growers’ and ‘non-growers’ but could not detect any such differences and hence, we conclude that such indicators of growth remain to be identified.
Bleeding is the most alarming complication of AML and this was actually the first symptom in 20% of our patients. However in the patients selected to AS, the risk of bleeding was low. That was especially true for sporadic AML with only one bleeding episode in 45 patients. Our findings seem to justify a 50 mm limit to trigger prophylactic intervention in this patient population in order to avoid overtreatment, at least in a setting where AE is available.
When this principle was applied in the more complex TSC-patients, although modified by patient and tumor characteristics, AE was eventually performed in all six patients on AS but the kidneys could be spared for several years in the majority of the patients.
Our results also indicate that a significant number of lesions will increase in size why follow-up is recommended to identify ‘growers’.
The retrospective nature of this single center study is of course a limitation. There might also be a selection bias towards larger lesions due to referrals to us being a tertiary referral center.
Conclusions
Bleeding occurred in 20% of AML at presentation. In patients selected for AS, we found a very low risk of bleeding in sporadic AML justifying our cut off size of 50 mm to trigger intervention. In TSC-associated AML individually tailored follow-up is needed due to a higher intervention rate.
Disclosure statement
The authors report no conflict of interests.
References
- Vos N, Oyen R. Renal angiomyolipoma: the good, the bad, and the ugly. J Belg Soc Radiol. 2018;102:41.
- Brandt MP, Tsaur I, Vallo S, et al. Ruptured angiomyolipoma of the kidney: a rare differential diagnosis of flank pain. Scand J Urol. 2017;51:342–344.
- Wang C, Li X, Peng L, et al. An update on recent developments in rupture of renal angiomyolipoma. Medicine (Baltimore). 2018;97:e0497.
- Buj Pradilla MJ, Balleste TM, Torra R, et al. Recommendations for imaging-based diagnosis and management of renal angiomyolipoma associated with tuberous sclerosis complex. Clin Kidney J. 2017;10:728–737.
- Bernstein J, Robbins TO, Kissane JM. The renal lesions of tuberous sclerosis. Semin Diagn Pathol. 1986;3:97–105.
- Fernandez-Pello S, Gonzalez Rodriguez I, Villamil LR, et al. Laparoscopic management of right renal angiomyolipoma with involvement of the inferior vena cava: case report and review of the literature. Scand J Urol. 2013;47:340–344.
- Oesterling JE, Fishman EK, Goldman SM, et al. The management of renal angiomyolipoma. J Urol. 1986;135:1121–1124.
- Murray TE, Doyle F, Lee M. Transarterial embolization of angiomyolipoma: a systematic review. J Urol. 2015;194:635–639.
- Wang C, Yang M, Tong X, et al. Transarterial embolization for renal angiomyolipomas: a single centre experience in 79 patients. J Int Med Res. 2017;45:706–713.
- Volpe A, Panzarella T, Rendon RA, et al. The natural history of incidentally detected small renal masses. Cancer. 2004;100:738–745.
- Bhatt JR, Richard PO, Kim NS, et al. Natural history of renal angiomyolipoma (AML): most patients with large AMLs >4cm can be offered active surveillance as an initial management strategy. Eur Urol. 2016;70:85–90.
- Seyam RM, Bissada NK, Kattan SA, et al. Changing trends in presentation, diagnosis and management of renal angiomyolipoma: comparison of sporadic and tuberous sclerosis complex-associated forms. Urology. 2008;72:1077–1082.
- Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668.
- Franz DN. Everolimus: an mTOR inhibitor for the treatment of tuberous sclerosis. Expert Rev Anticancer Ther. 2011;11:1181–1192.
- Sooriakumaran P, Gibbs P, Coughlin G, et al. Angiomyolipomata: challenges, solutions, and future prospects based on over 100 cases treated. BJU Int. 2010;105:101–106.
- Nelson CP, Sanda MG. Contemporary diagnosis and management of renal angiomyolipoma. J Urol. 2002;168:1315–1325.
- Mues AC, Palacios JM, Haramis G, et al. Contemporary experience in the management of angiomyolipoma. J Endourol. 2010;24:1883–1886.
- Northrup H, Krueger DA, Northrup H, et al. Tuberous Sclerosis Complex Consensus, Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243–254.
- Soulen MC, Faykus MH, Jr., Shlansky-Goldberg RD, et al. Elective embolization for prevention of hemorrhage from renal angiomyolipomas. J Vasc Interv Radiol. 1994;5:587–591.
- Ouzaid I, Autorino R, Fatica R, et al. Active surveillance for renal angiomyolipoma: outcomes and factors predictive of delayed intervention. BJU Int. 2014;114:412–417.
- Danforth TL, Lane BR, Novick AC. Conservative management of giant symptomatic angiomyolipomas in patients with the tuberous sclerosis complex. BJU Int. 2007;100:794–797.
- De Luca S, Terrone C, Rossetti SR. Management of renal angiomyolipoma: a report of 53 cases. BJU Int. 1999;83:215–218.
- Hadley DA, Bryant LJ, Ruckle HC. Conservative treatment of renal angiomyolipomas in patients with tuberous sclerosis. Clin Nephrol. 2006;65:22–27.