Abstract
Objective: To evaluate the extent and plausible effects of blood transfusions given during cisplatin-based neoadjuvant chemotherapy (NAC) on overall survival in patients with muscle-invasive urothelial bladder cancer (MIBC) undergoing NAC and radical cystectomy (RC).
Background: Several studies have demonstrated a decreased survival for MIBC patients receiving allogenic peri- and postoperative blood transfusions in conjunction with RC. No studies have previously investigated the effects of blood transfusions during NAC.
Materials and methods: 120 patients with MIBC (cT2-T4aN0M0) undergoing NAC and RC between 2008 and 2014 at four Swedish cystectomy centers were retrospectively evaluated. Clinicopathological data were obtained, including data of allogenic blood administration. Survival data was analyzed by Kaplan–Meier plotting and Cox regression.
Results: One third of the cohort received blood transfusions during the period of NAC. In univariate analysis, blood transfusions during NAC, nodal stage and advanced tumor stage (pT >2) were negative prognostic factors for survival. In multivariate analysis, only pNx and pT >2 remained significant negative prognostic factors. In a subgroup analysis consisting of patients with localized tumors without dissemination (n = 96), patients that received transfusions during NAC showed an 18.5% absolute risk increase of death at five years of observation, although without statistical significance (p = .197).
Conclusions: This is the first time that the extent and plausible effects of allogenic blood transfusions during NAC is examined in MIBC. Data suggest that there may be an association between blood transfusion and poor pathological and oncological outcome. Firm conclusions are difficult to draw due to few study participants and the retrospective nature of the study.
Background
Muscle-invasive bladder cancer (MIBC) is a deadly disease with an overall survival of 50% at five years [Citation1,Citation2]. In case of localized disease (pT2-T4aN0M0), the mainstay of the treatment is radical cystectomy. Two large randomized prospective studies have shown a 5–8% absolute risk reduction for death when adding cisplatin based neoadjuvant chemotherapy (NAC) [Citation3,Citation4]. As a result, more than a third of all cystectomized patients in Sweden receive NAC prior to surgery [Citation5].
Anemia is a common finding in cancer patients [Citation6] which makes this group at high risk of receiving allogenic blood transfusions. However, a growing number of studies have demonstrated an association between peri- and postoperative blood transfusion and worse long term survival after cancer surgery [Citation7]. In a meta-analysis of more than 15 000 patients undergoing radical cystectomy, a 27% increase of mortality was seen in patients receiving perioperative blood transfusion [Citation8]. The underlying mechanism by which allogenic blood impacts cancer survival is thought to be due to suppression of the immune system. The process is called transfusion-related immunomodulation, TRIM, and the exact mechanism is not entirely understood. Overall, allogenic blood appears to enhance the effects of immunosuppressive cells while inhibiting the effects of immunostimulatory cells [Citation9–12].
The quantity and the consequences of blood transfusions at the time of NAC in patients with MIBC have not been addressed before. Therefore, we decided to retrospectively map the extent and plausible effects of blood transfusions during NAC with regard to pathological and clinical outcomes.
Materials and methods
Procedure
A retrospective review of medical records from all patients cystectomized for urinary bladder cancer between the years of 2008 and 2014 was conducted. The cystectomies were performed at four urological centers in Sweden (the University Hospital of Umeå, Sundsvall-Härnösand County Hospital, Gävle County Hospital and Västmanland County Hospital) (n = 372). The criteria for inclusion were: localized urothelial MIBC (cT2-T4aN0M0) and participation in treatment with 1–4 cycles of NAC followed by radical cystectomy with intended lymph node dissection (). Clinicopathological data are summarized in . Evaluations of transfusions were limited to erythrocyte transfusions. Plasma and thrombocyte transfusions were not taken into account. One center (Sundsvall-Härnösand County Hospital) used a Cell Saver autologous blood recovery system intraoperatively at seven cystectomies. No Cell Saver was used during the period of NAC. Primary outcome was number of blood transfusions, pathological outcome and overall survival. Overall survival was estimated as time between radical cystectomy to death of any cause. The final date for follow-up was 8 June 2018.
Figure 1. Flow chart of inclusion of all evaluated patients. UBC: Urinary bladder cancer. MIBC: Muscle-invasive bladder cancer. cT: Clinical staging of the primary tumor; cN: Clinical staging of regional lymph nodes; cM: Clinical staging of distant metastases.
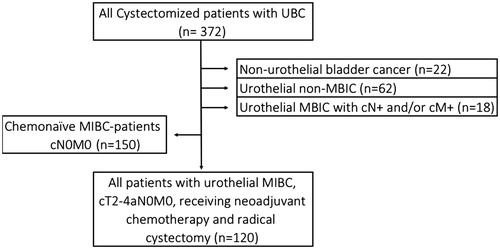
Table 1. Patient Characteristics and group comparison (%).
Participants
The final cohort consisted of 120 patients, the majority of which (58.3%) were treated at the largest urological center among the four, the University Hospital of Umeå. The second largest urological center, Sundsvall-Härnösand County Hospital, did not implement NAC-treatment as a clinical routine until 2012, and therefore only contributed with 11.7% of the patients (data not shown).
Lymph node dissection (LND)
The included patients underwent cystectomy with curative intention. The extent of lymph node dissection (LND) varied according to local traditions. To our knowledge, LND was part of the preoperative planning for all patients. There were 12 patients in which LND was not performed (pNx) (). The reasons for not performing LND was either local tumor spread (n = 2), extensive fibrosis in the area (n = 5), or reasons unknown (n = 5).
Statistical methods
Statistical analyses were performed using IBM SPSS Statistics, version 24 and version 26. The cohort was divided into two groups depending on if the patients received blood transfusions during NAC, or not (blood yes and blood no). Differences in clinicopathological variables were tested by t-test for continuous variables and Chi-Square Test for categorical variables. In the analyses of categorical variables where the criteria of Chi-Square Test were not fulfilled, Fisher’s Exact Test was used (). Survival rates were compared with the Kaplan–Meier Estimator and the Log-Rank Test. Cox Proportional Hazard Regression Model was used to identify prognostic factors for survival. For all analyses, p < .05 was considered statistically significant.
Results
One third of the cohort (n = 40) received allogenic blood transfusion during NAC. The cohort was divided into two groups depending on if blood transfusions were administrated during NAC, or not (blood yes and blood no). There were no statistical differences in age, cTNM-stages, comorbidity (CACI/ASA), NAC-cycles or peri- and postoperative blood transfusions (). Yet, the percentage of cT4a in the blood yes was more than doubled compared to blood no patients. Furthermore, the blood yes group consisted of significantly more women and had a lower level of hemoglobin (Hb) at transurethral resection of the bladder (TURB). In histopathological outcome after cystectomy, the blood yes group had half as many downstaged tumors (pT0N0M0) and 15% of the blood yes patients were upstaged to pT4b compared to 1.3% in blood no group (p = .044). In addition, 42.5% of the patients in the blood yes group were pN+/pNx compared to 17.5% in the blood no group (p = .007) ().
Survival analysis of the whole cohort (n = 120, number of events = 62) showed an overall survival of 58.9% in the blood no group versus 39.7% in the blood yes group at five years of observation (p = .047) (). Predictors for survival were examined in a Cox Proportional Hazard Model. In univariate analysis, blood yes, nodal status and locally advanced tumor (pT >2), were negative prognostic factors for survival (). In a multivariate analysis, locally advanced tumor (pT >2) and unknown nodal status (pNx) remained independent negative prognostic factors ().
Figure 2. Kaplan–Meier overall survival curve for all patients with urothelial muscle-invasive bladder cancer (cT2-T4aN0M0) undergoing neoadjuvant chemotherapy and radical cystectomy between 2008 and 2014 at four Swedish cystectomy centers (n = 120). The cohort was divided into two groups depending on if the patients received blood transfusions during NAC, or not (blood yes and blood no).
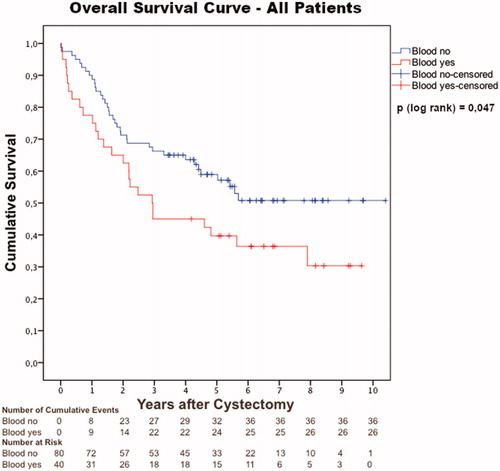
Table 2. Cox proportional hazard model.
Discussion
One third of the patients received allogenic blood during neoadjuvant chemotherapy (NAC). These patients showed no difference in age or comorbidity compared to patients not receiving transfusions during NAC. Yet, they demonstrated distinctly worse postoperative pathological outcomes and a 19.2% absolute risk increase of death at five years of observation. The current study is, to our knowledge, the first to examine the extent of blood transfusions during neoadjuvant chemotherapy with regard to pathological and clinical outcomes.
Allogenic blood transfusion and efficacy of chemotherapy
It has been shown that pathological downstaging is significantly increased after neoadjuvant chemotherapy and that it can be regarded as a surrogate marker for response to NAC [Citation13]. In this cohort, the percentage of completely downstaged tumors (pT0N0M0) was doubled in the blood no group, whereas the proportion of advanced postoperative tumor stage was larger in the blood yes group. Although there was no statistical difference in the preoperative tumor staging between the groups, the proportion of patients with cT4a tumors was more than doubled in the blood yes group. Thus, the difference in pathological staging might primarily reflect the blood yes group consisting of more advanced tumors prior to blood transfusion. Yet, recent published data have revealed that chemotherapy elicits an immunostimulatory response that partly accounts for its efficacy [Citation14–16]. Therefore, the maximal therapeutic benefits of NAC might be reduced if the immune system is suppressed, as it may be after allogenic blood administration.
The idea that the allogenic blood acts as an immunosuppressant dates back to 1973 when Opelz et al. showed that patients receiving blood transfusions prior to renal transplantation had a lower rate of organ rejection compared to non-transfused patients [Citation17]. Since then, numerous studies have confirmed the immunosuppressive effect of allogenic blood, a phenomenon which is often referred to as transfusion-related immunomodulation (TRIM) [Citation12]. If TRIM reduces the efficacy of NAC and mediates a general immunosuppressive effect in the patient, this could be a plausible explanation to the more advanced tumor stage and the lower overall survival seen in the blood yes group compared to the blood no group.
Long-term outcomes
Patients in the blood yes group had worse survival rates five years after cystectomy. This aligns with earlier studies showing negative oncological outcomes in patients receiving perioperative blood transfusions [Citation7,Citation8]. Interestingly, blood transfusion during NAC was not an independent prognostic factor in the multivariate survival analysis. The reason for this could either be that no causal relationship exists between allogenic blood and overall survival, or that the negative effect of blood on survival is primarily conveyed in its effect on tumor progression. This hypothesis may explain why no independent effect of blood is seen when adjusted for pT and pN status, since the link between blood and survival would be mediated by tumor progression.
Lymph node status
It is well established that nodal dissemination is associated with poor survival [Citation18]. Yet, in this cohort, only unknown nodal status (pNx) was an independent negative prognostic factor. The reasons for not performing lymph node dissection (LND) in pNx patients were due to local tumoral spread (n = 2), extensive fibrosis in the area (n = 5), and reasons unknown (n = 5). This uncertainty clearly signals a limitation of the study, especially when 20% of the blood yes group lacked nodal staging compared to 5% in the blood no group. Since pNx was a stronger negative predictor for survival than pN + in both uni- and multivariate analyses, we suspect pNx to be a surrogate marker for regionally advanced disease. The high proportion of pT4b, pN + and pNx in the blood yes group clearly signals a more advanced disease in this stratum of patients.
Limitations
There is a risk of bias when variables are selected and analyzed retrospectively. This cohort was stratified according to transfusion status during NAC. Although the two patient groups showed similar clinicopathological characteristics at the preoperative staging, they did differ in the distribution of sex and hemoglobin status at time of primary TURB. This imbalance might affect clinical outcome as it is well known that women tend to have more aggressive urinary bladder cancer compared to men [Citation19] and anemia is associated with shorter survival times [Citation20]. In addition, it is plausible that the patients receiving transfusions had lower hemoglobin levels and received blood as a consequence of having an underdiagnosed, more advanced cancer from the start. Furthermore, patients included after June 2013 have contributed with less than five years of observation at time of analysis and postoperative TNM-staging was not fully completed in terms of nodal staging.
Concluding remarks
To our knowledge, this is the first time that patients with muscle-invasive urothelial bladder cancer undergoing neoadjuvant chemotherapy and radical cystectomy are examined with regard to allogenic blood transfusions during neoadjuvant chemotherapy. The findings may indicate a plausible association between allogenic blood products during chemotherapy and adverse pathological and clinical outcomes. The findings warrant studies on larger cohorts and further experimental translational research on the effects of blood transfusions in cancer patients undergoing chemotherapy.
Ethical approval and consent to participate
The study was approved by the Regional Ethical Committee in Umeå (EPN-Umeå, dnr: 2015/395-32 and dnr: 2016/403-32 M). Informed consent was specifically not needed according to the regional ethical committee and was therefore not obtained.
Acknowledgements
We would first of all, like to acknowledge the valuable assistance and cooperation of our dear colleague, Dr. Johan Hanson (Gävle County Hospital and Uppsala University), who passed away during the preparations of this mansucript. We would also like to thank Roger Brännström (Sunderbyn Hospital, Luleå), Martin Odmark (Sundsvall-Härnösand County hospital, Sundsvall), Annika Axelsson Åhs (Gävle County Hospital), Botan Hawas (Västmanland County Hospital), and Maud Nilsson (University Hospital of Umeå) for valuable assistance in the process of data collection. We also acknowledge the valuable assistance from statistician Marcus Thuresson at Statisticon AB, Uppsala, Sweden and the important comments from our colleague Dr. Michael Mints, MD, PhD, the Department of Surgical and Perioperative Sciences, Urology and Andrology, Umeå University, Umeå, Sweden.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2011;19:666–675.
- Dalbagni G, Genega E, Hashibe M, et al. Cystectomy for bladder cancer: a contemporary series. J. Urol. 2001;165(4):1111–1116.
- Meta-analysis Group. European Urology Neoadjuvant Chemotherapy in Invasive Bladder Cancer: Update of a Systematic Review and Meta-Analysis of Individual Patient Data. Eur Urol. 2005;48:202–206.
- Sherif A, Holmberg L, Rintala E, et al. European urology neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two nordic studies. Eur Urol. 2004;45:297–303.
- Regionala Cancercentrum. Urineblåse- och urinvägscancer. Årsrapport nationellt kvalitetsregister. [Internet]. 2017 [cited 2020 Jan 13]. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/urinvagar/urinblase–och-urinrorscancer/rapporter/urinblasa_arsrapport_2017_final.pdf?v=2e6448163bfb4814b2e9344b2e4e9cb6.
- Ludwig H, Van Belle S, Barrett-Lee P, et al. The European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur. J. Cancer. 2004;40(15):2293–2306.
- Iqbal N, Haider K, Sundaram V, et al. Red blood cell transfusion and outcome in cancer. Transfus. Apher. Sci.. 2017;56(3):287–290.
- Cata JP, Lasala J, Pratt G, et al. Association between perioperative blood transfusions and clinical outcomes in patients undergoing bladder cancer surgery: a systematic review and meta-analysis study. J. Blood Transfus. 2016;2016:1–8.
- Goubran H, Sheridan D, Radosevic J, et al. Transfusion-related immunomodulation and cancer. Transfus. Apher. Sci. 2017;56(3):336–340.
- Vallion R, Bonnefoy F, Daoui A, et al. Transforming growth factor-beta released by apoptotic white blood cells during red blood cell storage promotes transfusion-induced alloimmunomodulation. Transfusion. 2015;55(7):1721–1735.
- Karam O, Tucci M, Toledano BJ, et al. Length of storage and in vitro immunomodulation induced by prestorage leukoreduced red blood cells. Transfusion. 2009;49(11):2326–2334.
- Hellings S, Blajchman MA. Transfusion-related immunosuppression. Anaesth. Intensive Care Med. [Internet]. 2009;10(5):231–234.
- Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur. Urol. 2012;61(6):1229–1238.
- Krantz D, Hartana CA, Winerdal ME, et al. Neoadjuvant chemotherapy reinforces antitumour T cell response in urothelial urinary bladder cancer. Eur. Urol. 2018;74(6):2–6.
- Zirakzadeh AA, Kinn J, Krantz D, et al. Doxorubicin enhances the capacity of B cells to activate T cells in urothelial urinary bladder cancer. Clin. Immunol. [Internet]. 2017;176:63–70.
- Hu J, Kinn J, Zirakzadeh AA, et al. The effects of chemotherapeutic drugs on human monocyte-derived dendritic cell differentiation and antigen presentation. Clin Exp Immunol. 2013;172(3):490–499.
- Opelz G, Sengar D, Mickey M, et al. Effect of blood transfusions on subsequent kidney transplants. Transplant. Proc. 1973;5(1):253–259.
- Jewett HJ. Proceedings: cancer of the bladder. Diagnosis and staging. Cancer. 1973;32(5):1072–1074.
- Scosyrev E, Trivedi D, Messing E. Female bladder cancer: incidence, treatment, and outcome. Curr. Opin. Urol. 2010;20(5):404–408.
- Caro JJ, Salas M, Ward A, et al. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer [Internet]. 2001;91(12):2214–2221.
