Abstract
Objective
Tumour associated trypsin inhibitor (TATI) is a peptide that is a marker for several tumours. TATI may also behave as an acute phase reactant in severe inflammatory disease. Overexpression of TATI predicts an unfavourable outcome for many cancers. This study aimed to evaluate the prognostic value of pre- and postoperative concentration of TATI in serum (S-TATI) of patients with renal cell carcinoma (RCC).
Materials and methods
S-TATI was determined by time resolved immunofluorometric assay in preoperative and postoperative samples that were collected from 132 RCC patients, who underwent partial or complete nephrectomy in Helsinki University Hospital from May 2005 to July 2010.
Results
Preoperative S-TATI was significantly associated with tumour stage, lymph-node involvement, metastatic stage, Chronic Kidney Disease Stage (CKD grade), and preoperative C-reactive protein level (p < 0.05). Postoperative S-TATI was significantly associated only with CKD grade (p < 0.001). Multivariate Cox regression analysis of postoperative S-TATI, as a continuous variable, was an independent prognostic factor for overall survival (HR = 1.01, 95% CI = 1.00−1.01, p = 0.03) and cancer-specific survival (CSS) (HR = 1.01, 95% CI = 1.00−1.02, p = 0.004).
Conclusions
Our data suggest that elevated postoperative S-TATI may be associated with adverse prognosis in RCC patients.
Introduction
Renal cell carcinoma (RCC) is the most lethal urological malignancy. Merely 49% of all RCC patients survive beyond 5 years [Citation1]. To decide who benefits from nephrectomy or more complication-prone partial nephrectomy, there is a need to estimate the survival of each patient. The postoperative follow-up for local RCCs aims to find these 20–40% of RCC patients, operated on for curative intention, whose disease recur [Citation2]. Abundance of prognostic nomograms combining laboratory parameters, clinical, anatomical and histopathological features exist to predict the likelihood of progression or mortality of patients with local and advanced RCC [Citation3,Citation4]. Despite the surge for optimum prognostic markers, the estimates of survival and recurrence still rely on the Tumor, Node, Metastasis (TNM) classification, being the most robust predictor of adverse oncological outcomes. Currently, no serum biomarker for RCC is recommended for routine diagnostic or monitoring use in clinic [Citation5].
Tumour-associated trypsin inhibitor (TATI), also called serine peptidase inhibitor Kazal-type 1 (SPINK1) or pancreatic secretory trypsin inhibitor (PSTI), was identified in the urine of an ovarian cancer patient [Citation6]. A single gene on chromosome 5, SPINK1, encodes TATI [Citation7].
The reference values for serum of TATI (S-TATI) in a healthy adult were 3–16 µg/l, corresponding to 0.5–2.6 nmol/l [Citation7]. A cut-off of 16 µg/l has been shown to be a useful threshold for predicting a prognosis in patients with RCC [Citation8,Citation9]. S-TATI may be elevated in patients with various malignancies. S-TATI may also be elevated by reduced kidney function (GFR < 60 ml/min) [Citation7,Citation10,Citation11] and by severe inflammatory diseases [Citation12,Citation13].
S-TATI seems to predict survival in several cancers [Citation7,Citation14–16]. An increase in S-TATI levels after radically intended surgery of ovarian cancer predicts the recurrence and occurrence of metastases [Citation16]. A recent study by Peltonen et al. [Citation15] reported that S-TATI, taken at 3 months after resection of liver metastasis of colorectal carcinoma, was not associated with shorter disease-free survival or overall survival (OS). Preoperatively elevated S-TATI determinations have been shown to predict shorter survival in patients with RCC [Citation8]. The prognostic value of postoperative S-TATI of RCC patients, however, has not been studied.
Preoperative and postoperative identification of patients at high risk of cancer recurrence is of paramount importance for choosing the optimal follow-up protocol and adjuvant treatment [Citation17]. In the present study, we evaluated the prognostic significance of preoperative and postoperative serum concentration of TATI in patients with RCC.
Materials and methods
Patient data
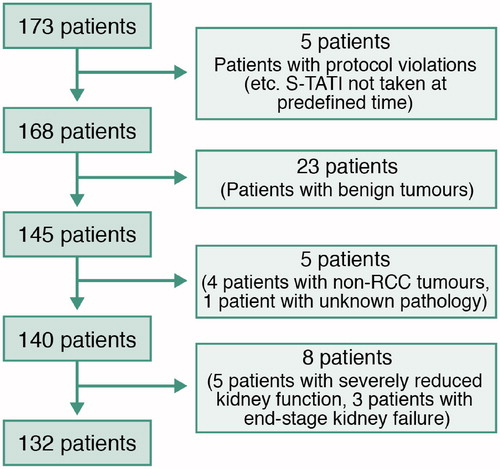
After we received institutional review board approval (The Ethics Committee of Helsinki University hospital, permission number 8/28/1/2004), patients that had been diagnosed with a radiological mass suspected to be a renal tumour and scheduled to be operated on in Helsinki University Hospital (HUCH) from May 2005 to July 2010 were asked to participate in the study. Patients with a renal tumour and who had provided their written informed consent were included in the study. No exclusions based on patient or tumour anatomical characteristics were applied at the time of recruitment. Serum samples were collected from 173 patients before and at a minimum of 3 weeks after the operation. Patients, whose final pathology was benign or non-RCC cancer, were excluded. As described before by Tramonti et al. [Citation10,Citation11], increased S-TATI values have been associated with reduced kidney function. After confirming the correlation between Chronic Kidney Disease Stage (CKD) grades and S-TATI, five patients (4%) with CKD grade 4 and three patients (2%) with CKD grade 5 postoperatively were excluded from the analysis of postoperative samples. Moreover, patients with severely reduced renal function preoperatively (CKD grade > 4) were excluded from analysis of preoperative samples. None of the patients had acute pancreatitis or any acute infection at the time of TATI measurement. After these exclusions, a total of 132 patients treated with radical or partial nephrectomy for RCC were included in the study-group ().
Histology was based on the WHO 2004 classification [Citation18] and the tumors were staged according to the TNM 2009 classification [Citation19]. For statistical analyses, the Fuhrman grade was dichotomized (grades 1−2 and 3−4 combined) as some studies indicate the Fuhrman grading system to be a better prognostic factor when grouped [Citation20]. The T-stage was correspondingly dichotomized into local T-stages (T-stage 1−2) and locally advanced stages (3−4). Preoperative assessment of metastatic status was based on computed tomography of the thorax, abdomen, and pelvis. Pathological lymph node status was evaluated either radiologically or confirmed pathologically by lymphadenectomy.
Patients were followed at the HUCH outpatient department with routine follow-up that included CT and MRI imaging at 6–12-month intervals. Information on disease progression, survival, and official cause of death certificates up to June 2017 were obtained from the HUCH patient registry and Statistics Finland. No patients were lost to follow-up.
Blood samples and serum assay protocols
Blood samples for serum analyses were drawn at a median of 1.0 day (range = 0−50 days) prior to and at a median of 52 days (range = 25−176 days) after surgery. Serum was stored at −20 °C before assay of S-TATI by time-resolved immunofluorometry (TR-IFMA), as previously described [Citation21].
Statistical analyses
Statistical analyses were performed using SPSS® Statistics software (Version 24; IBM Corp., Armonk, NY). The S-TATI concentrations in patients with various disease stages and grades were non-normally distributed, therefore they were analyzed by non-parametric tests. Correlations between S-TATI, as a continuous variable, and clinical parameters were calculated using Spearman’s rho, Mann-Whitney U-test and Kruskal-Wallis test when appropriate. Differences in median pre- and postoperative TATI measurements were analysed using the Wilcoxon signed-rank test.
Cancer-specific survival (CSS), OS and disease recurrence were analysed by the Kaplan-Meier method. Statistically significant covariates in univariate analysis (UVA) were also studied by multivariate analysis (MVA) with the Cox proportional hazards model. A two-sided p < 0.050 was considered statistically significant.
Results
Patient and clinical characteristics
The clinical characteristics of the patients at the time of diagnosis are presented in . The median age was 64 years (range = 24−87). Eighteen patients (14%) underwent partial nephrectomy and 114 (86%) radical nephrectomy. Fifty-eight patients (44%) had local disease (T1a−T2c) and 74 (56%) locally advanced disease (T3a−T4). Fourteen (11%) had lymph node involvement and 25 (19%) had metastatic disease. A majority of the patients (n = 114, 86%) had clear-cell renal cell carcinoma (ccRCC).
Table 1. Table of demographic and histological features of patients and tumours.
The median concentration of S-TATI was 13.0 µg/l preoperatively (interquartile range (IQR) = 9.9−19.5) and 18.3 µg/l postoperatively (IQR = 13.6−24.3) for all patients (Wilcoxon signed-rank test, Z = −5.70, p < 0.001). Postoperative median S-TATI value was 16.9 µg/l (IQR = 13.5−23.2) for patients who underwent tumour excision with radical intent.
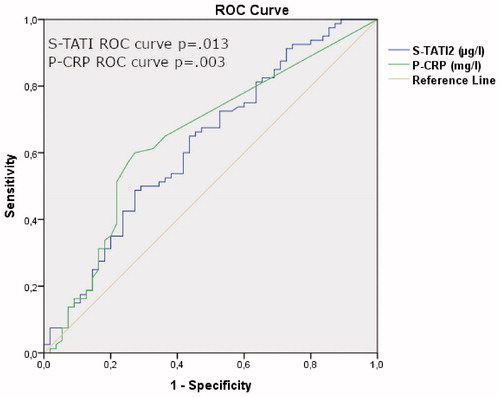
Based on ROC analysis the optimal cut-off is 16.5 µg/l. The AUC value for prediction of OS was 0.633 for S-TATI (p = 0.01) and 0.655 for P-CRP (p < 0.001) for all patients ().
Figure 2. ROC curve analysis. ROC curve analysis of S-TATI and P-CRP for overall mortality of RCC patients.
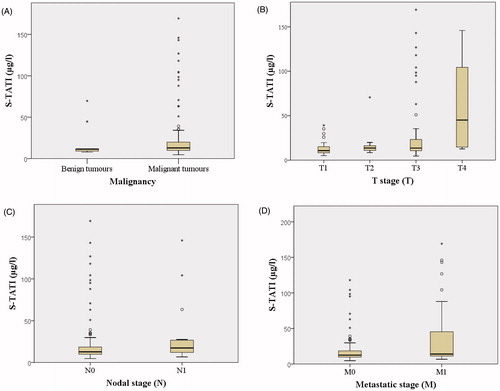
As a continuous variable, preoperative S-TATI was significantly associated with T-stage (p = 0.001), lymph-node involvement (p = 0.04), metastatic stage (p = 0.04), CKD grade (p < 0.001) and preoperative CRP level (p = 0.01), but not with Fuhrman grade (p = 0.08). Preoperative S-TATI was not significantly associated with malignancy (p = 0.08). The distribution of preoperative S-TATI measurements in benign and malignant diseases are presented in . Postoperative S-TATI was associated significantly only with CKD grade (p < 0.001).
Figure 3. Distribution of S-TATI according to disease. Box plots displaying s-TATI distribution according to malignancy (a), tumour stage (b), nodal stage (c) and metastatic stage (d). Boxes show 25th, 50th and 75th percentiles, whiskers show 5th and 95th percentiles. Outliers are represented with circles while black stars represent extreme outliers. Figure (a) includes benign tumours, but other figures include only malignant tumours.
Median serum creatinine concentration was 78 µmol/l (IQR = 64−90) preoperatively and 105 µmol/l (IQR = 91−125) postoperatively. As a continuous variable, postoperative S-TATI concentrations correlated with CKD grade (rho = 0.52, p < 0.001) better than with glomerular filtration rate (GFR) (rho = 0.50, p < 0.001) or creatinine concentration (rho = 0.37, p < 0.001).
Survival analysis
The median follow-up time after operation was 84 months (range = 3−153 months) for the whole study group and 97 months (range = 5−153 months) for N0M0 patients. At the end of the follow-up, 55 patients (41.4%) were alive and 78 (58.6%) were dead. Forty-one patients (53%) died of RCC and 37 (47%) of other causes.
UVA and MVA of OS expressed as hazard ratios and TATI as a continuous variable are presented in . For OS, patient age, N-stage, M stage, T stage and postoperative S-TATI were significant in UVA. In MVA, postoperative S-TATI remained significant along with age, lymph-node involvement and metastatic state.
Table 2. Univariate and multivariate analyses of postoperative S-TATI, other prognostic factors and RCC overall survival.*
For CSS postoperative S-TATI, N-stage, M-stage and T-stage were statistically significant for UVA. In MVA, postoperative S-TATI remained significant along with N-stage, M-stage and T-stage ().
Table 3. Univariate and multivariate analyses of postoperative S-TATI, other prognostic factors and RCC cancer-specific survival.*
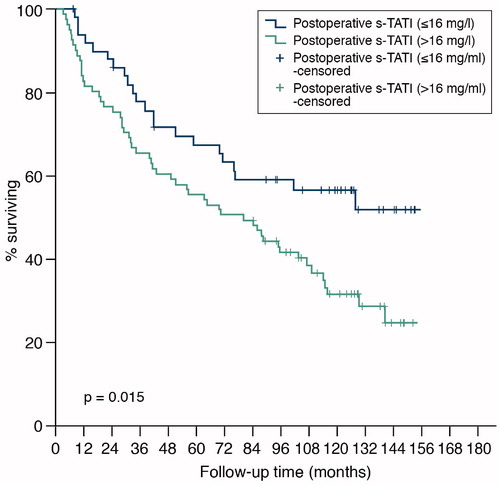
As a dichotomized variable with a cut-off > 16 µg/l, postoperative S-TATI was also a statistically significant predictor of OS in Kaplan-Meier survival analysis (log-rank test p = 0.02) () and in Cox univariate logistic regression (HR = 1.83, 95% CI = 1.12−3.00, p = 0.02). In MVA, only age (HR = 1.03, 95% CI = 1.01−1.05, p = 0.007), N-stage (HR = 3.29, 95% CI = 1.63−2.35, p = 0.001) and M-stage (HR = 2.48, 95% CI = 1.34−4.60, p = 0.004) remained significant predictors for OS.
Figure 4. Overall survival of 132 patients with renal cell carcinoma by concentration of postoperative s-TATI.
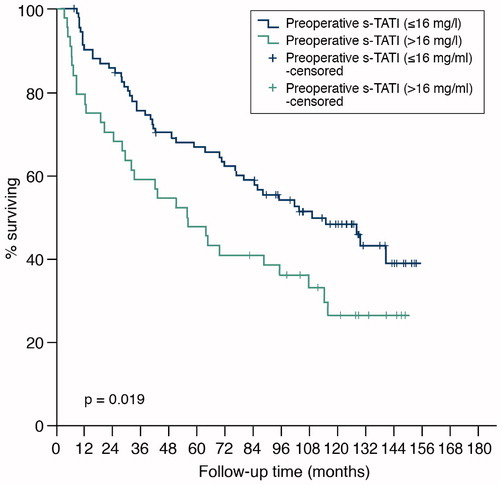
With a cut-off of 16 µg/l, the preoperative concentration of S-TATI was also shown to predict both CSS and OS in Kaplan-Meier (p = 0.04 for CSS and p = 0.02 for OS) () and Cox UVA (HR = 1.91, 95% CI = 1.04−3.52, p = 0.04 for CSS and HR = 1.71, 95% CI = 1.09−2.69, p = 0.02 for OS).
Figure 5. Overall survival of 136 patients with renal cell carcinoma by concentration of preoperative s-TATI.
Furthermore, the Cox proportional hazards model was run with no exclusions based on kidney dysfunction (n = 140). The postoperative S-TATI was able to predict CSS significantly both in univariate analysis with COX proportional hazards model (p = 0.026, HR = 1.007, 95% CI = 1.001–1.014) and in multivariate analysis (p = 0.007) together with N-stage (p = 0.001), M-stage (0.001) and T-stage (p = 0.01). Without exclusions for kidney dysfunction, postoperative S-TATI failed to predict OS significantly.
Disease recurrence
Of 101 patients operated on with radical intention, 26 (26%) had disease recurrence during follow-up. The histological subtype of these was ccRCC. Recurring patients had a median postoperative S-TATI of 18.3 µg/l (IQR = 14.5−20.2 µg/l). The median follow-up time to recurrence was 13 months (range = 2−112 months). A postoperative S-TATI cut-off limit of 16 µg/l did not significantly predict recurrence (p = 0.2) in patients with ccRCC.
Discussion
Our results show that S-TATI concentrations significantly predict OS and CSS both as dichotomized and as continuous variables. Continuous variables are essential when evaluating and implementing new biomarkers, as they are less vulnerable to statistical errors [Citation22]. However, dichotomized variables, with clear cut-off thresholds, are useful for clinicians when planning the follow-up scheme. To the best of our knowledge this is the first study in which S-TATI has been evaluated as a continuous variable during follow-up of RCC patients in a post-nephrectomy setting.
Previous studies have shown that preoperative S-TATI with a cut-off value of 16 µg/l predicted OS and CSS in RCC, although only in UVA [Citation8]. In line with previous studies, the preoperative S-TATI in our study population was also a significant prognostic factor with a cut-off point of 16 µg/l.
The carcinogenic pathways that affect TATI expression in RCC are not well understood. S-TATI concentrations increase with increasing stage of RCC [Citation8]. As a continuous variable, the preoperative S-TATI concentration also correlated with TNM-stage and with the preoperative C-reactive protein concentration, whereas postoperative S-TATI did not. S-TATI should be considered as an addition to established prognostic factors. Postoperative S-TATI may improve postoperative prognostication beyond traditional factors for patients with localized RCC.
Postoperatively, S-TATI concentrations may become elevated due to surgical stress or inflammation as S-TATI may behave as an acute phase reactant in severe inflammatory disease and trauma [Citation23]. However, S-TATI has been shown to normalize within 2 weeks after tissue injury [Citation12,Citation13]. TATI, a 6 kDa peptide, is eliminated through the kidneys and consequently serum concentrations are elevated in patients with markedly impaired renal function. Likewise, our analyses confirmed postoperative S-TATI measurements and CKD grade to be significantly associated. Radical and partial nephrectomy are known to impair renal function [Citation24,Citation25] and improvements in renal function tend to continue past the first postoperative year [Citation26]. Thus, patients with severely impaired renal function were excluded from our study and postoperative serum samples were taken after a minimum of 25 days after the operation to minimize the confounding effect of impaired kidney function and tissue injury. A previous study by Lukkonen et al. [Citation9] did not exclude any patients due to kidney deficiency; however, they tested only preoperative S-TATI.
To confirm the effect of kidney dysfunction on the prognostic impact of S-TATI, we tested S-TATI without exclusions based on renal dysfunction, and postoperative S-TATI was able to predict CSS significantly in multivariate analysis. However, post-operative S-TATI could not predict overall survival. As end-stage renal disease carries a high risk of death and long-term haemodialysis shortens the life-expectancy with RCC patients [Citation27], there are several confounding factors when it comes to overall mortality. With such a limited patient material, we could not evaluate factors possibly affecting the overall mortality of ESRD patients. Therefore, without further studies, we cannot recommend the use of postoperative S-TATI as a prognostic marker for ESRD patients. Based on current knowledge, postoperative S-TATI can be used as an additional prognostic marker for patients with CKD grade 1–3.
We expected elevated concentrations of TATI after surgery of N0M0 tumours to indicate the presence of an otherwise undetectable tumour and be a prognosticator for early recurrence. However, postoperative TATI with a cut-off limit of 16 µg/l did not significantly predict the recurrence in patients operated on for N0M0 disease in the present study. No firm conclusions regarding recurrence can be drawn from this subset of patients because of the limited sample size of recurring patients (n = 26) in our study.
There is a growing need for biomarkers that, in combination with clinico-pathological risk algorithms, can discriminate between indolent and aggressive renal cell cancers. There are limitations with the use of current risk algorithms. No serum biomarker is currently available for postoperative follow-up of RCC patients. Biomarker-based postoperative monitoring is continuously studied using various markers [Citation28,Citation29]. A clinically applicable biomarker would optimally be easy to measure and inexpensive to use. Our results indicate that S-TATI might have the potential to be integrated in currently used postoperative follow-up protocols. In the future, TATI has been suggested to have the potential to become a target molecule for cancer therapy, emphasizing its importance also in RCC [Citation30].
Limitations of this study include a relatively small sample size that yielded a small number of ccRCC patients with tumour recurrence. We did not perform serial S-TATI measurements and postoperative S-TATI samples were not taken at the same fixed time-point after surgery, which limits the interpretation of our findings. If the results of this study can be validated, then the postoperative concentrations of S-TATI might facilitate detection of otherwise non-detectable residual tumour tissue and also to predict both recurrence and mortality in RCC patients without severe kidney dysfunction. In the future, comparison between S-TATI and other cost-effective and easy-to-use prognostic markers predicting survival and recurrence, such as neutrophil-to-lymphocyte ratio [Citation31] or platelet-to-lymphocyte ratio [Citation31], would be of utmost interest.
Conclusions
Our results suggest that postoperative S-TATI could be a useful prognostic marker in RCC either as an independent biomarker or when used in combination with clinical and histological parameters.
Acknowledgements
The authors would like to thank statisticians Taija Alatalo and Paula Bergman for their assistance in the statistical analyses.
Disclosure statement
Kilpeläinen and Visapää have no conflict of interest to declare. Tornberg declares unrestricted financial grants from the Finnish Urological Association and reimbursements from SwanMedica for attending a scientific meeting. Järvinen P. declares a fee from Elypta, outside the submitted work, Taari declares research funding from Medivation/Astellas/Pfizer, Orion and Myovant, outside the submitted work. Järvinen R. declares research funding from Photocure, Bristol Myers Squibb, Roche and Nektar, outside the submitted work. Nisen has received travel grants from Olympus, Novartis, Pfizer, Astellas-Pharma, consultation fees from Ipsen, Pfizer and a fee from Elypta, outside submitted work. U-H Stenman is the holder of a patent for TATI, which has expired.
Additional information
Funding
References
- Wahlgren T, Harmenberg U, Sandstrom P, et al. Treatment and overall survival in renal cell carcinoma: a Swedish population-based study (2000–2008). Br J Cancer. 2013;108(7):1541–1549.
- Janzen NK, Kim HL, Figlin RA, et al. Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am. 2003;30(4):843–852.
- Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97(7):1663–1671.
- Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Cin Oncol. 2009;27(34):5794–5799.
- Ljungberg B, Albiges L, Abu-Ghanem Y, et al. European Association of Urology guidelines on renal cell carcinoma: the 2019 update. Eur Urol. 2019;75(5):799–810.
- Huhtala ML, Pesonen K, Kalkkinen N, et al. Purification and characterization of a tumor-associated trypsin inhibitor from the urine of a patient with ovarian cancer. J Biol Chem. 1982;257(22):13713–13716.
- Itkonen O, Stenman UH. TATI as a biomarker. Clin Chim Acta. 2014;431:260–269.
- Paju A, Jacobsen J, Rasmuson T, et al. Tumor associated trypsin inhibitor as a prognostic factor in renal cell carcinoma. J Urol. 2001;165(3):959–962.
- Lukkonen A, Lintula S, von Boguslawski K, et al. Tumor-associated trypsin inhibitor in normal and malignant renal tissue and in serum of renal-cell carcinoma patients. Int J Cancer. 1999;83(4):486–490.
- Tramonti G, Ferdeghini M, Donadio C, et al. Serum levels of tumor associated trypsin inhibitor (TATI) and glomerular filtration rate. Ren Fail. 1998;20(2):295–302.
- Tramonti G, Ferdeghini M, Donadio C, et al. Tumor-associated trypsin inhibitor (TATI) and renal function. Kidney Int Suppl. 1997;63:S179–S81.
- Ogawa M, editor Serum Pancreatic Secretory Trypsin Inhibitor as a Sensitive Marker of Tissue Damage. The Strangeways Research Laboratory 75th Anniversary Symposium; 1987 the 6–8th of April 1987; Cambridge: Arthritis & Rheumatism.
- Ogawa M. Pancreatic secretory trypsin inhibitor as an acute phase reactant. Clin Biochem. 1988;21(1):19–25.
- Paju A, Vartiainen J, Haglund C, et al. Expression of trypsinogen-1, trypsinogen-2, and tumor-associated trypsin inhibitor in ovarian cancer: prognostic study on tissue and serum. Clin Cancer Res. 2004;10(14):4761–4768.
- Peltonen R, Osterlund P, Lempinen M, et al. Postoperative CEA is a better prognostic marker than CA19-9, hCGβ or TATI after resection of colorectal liver metastases. Tumour Biol. 2018;40(1):1010428317752944.
- Kozakiewicz B, Chądzyńska M, Dmoch-Gajzlerska E, et al. Monitoring the treatment outcome in endometrial cancer patients by CEA and TATI. Tumour Biol. 2016;37(7):9367–9374.
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375(23):2246–2254.
- Eble JL, Sauter G, Epstein JI, et al. Pathology and genetics of the tumours of the genitourinary system and male genital organs. WHO classification of tumours. 2004 ed. Lyon: IARC; 2004.
- Sobim LG, Wittekind C. UICC International Union against cancer. TNM classification of malignant tumours. 7th ed. Oxford: Wiley-Blackwell; 2009.
- Ficarra V, Martignoni G, Maffei N, et al. Original and reviewed nuclear grading according to the Fuhrman system: a multivariate analysis of 388 patients with conventional renal cell carcinoma. Cancer. 2005;103(1):68–75.
- Osman S, Turpeinen U, Itkonen O, et al. Optimization of a time-resolved immunofluorometric assay for tumor-associated trypsin inhibitor (TATI) using the streptavidin-biotin system. J Immunol Methods. 1993;161(1):97–106.
- McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J Natl Cancer Inst. 2005;97(16):1180–1184.
- Lasson A, Borgstrom A, Ohlsson K. Elevated Pancreatic Secretory trypsin inhibitor levels during severe inflammatory disease, renal insufficiency, and after various surgical procedures. Scand J Gastroenterol. 1986;21(10):1275–1280.
- Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol. 2012;188(1):51–57.
- Mariusdottir E, Jonsson E, Marteinsson VT, et al. Kidney function following partial or radical nephrectomy for renal cell carcinoma: a population-based study. Scand J Urol. 2013;47(6):476–482.
- Winer AG, Zabor EC, Vacchio MJ, et al. The effect of patient and surgical characteristics on renal function after partial nephrectomy. Clin Genitourin Cancer. 2018;16(3):191–196.
- Tsuzuki T, Iwata H, Murase Y, et al. Renal tumors in end-stage renal disease: a comprehensive review. Int J Urol. 2018;25(9):780–786.
- Tusong H, Maolakuerban N, Guan J, et al. Functional analysis of serum microRNAs miR-21 and miR-106a in renal cell carcinoma. Cancer Biomark. 2017;18(1):79–85.
- Nuerrula Y, Rexiati M, Liu Q, et al. Differential expression and clinical significance of serum protein among patients with clear-cell renal cell carcinoma. CBM. 2015;15(4):485–491.
- Rasanen K, Itkonen O, Koistinen H, et al. Emerging roles of SPINK1 in cancer. Clin Chem. 2016;62(3):449–457.
- Semeniuk-Wojtaś A, Lubas A, Stec R, et al. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and C-reactive protein as new and simple prognostic factors in patients with metastatic renal cell cancer treated with tyrosine kinase inhibitors: a systemic review and meta-analysis. Clin Genitourin Cancer. 2018;16(3):e685–e693.