Abstract
Background
Previous studies have investigated [18F]-fluorocholine (FCH) positron emission tomography with computed tomography (PET/CT) in primary staging of men with intermediate or high-risk prostate cancer and have generally shown high specificity and poor sensitivity. FCH PET/CT is not recommended for the primary staging of metastases in the European guidelines for prostate cancer. However, it has been an option in the Swedish recommendations. Our aim was to assess PET/CT for primary staging of lymph node metastases before robotic-assisted laparoscopic prostatectomy (RALP) with extended pelvic lymph node dissection (ePLND) in patients with intermediate or high-risk prostate cancer.
Method
We identified all men with prostate cancer undergoing FCH PET/CT for initial staging followed by RALP and ePLND at Skåne University Hospital between 2015 and 2018. The result from PET/CT scan was compared with pathology report as the reference method for calculation of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV).
Results
In total, 252 patients were included in the final analysis. Among 85 patients with a suspicion of regional lymph node metastases on FCH PET/CT only 31 had pathology-proven metastases. The sensitivity was 43% (95% CI 0.32–0.55) and the specificity 70% (95% CI 0.63–0.76) for PET/CT to predict lymph node metastases. PPV was 36% and NPV was 75%. Risk group analyses showed similar results.
Conclusion
Our study emphasizes the poor performance of FCH PET/CT to predict lymph node metastasis in intermediate and high-risk prostate cancer. The method should be replaced with newer radiopharmaceuticals, such as prostate-specific membrane antigen ligands.
Background
Prostate cancer is one of the most diagnosed cancers in the world. Treatment options will depend on whether the cancer is organ-confined, i.e. contained within the prostate gland or has spread to regional lymph nodes, skeleton or to other metastatic sites. Most patients with newly diagnosed intermediate or high-risk prostate cancer undergo radiological examinations for staging of the disease with computer tomography and bone scintigraphy as recommended in guidelines [Citation1].
Positron emission tomography fused with computed tomography (PET/CT) is a widely used method for staging of patients with different malignancies. Several radiopharmaceuticals have been used in patients with prostate cancer, and the first tracers used were directed against [18F]-fluorodeoxyglucose (FDG) and later on replaced by [11C]-choline, [18F]-fluorocholine (FCH), [11C]-acetate and [18F]-fluoroacetate [Citation2,Citation3]. Previous studies have shown that FCH PET/CT has a high specificity for the detection of lymph node metastases, but a lower sensitivity [Citation4–7]. FCH PET/CT has not been recommended for primary metastasis staging in the guidelines from the European Association of Urology (EAU); however, it is recommended for detection of metastases in patients with biochemical recurrence [Citation1,Citation8]. The current Swedish National Health Care Program state that high-risk and intermediate-risk prostate cancer with an unfavorable Gleason grade group should be examined for metastatic spread by bone scintigraphy, [18F]-sodium fluoride PET/CT, CT or magnetic resonance imaging (MRI), well aware of certain limitations with each of the methods [Citation9]. PET/CT with new radiopharmaceuticals, such as prostate-specific membrane antigen (PSMA) ligands are only recommended in clinical trials due to lack of studies on its accuracy.
In our county in southern Sweden, Region Skåne, patients with intermediate-high risk prostate cancer have routinely been referred for FCH PET/CT for initial staging for several years and results on its performance have been reported earlier [Citation4]. With access to a larger cohort, we have now retrospectively evaluated the accuracy of FCH PET/CT to detect pelvic lymph node metastases in patients with intermediate or high-risk prostate cancer using histopathology after extended pelvic lymph node dissection (ePLND) as a reference method.
Method and materials
Through the medical record systems at Region Skåne, all patients who had undergone an FCH PET/CT at Skåne University Hospital in Lund or Malmö, followed by robotic-assisted laparoscopic prostatectomy (RALP) with ePLND during 2015–2018 were identified. The study protocol was approved by the Regional Ethics Committee in Lund (#2018/690).
PET/CT imaging
FCH was administered intravenously with a dose of 4 MBq/kg. A PET-CT from the upper thigh to the base of the scull was acquired after an accumulation time of 60 min, using an acquisition time of 2.0 min per bed position. Three different PET-CT systems were used: A Philips Gemini TF (Philips Healthcare, Cleveland, OH, USA), a GE Discovery 690 (GE Healthcare, Milwaukee, WI, USA) or a GE Discovery MI (GE Healthcare, Milwaukee, WI, USA). Time-of-flight was used on all systems. The CT scans were acquired with diagnostic quality, using intravenous and per oral contrast.
The scans were interpreted by one nuclear medicine physician and one radiologist who jointly wrote a clinical report. The clinical report was used in this study in order to assess the presence or absence of suspected lymph node metastases from FCH PET/CT. Focal FCH uptake in pelvic lymph nodes exceeding that of the mediastinal blood pool was considered abnormal. A high uptake overruled the lymph node size in the interpretation. When the clinical report was ambiguous (for example possible but not certain metastases), the patient was regarded as having abnormal lymph nodes in the further analysis.
Surgical technique
Surgery was performed with RALP with bilateral ePLND defined as removal of tissue from the bifurcation of the common iliac artery and distally along the external iliac vessels and deep in the obturator fossa. Tissue from the left and right side was sent separately for histopathological examination. Surgery was performed by four high-volume surgeons using the identical technique.
Histological examination
The specimens were processed according to standard protocols and examined by experienced uro-pathologists. All lymph nodes were counted, and the sizes of pathological lymph nodes and the cancer area were measured.
Statistical analysis
The result from PET/CT scan was compared to the result of histology outcome in lymph node dissection as reference for calculation of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV). Confidence intervals were 95% for respective groups. A p-value less than 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS version 26 (IBM, Armonk, NY, USA).
Results
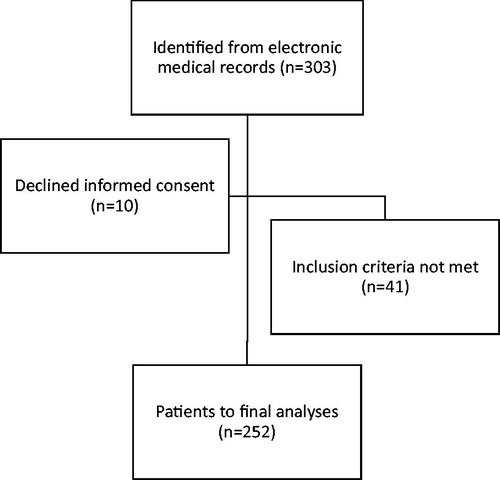
A consecutive series of 303 men diagnosed with prostate cancer with intermediate or high-risk prostate cancer underwent RALP with ePLND surgery with prior FCH PET/CT and without prior treatment for prostate cancer. After exclusion for different reasons as given in , a total of 252 patients remained for final analyses.
Characteristics of the 252 patients are presented in . The median age was 68 years and the median preoperative PSA concentration in blood was 9 mg/mL. Patients were sub-grouped according to the D’Amico risk classification. Most patients had a palpable tumour prior to surgery with clinical stage T2 (51.6%) and (11.1%) patients had locally advanced disease at stage T3–T4. The most common Gleason score was 9–10, ISUP grade 5 (26.2%). During ePLND, a total of 6049 lymph nodes were obtained from all patients with a median number of 20 (range 1–83). Metastases were detected in 170 (2.8%) of the removed lymph nodes ().
Table 1. Characteristics of patients.
Table 2. Characteristics of lymph nodes removed at surgery (ePLND).
The results of FCH PET/CT and ePLND are summarized in . Among the 252 patients, 85 (34%) had a suspicion of lymph node metastases on PET/CT but only 31 (12%) of those had proven metastases at the surgery. Thus 54 patients (21%) were false positive. A similar distribution was noted in the high-risk group. Outcome prediction of PET/CT was better in the intermediate-risk group where PET/CT scan was true positive according to pathology in 7 out to 20 patients (35%) and 10% false positive (7/70). Only 20 were classified as low-risk which makes statistical analyses less reliable in this group.
Table 3. Lymph node status by histopathology and by PET/CT.
In the group of 167 patients with negative PET/CT scan, as many as 41 (25%) had metastases at histopathological examination. All risk groups showed similar results.
The number of patients with pathology-proven lymph node metastases was 72 (29%) and PET/CT scan missed the metastases in 41 among these patients (57%). Risk group stratification revealed that PET/CT did not detect metastases in 56% (27/48) of patients in the high-risk group and in 41% and 42% in the intermediate and low-risk groups, respectively.
The calculated sensitivity for PET/CT to predict lymph node metastases was 43% and the specificity 70%, PPV 36% and NPV 75% (). The results were similar in the high-risk and intermediate-risk groups. Analyses in the low-risk group must be interpreted with caution due to the limited number of events.
Table 4. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for prediction of lymph node metastases by PET/CT (95% confidence interval) with histopathology report as reference method.
Discussion
The main objective of this study was to evaluate the diagnostic performance of FCH PET/CT for detection of pelvic lymph node metastases for initial staging of intermediate and high-risk prostate cancer patients, using histopathology from ePLND as a reference method. We found a relatively low sensitivity (43%) and specificity (70%). Based on our results, FCH PET/CT does not reach clinically acceptable diagnostic accuracy for detection of lymph node metastases, or to rule out a nodal dissection based on risk factors or nomograms. Poor performance was observed across different risk groups ( and ). FCH is not specific for prostate cancer and can also show increased uptake in benign pathological conditions such as inflammation [Citation10].
Previous studies have in general shown higher specificity compared with our study, whereas sensitivity has varied. Kjölhede et al. [Citation4] found a specificity of 92% and a sensitivity of 33% for lymph node metastases within the ePLND template in high-risk prostate cancer patients, using patients in the same part of Sweden as in this study, but earlier (2008–2011). Three different meta-analyses have found a sensitivity of 49%, 62%, 84% and specificity of 95%, 92%, and 79%, respectively [Citation5–7]. Similar results with a high specificity but lower sensitivity have been reported for [11C]-acetate [Citation11]. Different selection of patients, different PET-CT systems and different criteria when interpreting the PET-CT images might explain the different results obtained. The present investigation is retrospective but it represents a ‘real-world study’ as opposed to a randomized clinical trial. In studies regarding treatment, the results obtained in the randomized clinical trials are often different (often higher treatment efficacy) compared with subsequent studies when the treatment is used in the real world. This has been attributed to the very strict inclusion and exclusion criteria of patients for randomized clinical trials. One can speculate that the same is true for studies of diagnostic methods, due to both different inclusion/exclusion criteria for patients as well as less strict criteria used for image interpretation. Real-world studies have in recent years become increasingly important in health science, as an adjunct to clinical randomized trials [Citation12–14].
Our study emphasizes the poor performance of FCH PET/CT to predict lymph node metastasis in prostate cancer and should be compared with results from PMSA PET/CT. [68Ga]- or [18F]-PSMA PET/CT is increasingly used, since it has demonstrated superior performance compared with FCH PET/CT as well as other imaging modalities, in particular in biochemical recurrence at low PSA levels [Citation15–20]. So far, a limited number of studies exist that investigate the diagnostic performance of PSMA PET/CT with histopathology as the reference method for initial staging. Grubmüller et al. [Citation21] prospectively investigated [68Ga]-PSMA-11 PET/MRI before pelvic lymphadenectomy in 80 patients and found a sensitivity of 69% and a specificity of 100%. The metastatic lymph nodes missed by PET were smaller than 4 mm. Another small, prospective study of 30 patients using [68Ga]-PSMA-11 PET/CT found the sensitivity of 64% and a specificity of 95% [Citation22]. A recent meta-analysis of [68Ga]-PSMA-11 PET accuracy for detection of pelvic lymph nodes showed a sensitivity of 74% and a specificity of 96%, using nodal pathology as the gold standard [Citation23].
Limitations
The limitations of the study include the retrospective nature of the study. Management of patients was performed knowing the results of the FCH PET/CT and patients regarded as having extensive metastases were excluded from prostatectomy with ePLND. The analysis could only be performed on a per-patient and per-side level, not on a per-lymph node level, which is another limitation. We did not have information on the size of lymph nodes with metastases.
Conclusions
Our study emphasizes the poor performance of FCH PET/CT to predict lymph node metastasis in prostate cancer. The method should be replaced with newer radiopharmaceuticals, such as PSMA ligands.
Disclosure statement
No financial interest or benefit related to this study.
Additional information
Funding
References
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262.
- Jadvar H. Molecular imaging of prostate cancer with PET. J Nucl Med. 2013;54(10):1685–1688.
- Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52(1):81–89.
- Kjolhede H, Ahlgren G, Almquist H, et al. 18F-fluorocholine PET/CT compared with extended pelvic lymph node dissection in high-risk prostate cancer. World J Urol. 2014;32(4):965–970.
- Umbehr MH, Muntener M, Hany T, et al. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64(1):106–117.
- Evangelista L, Guttilla A, Zattoni F, et al. Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur Urol. 2013;63(6):1040–1048.
- von Eyben FE, Kairemo K. Meta-analysis of (11)C-choline and (18)F-choline PET/CT for management of patients with prostate cancer. Nucl Med Commun. 2014;35(3):221–230.
- Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71(4):630–642.
- Swedish National Health Care Program for Prostate Cancer 2020. Available from: https://kunskapsbanken.cancercentrum.se/globalassets/cancerdiagnoser/prostatacancer/vardprogram/nationellt-vardprogram-prostatacancer.pdf
- Schillaci O, Calabria F, Tavolozza M, et al. 18F-choline PET/CT physiological distribution and pitfalls in image interpretation: experience in 80 patients with prostate e cancer. Nucl Med Commun. 2010;31(1):39–45.
- Daouacher G, von Below C, Gestblom C, et al. Laparoscopic extended pelvic lymph node (LN) dissection as validation of the performance of [(11) C]-acetate positron emission tomography/computer tomography in the detection of LN metastasis in intermediate- and high-risk prostate cancer. BJU Int. 2016;118(1):77–83.
- Tashkin DP, Amin AN, Kerwin EM. Comparing randomized controlled trials and real-world studies in chronic obstructive pulmonary disease pharmacotherapy. Int J Chron Obstruct Pulmon Dis. 2020;15:1225–1243.
- Monti S, Grosso V, Todoerti M, et al. Randomized controlled trials and real-world data: differences and similarities to untangle literature data. Rheumatology (Oxford). 2018;57(57 Suppl 7):vii54–vii8.
- Blonde L, Khunti K, Harris SB, et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774.
- Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70(6):926–937.
- Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol. 2020;77(4):403–417.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208–1216.
- Anttinen M, Ettala O, Malaspina S, et al. A prospective comparison of (18)F-prostate-specific membrane antigen-1007 positron emission tomography computed tomography, whole-body 1.5 T magnetic resonance imaging with diffusion-weighted imaging, and single-photon emission computed tomography/computed tomography with traditional imaging in Primary Distant Metastasis Staging of Prostate Cancer (PROSTAGE). Eur Urol Oncol. 2020. doi: https://doi.org/10.1016/j.euo.2020.06.012.
- Schwenck J, Rempp H, Reischl G, et al. Comparison of 68Ga-labelled PSMA-11 and 11C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44(1):92–101.
- Afshar-Oromieh A, Zechmann CM, Malcher A, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11–20.
- Grubmuller B, Baltzer P, Hartenbach S, et al. PSMA ligand PET/MRI for primary prostate cancer: staging performance and clinical impact. Clin Cancer Res. 2018;24(24):6300–6307.
- van Leeuwen PJ, Emmett L, Ho B, et al. Prospective evaluation of 68Gallium-prostate-specific membrane antigen positron emission tomography/computed tomography for preoperative lymph node staging in prostate cancer. BJU Int. 2017;119(2):209–215.
- Hope TA, Goodman JZ, Allen IE, et al. Metaanalysis of 68Ga-PSMA-11 PET accuracy for the detection of prostate cancer validated by histopathology. J Nucl Med. 2019;60(6):786–793.