Abstract
Objectives
The aim of this study was to assess the incidence of infection after transrectal prostate biopsy (TRbx). Secondary objectives were to describe infection characteristics, antibiotic resistance patterns, ICD-10 coding, and costs.
Methods
TRbx carried out at the hospitals of Ängelholm and Helsingborg, Scania, Sweden, between October 2017 and March 2019, were identified based on the NOMESCO Classification of Surgical Procedures code for TRbx, TKE00. All patients received per oral antibiotic prophylaxis, usually 750 mg ciprofloxacin at biopsy. Other preventative measures were not used. Medical care within 30 days of the biopsy was evaluated through a manual retrospective medical chart review. Data on patient and infection characteristics were collected. The costs of infections causing hospitalization were estimated.
Results
After 36 (5.4%) of 670 biopsies, the patient developed post-biopsy infection within 30 days after TRbx. Twenty-six patients (3.9%) required hospitalization for an average of 6 days, at an estimated direct cost of USD 9174 (EUR 8031) per patient. Nine patients (1.3%) had a complicated infection leading to intensive care, multiple hospitalizations or emergency department visits. The inpatient care episodes for the 26 hospitalized patients were categorized with 15 different ICD-codes. In 6 episodes no ICD-code related to infection was used.
Conclusions
In this study, we found an infection rate of 5.4% after TRbx; 3.9% of the patients were hospitalized for a post-TRbx infection and 1.3% had complicated infections. A specific ICD code for post-TRbx infections would facilitate evaluation and monitoring of this common, costly, and sometimes serious complication.
Introduction
Prostate cancer (PC) is one of the most common cancers, with an estimated 1.28 million new cases worldwide in 2018 [Citation1]. PC is mainly diagnosed and sometimes monitored with repeated prostate biopsies, resulting in approximately 3.8 million biopsies annually in the US and Europe [Citation2,Citation3]. Prostate biopsies are most commonly performed transrectally (TRbx), using a tru-cut biopsy needle to puncture the prostate [Citation4]. The procedure may cause infection by transferring colonic bacteria with the biopsy needle, or by exacerbating an ongoing urinary tract infection. [Citation4–6]. The reported incidence of infection after TRbx is 0.1–13.8% [Citation3,Citation4]. Infections range in severity from mild urinary tract infections to potentially fatal sepsis [Citation6] and are the most common cause of hospitalization after TRbx [Citation7].
Fluoroquinolones (e.g. ciprofloxacin) are generally used for antibiotic prophylaxis in TRbx owing to their broad coverage and high penetration of the prostate [Citation8], although currently questioned because of side effects such as aortic aneurysm [Citation9]. A single dose is often used, but prolonged prophylaxis is recommended for patients with known risk factors for infection [Citation10]. Other preventive measures include rectal cleansing [Citation11,Citation12] and targeted antibiotic prophylaxis based on a pre-biopsy rectal culture [Citation13,Citation14]. Rectal cleansing with povidone-iodine is reported to significantly reduce infection rates, but is an uncomfortable and user-dependent procedure [Citation11,Citation12].
Despite the use of these preventative measures, the incidence of post-TRbx infections is rising [Citation3,Citation7,Citation15,Citation16]. For instance, a study of over 75,000 patients showed an increase in infections from 0.6% to 3.6% between 1996 and 2005 [Citation7]. The observed rise of infection rates is to a large part caused by the increasing antibiotic resistance among colonic bacteria; in 2015 it was estimated that 42% of post-TRbx infections in the US were caused by fluoroquinolone resistant bacteria [Citation17]. However, because of the wide range in reported infection rates and varying definitions of infection, it is challenging to compare results across studies and over time.
In this study, the primary aim was to evaluate the rate of post-TRbx infections. Secondary aims were to evaluate the severity, treatment, characteristics, and costs of post-Trbx infections. We used a rigorous method based on a medical chart review to identify post-TRbx infections at two hospitals serving a geographically defined population. As ICD-10 codes [Citation18] are the basis for register-based studies, we also evaluated what codes were used following TRbx infections.
Materials and methods
TRbx identification
We included all transrectal prostate biopsies between October 2017 and March 2019 at two Swedish hospitals serving a geographically defined population of about 300,000 people (Helsingborg and Ängelholm hospitals). We set the time of 18 months inclusion prior to study start, as we estimated that this would yield around 650 patients, which would be adequate for calculating the rate of post-biopsy infections with a reasonable statistical precision. The biopsies were identified through the electronic medical record by a search for the NOMESCO Classification of Surgical Procedures (NCSP) code TKE00 in the Melior medical record system. A qualified medical secretary conducted the search providing a defined list of patients who underwent prostate biopsies at the two hospitals. We did not include biopsies carried out by private practitioners, which we estimate to have been around 150 during the study period. We only had access to the hospital charts in Region Skåne and not data from general practitioners or company health care journals. No analytical methods were applied to account for potential missed cases from these sources.
Outcomes
The primary outcome measure was clinical infection after TRbx. Clinical infection was defined as fever (≥38 °C) or a positive blood or urine culture within 30 days of TRbx, in combination with manual chart review with clinical suspicion of the infection arising from the urinary tract.
Urinary tract symptoms without fever (≥38 °C) or a positive culture alone were not considered as infection. Likewise were infections with other primary focuses such as airways or bowel were excluded. We did not use antibiotic prescriptions as an indication of infection. Furthermore, emergency department (ED) visits and hospital admissions for other reasons than infection were excluded. Sepsis was defined as sequential organ failure assessment (SOFA) score ≥2 [Citation19]. Patients with infection were further divided into three categories based on severity: (1) patients not requiring hospitalization (2) patients requiring hospitalization who recovered at their first admission (3) patients with complicated infections requiring intensive care unit (ICU) treatment, repeated hospitalizations or repeated ER visits for recurring infection after hospital discharge. Secondary outcome measures were infections leading to hospital admission, infections leading to admission to an ICU, infection characteristics, antibiotic resistance patterns and clinical path of patients with infection, as well as ICD-10 codes [Citation18] used for hospitalized patients.
Patient data
The following information was retrieved from the medical charts for each patient: date and hospital for TRbx, age, prostate volume, PSA value, biopsy result (cancer or no cancer), comorbidity (heart failure, cardiac arrhythmias, previous heart attack, chronic obstructive pulmonary disease, or kidney failure; evaluated based on diagnoses and medical treatments/prescriptions), immunosuppressing treatment, number of previous TRbx, previous infection after TRbx, and type and duration of antibiotic prophylaxis. A randomized controlled study of antibiotic prophylaxis with either single-dose 750 mg Ciprofloxacin or 800/160 mg Trimethoprim/sulfamethoxazole was ongoing at both hospitals during the studied time period; 93 of the included patients were included in the present study. As the antibiotic prophylaxis was blinded in the randomized study, it is unknown for these 93 patients.
Infection data based on ICD-10 codes
Data on inpatient care, bacterial resistance patterns in blood and urine cultures, ICD-10 codes at discharge, and deaths within 30 days after biopsy were collected in the medical chart review. Multiple ICD-10 codes were used for some of the inpatient episodes. Infection-related codes are presented if present. Primary codes are reported for discharges without infection-related codes.
Cost analysis
The cost of inpatient care was based on the average daily costs for health care-associated infections in Region Skåne, Sweden (12,544 SEK in 2015) [Citation20]. The cost was adjusted for inflation (6.7% between 2015 and 2019) and converted to USD (8.75 SEK/USD) and to EUR (10.00 SEK/EUR), resulting in a daily inpatient care cost of USD 1529 or EUR 1338. We did not calculate indirect costs such as loss of work days or need for home care.
Statistics
Continuous data are reported as median and range. Categorical data are reported as numbers and percentages and 95% confidence intervals.
Ethics
This study was approved by the Swedish Ethical Review Authority (dnr 2019-00712). Owing to the study design patient consent was waived.
Results
Patient characteristics
Six-hundred-seventy (670) TRbx procedures in 566 men were identified by the NCSP code TKE00 (). Median age at the time of biopsy was 69 years, median prostate volume was 44 cm3, and median PSA level 7 µg/l (). Cancer was present in 60% of the biopsies. Twelve of the patients had previously developed an infection following a prostate biopsy. All these 12 patients received extended prophylaxis; one of them had a new infection.
Figure 1. Identification of patients with infection after biopsy. Data on primary care visits for reasons unrelated to post-biopsy infection was not collected. ED: emergency department; PO: per oral; TRbx: transrectal prostate biopsy.
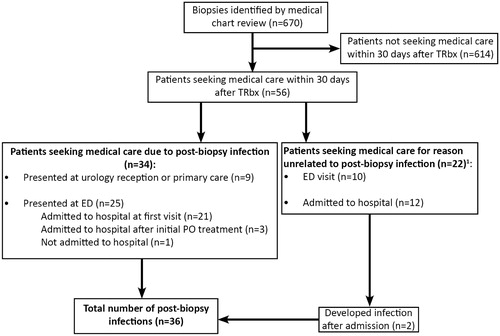
Table 1. Patient characteristics and potential risk factors.
Rate of post-biopsy infections
Fifty-six TRbx procedures (8.4%) led to the patient seeking medical care within 30 days. We found a total number of 36 infection episodes after TRbx (5.4% of the 670 TRbx procedures), of whom 26 (3.9%) were admitted to hospital. Nine patients came with infectious symptoms to the urology outpatient clinic or to primary care, and were thereafter managed in outpatient care. Twenty-six patients were hospitalized; details are described in .
Antibiotic prophylaxis
The type and duration of antibiotic prophylaxis are shown Supplementary Table 1. Most patients (77%; n = 516) received antibiotic prophylaxis on the day of the biopsy only. The Swedish guidelines recommend a single dose of Ciprofloxacin (750 mg) at the time of biopsy in patients without risk factors for post-TRbx infection, such as previous post-biopsy infection, diabetes, or catheterization [Citation21]. The infection rates for patients who received 1-day antibiotic prophylaxis (single or two doses) and those who received prolonged prophylaxis (≥2 days) were similar (Supplementary Table 1). Targeted antibiotic treatment was given to the 3% of patients who had a positive urine culture prior to TRbx.
Infection characteristics
Median antibiotic treatment time was 12.5 days, ranging from 5 to 63 days (; Supplementary Figure 1). Median time between biopsy and first ED visit was 2 days (0–11 days). Twenty-six patients were admitted to hospital for a median of 5 days (2–14 days, ). Five of them fulfilled sepsis criteria (SOFA-score ≥ 2) and one required ICU treatment. Four patients were readmitted because of recurring infection and 2 patients had repeated ED visits after hospitalization for the same reason. No patients died because of a confirmed post-biopsy infection, but one elderly patient was found dead at home 12 days after biopsy with unknown cause of death.
Table 2. Infection characteristics.
Bacterial cultures in infected patients
Positive bacterial cultures were found in 28 of 36 patients with a post-biopsy infection (), of which 26 had a positive urine culture and 9 had a positive blood culture. Escherichia coli (E. coli) was by far the most common pathogen and was found in 23 of 28 cultures (82.1%). Other detected bacteria included Klebsiella pneumoniae, Citrobacter koseri, Enterobacter cloacae and Enterococcus faecalis. Fourteen (61%) of the E. coli cultures were Ciprofloxacin resistant. Trimethoprim/sulfamethoxazole resistant E. coli were found in 9 cultures and extended spectrum beta lactamase (ESBL) carrying E. coli in 3 cultures ().
Table 3. Bacteria found in blood and urine cultures.
Costs of post-TRbx infections
Cost analysis was performed for hospitalizations only. With a daily inpatient cost for health care-associated infections of USD 1529 (EUR 1338) and an average hospital stay of 6 days, total direct cost is estimated to USD 9174 (EUR 8031) per hospitalization. The additional hospital cost of infection per each performed TRbx is thus estimated to USD 358 (EUR 313) for a hospitalization rate of 3.9%.
ICD-10 coding
Of the 26 inpatient episodes, 20 (77%) had an infection-related code at discharge (; Supplementary Table 2). Six (23%) of the inpatient episodes that according to the medical chart review were related to a post-biopsy infection, were not labeled with an infection-related ICD-10 code at discharge. Five (19%) of these patients suffered from synchronous diseases such as stroke, heart failure or pulmonary embolism. In the remaining one case (4%) a non-infectious code attempting to describe TRbx infection was used.
Table 4. List of ICD-10 codes at hospital discharge given to patients with post-biopsy infection at the first hospitalization.
Discussion
The overall risk of post-biopsy infection was 5.4%; 3.9% of the patients were admitted to hospital for the infection and 0.7% developed sepsis. Four patients were hospitalized multiple times and two had repeated ED visits for recurrent infection after discharge from hospital. Furthermore, three of the four patients sent home after an initial ED visit were later hospitalized due to failure of PO antibiotic treatment. Our results are similar to a previous report of sepsis after prostate biopsy [Citation22], although comparisons are complicated by varying definitions of post-biopsy infection and sepsis. Hospitalization may be a better outcome measure since it is strongly associated with severe illness, costs and the health-care resource utilization.
The infection rate after TRbx is increasing [Citation7,Citation15,Citation16]. A study by Lundström et al. showed a 1% hospital admission for post-biopsy infection in Sweden between 2006 and 2011 [Citation23], considerably lower than the 3.9% found in the present study. Moreover, another recent Scandinavian study, with a similar design as the present study, reported a 6.1% rate of hospital admissions for infection [Citation24]. These studies indicate that the risk of hospitalization for post-TRbx infections may have increased significantly over the past ten years. The rising infection rates are at least partially a consequence of increasing antibiotic resistance [Citation17]. According to the Public Health Agency of Sweden, 12.8% of E. coli in blood cultures in Scania from 2014 were resistant and 0.9% intermediately sensitive to ciprofloxacin [Citation25]. The proportion of ciprofloxacin-resistant cultures remained relatively stable during the study period, having risen to 13.3% in 2017. Our results show that nearly two thirds (60.9%) of E. coli cultured from patients with post-TRbx were resistant to Ciprofloxacin, the most commonly used antibiotic for prophylaxis.
In a recent meta-analysis of healthcare costs related to prostate biopsy, the average cost of an infection was estimated to USD 8672–19,100 [Citation26]. In our calculation we used an average inpatient cost of USD 1529 (EUR 1338)/day and a mean hospitalization duration of 6 days, rendering a mean direct cost per hospitalization episode of USD 9174 (EUR 8031). With a hospitalization rate of 3.9% we estimate the average excess cost of infection to be USD 358 (EUR 313) for every patient undergoing a standard TRbx. However, this cost is likely to be an underestimation as we did not account for indirect costs such as sick leave, loss of work days, or care after hospital discharge.
Population-based studies commonly use ICD codes to identify patients with post-biopsy infection. The codes used include acute cystitis (N30.0), urinary tract infection (N39.0), and acute prostatitis (N41.0) [Citation7,Citation23,Citation27,Citation28]. In the current study, 23% of the inpatient episodes for a post-TRbx infection did not receive an infection-related code at discharge. The results imply that the infection rate may be underestimated in studies relying solely on ICD-10 [Citation18]. An ICD code for post prostate biopsy infection would facilitate future studies and monitoring of post-biopsy infections.
A limitation of this study is that we only had access to the hospital charts in Region Skåne and not data from general or private practitioners. Some post-biopsy infections may thus have been missed if patients were treated at hospitals outside Region Skåne, or were culture negative and treated in primary care without any note about the infection in the Region Skåne hospital charts. We used a definition of post-TRbx infections that we believe is sensitive for capturing clinical infections, but it may limit the comparability of our results with those from other studies. Furthermore, the time limit for a post-biopsy infection was defined as up to 30 days after TRbx, a limit commonly used in other studies [Citation7,Citation24]. Here, all admissions to the ward occurred within 11 days, yet some patients developed urinary tract infections more than 20 days after biopsy, all successfully treated by per oral antibiotics. We raise the question if all these infections were truly related to the TRbx. Additionally, the study was not population-based as only biopsies taken at the hospitals were included. Finally, antimicrobial resistance (AMR) is still relatively low in the study area, with 12.8–13.1% of E. coli resistant to ciprofloxacin [Citation24], and our results may not be applicable for centres where AMR is much more common (AMR vary from 8.4% to 90.2% worldwide [Citation29]).
In conclusion, post-biopsy infections (in a low AMR situation) occurred in 5.4% of men after TRbx, of which most (3.9%) were hospitalized with infection and some (1.3%) had a complicated infection. The hospital cost of these infections was estimated to USD 9174 (EUR 8031) per hospitalization. These increasingly common infections may thus not only have severe consequences for the affected patients, but are also be difficult to treat, resource-demanding and costly. A specific ICD-code for infection after prostate biopsy would facilitate monitoring of this iatrogenic complication.
Supplemental Material
Download MS Word (428.8 KB)Acknowledgments
We thank Daniela Grassi (AdvanSci Research Consulting) for writing assistance and Jane Fisher for revision of the manuscript.
Disclosure statement
AF is listed as an inventor in patents of a novel biopsy needle aiming to reduce infection complications in TRbx and also the founder of Saga Surgical AB, a company owning the patent rights. Other authors have nothing to disclose.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Tandoğdu Z. The dilemma of increasing antibiotic resistance in the prostate: Increasing prevalence of biopsy-related complications and increasing incidence of antibiotic resistance. Presented at: Joint meeting of the EAU Section of Infections in Urology (ESIU) and the EAU Section of Urological Imaging (ESUI) - Prepare for the future: Prevent, detect, strike back! 34th annual European Association of Urology Congress 2019; 2019 March 15–19; Barcelona, Spain.
- Johansen TEB, Zahl PH, Baco E, et al. Antibiotic resistance, hospitalizations, and mortality related to prostate biopsy: first report from the Norwegian Patient Registry. World J Urol. 2020;38(1):17–26.
- Liss MA, Ehdaie B, Loeb S, et al. An update of the American Urological Association white paper on the prevention and treatment of the more common complications related to prostate biopsy. J Urol. 2017;198(2):329–334.
- Liss MA, Johnson JR, Porter SB, et al. Clinical and microbiological determinants of infection after transrectal prostate biopsy. Clin Infect Dis. 2015;60(7):979–987. 1
- Loeb S, Vellekoop A, Ahmed HU, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64(6):876–892.
- Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol. 2013;189(1 Suppl):S12–S17. discussion S17–S18.
- Roberts MJ, Bennett HY, Harris PN, et al. Prostate biopsy-related infection: a systematic review of risk factors, prevention strategies, and management approaches. Urology. 2017;104:11–21.
- Rawla P, El Helou ML, Vellipuram AR. Fluoroquinolones and the risk of aortic aneurysm or aortic dissection: a systematic review and meta-analysis. Cardiovasc Hematol Agents Med Chem. 2019;17(1):3–10.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262.
- Raman JD, Lehman KK, Dewan K, et al. Povidone iodine rectal preparation at time of prostate needle biopsy is a simple and reproducible means to reduce risk of procedural infection. J Vis Exp. 2015;103:52670.
- Pilatz A, Veeratterapillay R, Koves B, et al. Update on strategies to reduce infectious complications after prostate biopsy. Eur Urol Focus. 2019;5(1):20–28.
- Fahmy A, Rhashad H, Mohi M, et al. Optimizing prophylactic antibiotic regimen in patients admitted for transrectal ultrasound-guided prostate biopsies: a prospective randomized study . Prostate Int. 2016;4(3):113–117.
- Dai J, Leone A, Mermel L, et al. Rectal swab culture-directed antimicrobial prophylaxis for prostate biopsy and risk of postprocedure infection: a cohort study. Urology. 2015;85(1):8–14.
- Carignan A, Roussy JF, Lapointe V, et al. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol. 2012;62(3):453–459.
- Lahdensuo K, Rannikko A, Anttila VJ, et al. Increase of prostate biopsy-related bacteremic complications in southern Finland, 2005-2013: a population-based analysis. Prostate Cancer Prostatic Dis. 2016;19(4):417–422.
- Teillant A, Gandra S, Barter D, et al. Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modelling study. Lancet Infect Dis. 2015;15(12):1429–1437.
- World Health Organization. ICD-10: International Statistical Classification of disease and related health problems: 10th revision. 2016. [cited 2020 Oct 29]. Available from: https://icd.who.int/browse10/2016/en.
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):762–774.
- Sveriges Kommuner och Regioner. Vårdrelaterade infektioner. 2017.
- Nationellt vårdprogram. Prostatacancer. 2020. [cited 2020 Oct 21]. Available from: https://www.cancercentrum.se/globalassets/cancerdiagnoser/prostatacancer/vardprogram/nationellt-vardprogram-prostatacancer.pdf.
- Bennett HY, Roberts MJ, Doi SAR, et al. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect. 2016;144(8):1784–1791.
- Lundstrom KJ, Drevin L, Carlsson S, et al. Nationwide population based study of infections after transrectal ultrasound guided prostate biopsy. J Urol. 2014;192(4):1116–1122.
- Danielsen L, Faizi G, Snitgaard S, et al. Infections after transrectal ultrasonic guided prostate biopsies – a retrospective study. Scand J Urol. 2019;53(2–3):97–101.
- Antibiotikaresistensrapport för Escherichia coli: Årsrapport för Region Skåne (SE120) från blododling 2017. Folkhälsomyndigheten 2017.
- Gross MD, Alshak MN, Shoag JE, et al. Healthcare costs of post-prostate biopsy sepsis. Urology. 2019;133:11–15.
- Roth H, Millar JL, Cheng AC, et al. The state of TRUS biopsy sepsis: readmissions to Victorian hospitals with TRUS biopsy-related infection over 5 years. BJU Int. 2015;116(Suppl 3):49–53.
- Anastasiadis E, van der Meulen J, Emberton M. Hospital admissions after transrectal ultrasound-guided biopsy of the prostate in men diagnosed with prostate cancer: a database analysis in England. Int J Urol. 2015;22(2):181–186.
- Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2020. Geneva: World Health Organization; 2020.
