Abstract
Background
Control computerized tomography (cCT) is routinely used in many cystectomy centres before the final treatment cycle in patients with muscle-invasive urinary bladder cancer (MIBC) undergoing neoadjuvant chemotherapy (NAC). This is for evaluating response or nonresponse to NAC treatment. In a real-world retrospective cohort, we intended to evaluate the frequency of changed individual treatment strategies following cCT and to investigate any discrepancies between cCT-results on nodal staging and final pN-stages.
Methods
We performed a retrospective data-based, multicenter study of 242 MIBC-patients, staged cT2N0M0-cT4aN0M0, having undergone NAC and radical cystectomy (RC) between 2008 and 2019 at four Swedish cystectomy centres. Statistical analysis was performed using IBM SPSS statistics 26.
Results
Overall, 139/242 patients were examined with cCT. Six patients were staged as progressive at cCT and 5/139 (3.6%) underwent a change of previously planned treatment strategy. 2/6 patients with suspected progression (33%) did not change strategy and underwent all preplanned NAC-cycles plus RC. Only 1/6 patients assigned as progressive at the cCT, showed progression in the postoperative pathology specimen. In total 133/139 patients were considered being without progress on cCT, yet 28/133 (21%) presented with nodal progression at postoperative pathology examinations. Only 1/29 patients with histopathologically verified nodal dissemination were detected with cCT, thus 28/29 (96.6%) with pN + were undetected. The sensitivity for cCT to predict pTNM was 17% CI [0%–64%] and the specificity was 78% CI [71%–86%].
Conclusions
CCT prior to the final treatment cycle of NAC in MIBC, leads to a low percentage of treatment strategy changes and cCT cannot accurately predict pN-status.
Introduction
In the western world, urinary bladder cancer (UBC) is the 4th most common malignancy in men and the 8th in women [Citation1]. The spectrum of UBC includes non-muscle-invasive, muscle-invasive (MIBC), and metastatic disease, each with its own clinical behaviour, biology, prognosis, and treatment [Citation2]. UBC is predominantly of urothelial origin. Squamous cell carcinoma, adenocarcinoma, and other tissue of origins also occur but only account for a few percent [Citation3]. Urothelial MIBC accounts for ∼25% of all UBC with clinical stages T2–T4 [Citation4]. MIBC has a 5-year overall survival (OS) of ∼50% after radical cystectomy (RC) [Citation3]. MIBC is associated with a high risk of regional and distant metastatic spread, the latter with a median survival of 15 months albeit maximum oncological treatment [Citation4]. To improve the outcomes, a combination of cisplatin-based combination neodjuvant chemotherapy (NAC), followed by RC, including regional lymph node dissection and urinary diversion, is the gold standard in medically fit patients [Citation3,Citation5,Citation6]. The main role of NAC is to eradicate micrometastatic disease at the best point of time and to produce a downstaging of the primary tumor. Downstaging of the primary tumor is considered being a positive surrogate marker for the efficacy of micrometastatic dissemination, which then is reflected upon the improved overall OS. The highest OS is found in patients with complete response (CR), with an absolute risk reduction for death (ARR) of 31% at 5 years median observation time [Citation7]. The concept CR in MIBC-patients undergoing NAC, is based on the combined histopathology of post-RC specimens showing pT0N0 in combination with radiological pM0 [Citation8].
In the Swedish Northern health region, as well as in the rest of the country, all newly diagnosed MIBC-patients are discussed regularly at the regional multidisciplinary team (MDT) meetings where the decision of treatment is reviewed and proposed. The patient's general condition, biological age, and comorbidities are also taken into consideration. The MDT takes place on at least two occasions for NAC patients; primarily after conclusive histopathology following TURB and primary CT evaluations and secondly, after the second of three to four planned NAC cycles. Organized and regular MDT meetings are highly recommendable for optimizing treatment of MIBC, with or without NAC. [Citation9]. The standard treatment of NAC is followed by RC, 4–6 weeks after the final treatment cycle [Citation3].
In some Swedish centres, including NUS (Norrlands university hospital in Umeå), there is a routine of performing a contrast-enhanced control computerized tomography (cCT) plus a CT over thorax, prior to the final NAC-cycle. The rational is to evaluate possible progression and in addition, any tumor response-status to NAC. This by radiological analysis of the primary tumor, nodal status, and possible distant dissemination. The primary baseline CT, obtained before the start of NAC, is compared to the cCT by the team radiologist. If the cCT shows progression, the regional recommendation is to proceed directly to RC or to abort RC and choose oncological palliative interventions instead, without performing the final NAC cycle [Citation9]. The major problem for the radiological evaluation of the urinary bladder is to have comparable examinations before and after NAC. Still, there are no existing and validated guidelines how to evaluate individual tumors with the background of alternating bladder filling, which can differ from occasion to occasion and between patients. The wide range of interindividual differences in terms of bladder volume and bladder capacity poses major challenges. Apart from one smaller retrospective single centre study, which suggested the routine of cCT [Citation10], there is a lack of solid evidence for the procedure. Control imaging, in NAC-treated MIBC-patients, has specifically been addressed by the European Association of Urology and its guidelines group on Muscle-invasive and Metastatic Bladder Cancer. A variety of imaging modalities (PET, CT, conventional MRI, or DCE MRI) are considered to lack scientific evidence to accurately predict NAC responses [Citation3]. The knowledge gap for cCT is wide, due to a lack of relevant studies, lack of algorithms, and no standardization of the whole procedure. Our group has recently published a single centre evaluation of a smaller cohort, displaying no correlation between cCT-evaluation and OS [Citation11]. We now proceed with the present multicentre retrospective study as a real-world experience, focusing on the results of actual decisions in clinical practice at four cystectomy centres. The cCT-evaluations in the present series are the actual radiological MDT evaluations which were performed and presented by several uro-radiologists performing their routine work at the different centres. The evaluations have not been scrutinized and re-evaluated for a scientific second opinion. The responses in NAC-patients who had undergone cCT, were categorized into complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) [Citation12]. Additionally, the categories Indefinable due to incompletely filled bladder and Inconclusive result were added. The two last-mentioned help categories are good examples of the heterogeneous nature of a practice that is unstandardized and lacking national and international consensus and has been implemented in clinical practice without any documented evidence-based protocols.
The study also entails a larger comparison between cCTs and post-RC pTN-stages. With the background of our previous study results, we hypothesized that cCT would be of low value in real-world treatment decisions and that there would be major discrepancies between cCT-evaluations and pTN-stages.
Material and methods
Patient information was collected from detailed local databases including all patients with MIBC at the participating hospitals, during the years 2008–2019. The regional ethics board had specifically decided that informed consent from the participants was to be considered redundant, especially due to the high mortality in MIBC, as well as due to the retrospective nature of the study.
Data from 242 patients, were collected from four different Swedish cystectomy centres, including NUS (n = 127), Sundsvall-Härnösands county hospital (n = 37), Västmanland county hospital (n = 38) and the University Hospital of Linköping (n = 40). The inclusion criteria for this study were patients diagnosed with MIBC (cT2N0M0–cT4aN0M0) receiving NAC followed by intended RC. Included clinical data were; sex, age at RC, histopathology, cTNM- and pTNM-staging, information regarding NAC (type and number of cycles), BMI pre-RC, CACI (Charlson’s age-adjusted Comorbidity Index) pre-RC, date of RC, date of first and second MDT, date of cCT, results from cCTs (based on RECIST-criteria), data on the officially recorded decision taken on the second MDT and date of last NAC-course. The primary cohort consisted of 244 patients, from which two patients had nodal dissemination (cN+) pre-NAC and were excluded from further analysis (). The baseline patient clinical characteristics (n = 242) are summarized in . The patients were further categorized into cCT-performed (cCT-yes) or cCT not performed (cCT-no) (). Thus, only patients who had undergone cCT before the final cycle of NAC (n = 139), were included in the final target group for analysis (). If a CT was performed after the last course of NAC, this was designated as preoperative CT (not having any impact on final NAC decisions) and those patients were not analysed.
Figure 1. Patient flow chart. From four participating cystectomy centres, a total of 244 patients with MIBC planned for neoadjuvant chemotherapy undergoing cystectomy (RC) 2008–2019 were identified. Two patients were excluded due to cN+. 139/242 (57%) underwent control computerized tomography (cCT) before the final NAC cycle and were finally analyzed for the study. 65 patients underwent RC following NAC without cCT and 38 patients underwent CT after the final NAC cycle and just before RC - none of these two subgroups entered analysis.
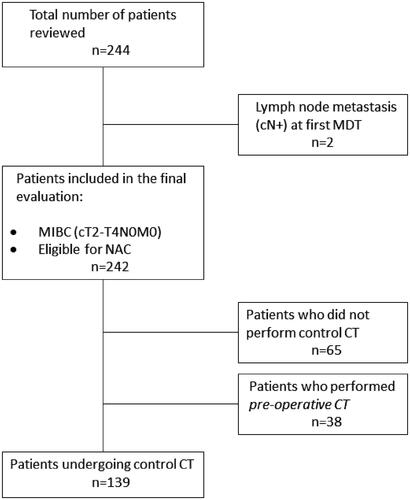
Table 1. Patient characteristics of the main cohorts.
Statistical analysis
Standard descriptive statistics were used to display the data in the result section. Patients were categorized based on if the radiological cCT was performed or not. Furthermore, they were divided into response groups. Descriptive statistics and crosstabs were used to identify patients with regress on cCT who had received alterations in treatment and those who had not. The cCT group was further divided into cCT-responses (Progress and No progress) and in crosstabs compared against treatment alterations and actual pTMN-progress. Confidence intervals for estimated probabilities are derived by using the exact (Clopper Pearson) method. All statistical analyses were made in IBM statistical SPSS version 26.
Results
82.7% of all cCT-examinations in the cCT-yes cohort (n = 139), were performed at NUS in Umeå, 9.4% in Linköping, 4.3% in Västmanland, and 3.6% in Sundsvall-Härnösand (). In the cCT-yes cohort, no missing values were noted. The radiological data were categorized into six different groups. Group 1 for CR (n = 4), Group 2 for patients with PR (n = 70), group 3 for SD (n = 38), group 4 for PD (n = 6), group 5 for radiological incapacity to assess the CTs due to the incomplete filled bladder (n = 15), group 6 for results difficult to interpret (n = 6) (). Of all cCT-yes patients, there were only six patients showing progress on cCT, and only four (4/139), received a change in treatment strategy (). Consequently, 2/6 patients (33%) with cCT-suggested progress did not receive any change in treatment strategy and continued instead with the last cycle of NAC, followed by RC. Additionally, one patient had poorly interpreted CT images which nevertheless led to a change of previously planned treatment, leading directly to RC. Thus, a total of five patients had an abrogation of pre-planned NAC treatment, leading to 134/139 (96.4%) of all patients undergoing cCT examinations, resulting in no treatment changes (). The six patients that had suggested progress on cCT are displayed in and only one of six (17%) was found progressive at histopathological analysis post-RC. Thus, in 5/6 patients, the suggested progress on cCT did not correlate to findings in the final post-RC staging (). In total 133 patients were considered being unprogressing in the evaluations of respective cCTs, 28 patients had progression in final post-RC histopathological staging. Thus, only 1/29 patients with nodal progression was detected at cCT. Consequently, the cCT-procedure was unable to detect 96.6% (28/29) of all patients with nodal progression (). The sensitivity for cCT in predicting pTNM was as low as 17% CI [0%–64%] and with a specificity of 78% CI [71%–86%].
Table 2. Differences in cCT-practices between the study centres.
Table 3. Groups based on the result of control CT imaging compared to baseline CT.
Table 4. Total patients who showed progress on control CT compared with total examined with control CT (%).
Table 5. Descriptive statistics over treatment alterations.
Table 6. Patient characteristics in the six patients who had suspicious progression at cCT.
Table 7. Displaying progress in pTNM-staging compared with assessed progress on cCT.
Calculated on the large primary cohort (n = 242), NAC was prematurely discontinued for a total of 24.4% due to NAC side effects. In the cCT-yes group, NAC was prematurely discontinued in 18.7% due to NAC side effects ().
Discussion
It has been shown that NAC combined with RC, is the treatment of choice in MIBC-patients who are medically fit up to a certain biological age and have an intact renal function [Citation3]. This treatment combination contributes to a major impact on improved OS, particularly in patients with CR (pT0N0M0) [Citation7]. In patients with NAC-naïve MIBC, a delay in surgery (RC) has been associated with a worse outcome on pathological, postoperative, staging [Citation13]. Higher pathological staging is consequently associated with a worse outcome for the patients and carries thereby a negative impact on long-term OS [Citation7]. The reason for the routine to perform a cCT before the last course of NAC would be to detect patients with MIBC who were unresponding to NAC and also patients with progressive disease (PD), with the consequence of refraining from the last cycle of NAC, and proceeding more quickly to RC. Alternatively, to consider non-surgical treatment and possible palliative oncological options, if warranted.
As noted in this real-world retrospective study, different centres have different approaches on how to plan the treatment for MIBC-patients being under assessment for NAC. As a standard, some centres perform 4 courses of NAC and other 3. The same differences concerning whether performing cCT is to be performed or not, are found. In Västerås and in Sundsvall, the initial strategy of performing cCT has been abandoned and there is no cCT in the present routines for MIBC-patients undergoing NAC. Instead, these centres perform a pre-operative CT–scan just prior to RC. The situation is similar in Linköping, but where it now is a routine to perform a preoperative CT on all MIBC patients prior to RC (both NAC-patients as well as NAC-naïve). Of the participating centres, NUS is the only centre that still performs cCT as a routine in the primary treatment plan. It should also be noted that in a significant proportion of the cases, ∼10.8% (15/139), it was impossible to make a correct CT assessment of the tumor burden in the urinary bladder. This is due to the lack of adequate bladder filling and the lack of standardization of the cCT, which further emphasizes the technical shortcomings of the procedure.
In the investigated cohort, our purpose was to evaluate the routine of using cCT to determine whether patients are responders and progressors to NAC or not. Non-responding patients with radiological signs of progression on cCT before the final NAC cycle would proceed directly to major surgery. In total, there were only 6 patients that were considered having a progression on control-CT and in 2/6 patients it was still decided to proceed with the original plan of final NAC-cycle and RC. In total, five patients proceeded directly to RC, this representing 2.1% (5/242) of all surveyed and 3.6% (5/139) of all patients undergoing cCT. Only 1/6 patients considered having progression, did actually progress. The majority of patients who discontinued NAC prematurely in the whole cohort (59/242), did so due to NAC side effects. The lack of predictive value for the radiological cCT concerning postoperative pathological parameters (pTNM), was obvious with a sensitivity of only 17% and a specificity of 78%. Another important aspect is that the cCT procedures were unable to detect ∼28/29 progressive patients, as displayed in the post-RC pathology evaluation. This rendering low practical value for real-life clinical decision-making according to the ascribed algorithm. We find that the results from this study further strengthens the rational for the EAU-Guidelines non-recommendations [Citation3].
For increasing the reliability of the study, it would be of great importance to include those patients who discontinued both NAC as well as RC and who instead continued solely with oncological treatment due to the results of the cCTs. The ethical permit does not cover evaluations of that small subgroup (estimated to be ∼20 patients totally, all centres included). Further, the study was retrospective, and a prospective study would be of greater value. Another possible limitation of the study is the fact that all radiological results were not entirely conclusive in interpretation, which also could rather be considered being a limitation of the method (cCT) and not of the study.
This study covers all patients that were diagnosed with MIBC, fulfilling inclusion criteria, in the years 2008–2019 at four Swedish cystectomy centres. From the start of the NAC-introduction, almost all Swedish centres used cCT as we still see at NUS today. Over time, individual decisions have been taken around at other centres around the country, in which cCT prior to the last NAC-cycle successively has been abandoned. All patient records have been carefully reviewed and in case of doubtful interpretation of CT results, a senior colleague has been consulted for second opinions. This study would not have been feasible by analysing variables from the existing national bladder cancer and cystectomy registers, whereas the specific data pertaining to radiological investigations (including cCT-data) is not collected in the national registration process [Citation14].
Conclusively, we found low evidence that cCTs could predict if the tumors were responding to neoadjuvant chemotherapy or not, and the examinations could not safely contribute to correct postoperative staging of the disease. Thus, the cCTs could not display credible results in terms of identifying true progressors. Consequently, real-life clinical decisions were not founded on reliable data. Prospective studies might be warranted. A clearer standardization is also required regarding the assessments and interpretations of cCT-images.
Ethics approval
The study was approved by the Regional Ethics Board in Umeå: EPN-Umeå, dnr; 2013/463-31 M with the amendment dnr; 2016/403-32. The study conforms to the provisions of the Declaration of Helsinki 1964 and to the revision in Fortaleza, Brazil, October 2013.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability statement
On reasonable request, the corresponding author can make available all codified data from the data base used for this study.
Additional information
Funding
References
- Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6):4–34.
- Lotan Y, Choueiri TK. Clinical presentation, diagnosis, and staging of bladder cancer. In: Lerner SP, Shah S, editors. UpToDate clinical presentation, diagnosis, and staging of bladder cancer; 2020. https://www.uptodate.com/contents/clinical-presentation-diagnosis-and-staging-of-bladder-cancer
- Witjes JA, Compérat E, Cowan NC, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475.
- von der Maase H, Hansen S, Roberts J, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18(17):3068–3077.
- Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two nordic studies. Eur Urol. 2004;45(3):297–303.
- Claire V. Advanced bladder cancer (ABC) Meta-analysis collaboration neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) Meta-analysis collaboration. Eur Urol. 2005; 48:202–205. discussion 205–6.
- Rosenblatt R, Sherif A, Rintala E, et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur Urol. 2012;61(6):1229–1238.
- Petrelli F, Coinu A, Cabiddu M, et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur Urol. 2014;65(2):350–357.
- Sherif A. The long perspective in emergence of neoadjuvant chemotherapy for bladder cancer in Ontario, Canada-space for improvement with regular and organized multidisciplinary team meetings. Transl Androl Urol. 2018;7(3):508–510.
- Fukui T, Matsui Y, Umeoka S, et al. Predictive value of radiological response rate for pathological response to neoadjuvant chemotherapy and post-cystectomy survival of bladder urothelial cancer. Jpn J Clin Oncol. 2016;46(6):560–567.
- Mogos H, Eriksson E, Styrke J, et al. Computerized tomography before the final treatment cycle of neoadjuvant chemotherapy or induction chemotherapy in muscle-invasive urinary bladder cancer, cannot predict pathoanatomical outcomes and does not reflect prognosis-results of a single Centre retrospective prognostic study. Transl Androl Urol. 2020;9(3):1062–1072.
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016; 62:132–137.
- Chang SS, Hassan JM, Cookson MS, et al. Delaying radical cystectomy for muscle invasive bladder cancer results in worse pathological stage. J Urol. 2003;170(4 Pt 1):1085–1087.
- Jerlström T, Chen R, Liedberg F, et al. No increased risk of short-term complications after radical cystectomy for muscle-invasive bladder cancer among patients treated with preoperative chemotherapy: a nation-wide register-based study. World J Urol. 2020;38(2):381–388.
