 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Androgens facilitate entrance of the severe acute respiratory syndrome coronavirus 2 into respiratory epithelial cells, and male sex is associated with a higher risk of death from corona virus disease (COVID-19). Androgen deprivation therapy (ADT) could possibly improve COVID-19 outcomes.
Methods
In a case–control study nested in the Prostate Cancer data Base Sweden (PCBaSe) RAPID 2019, we evaluated the association between ADT and COVID-19 as registered cause of death in men with prostate cancer. Each case was matched to 50 controls by region. We used conditional logistic regression to adjust for confounders and also evaluated potential impact of residual confounding.
Results
We identified 474 men who died from COVID-19 in March–December 2020. In crude analyses, ADT exposure was associated with an increased risk of COVID-19 death (odds ratio [OR] 5.05, 95% CI: 4.18–6.10); however, the OR was substantially attenuated after adjustment for age, comorbidity, prostate cancer characteristics at diagnosis, recent healthcare use, and indicators of advanced cancer (adjusted OR 1.25, 95% CI: 0.95–1.65). If adjustment has accounted for at least 85% of confounding, then the true effect could be no more than a 5% reduction of the odds for COVID-19 death.
Conclusions
The increased mortality from COVID-19 in men with prostate cancer treated with ADT was mainly related to high age, comorbidity, and more advanced prostate cancer. There was no evidence to support the hypothesis that ADT is associated with improved COVID-19 outcomes.
Introduction
Male sex is an independent risk factor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In humans, the risk of infection and hospitalization, critical care admission and death from the corona virus disease (COVID-19) is 50% higher among men than women [Citation1–4]. Sex influences several parts of the human immune system, specifically the response to viral infections [Citation5]. Androgen receptors are present in immune cells, and androgens have been shown to have mostly immunosuppressive effects [Citation6]. In vitro studies have shown that androgens facilitate cellular uptake of COVID-19 into host respiratory epithelial cells through transmembrane protease serine 2 protease inhibition [Citation7], thereby being one potential pathophysiological mechanism for an increased susceptibility of men to COVID-19.
Androgen deprivation therapy (ADT) has been hypothesized to be protective against COVID-19 [Citation8]. A previous observational study reported that men with prostate cancer not on ADT had a fourfold increased risk of COVID-19 and more severe disease compared with men on ADT [Citation9]. Based on these results, several clinical trials have been initiated to evaluate a potential benefit of ADT in men with COVID-19 [Citation10]. Recently, two other observational studies failed to replicate the protective effect of ADT [Citation11,Citation12]. These studies were, however, limited in terms of sample size and their ability to control for confounding.
Sweden, with a source population of more than 10 million people, was strongly affected by the COVID-19 pandemic in the spring of 2020. Sweden has a unique nationwide register data collection of men with prostate cancer, and in a previous cohort study of men with prostate cancer on ADT, we found no evidence to support a beneficial effect of ADT on excess mortality during the spring of 2020 compared to previous years [Citation13]. Overall mortality in a cancer population may, however, not be entirely reflective of COVID-19-specific mortality. We aimed to estimate the association between ADT and other risk factors, and mortality from COVID-19 in men with prostate cancer using nationwide data sources in Sweden that enabled good control of confounders.
Methods
Study design and participants
We conducted a case–control study among men with prostate cancer identified in the National Prostate Cancer Register (NPCR) of Sweden. Since 1998 NPCR has captured 98% of all cases of prostate cancer in Sweden and contains comprehensive data on cancer characteristics and primary cancer treatment [Citation14]. Data for men in NPCR diagnosed with prostate cancer between 1 January 1998 and 31 December 2019 was via the unique personal identify number [Citation15] given to every Swedish citizen linked to several other national registers held at The National Board of Health and Welfare to create the Prostate Cancer data Base Sweden (PCBaSe) RAPID 2019. These linked registers included the Swedish Cancer Register [Citation16], the Cause of Death Register [Citation17], the Swedish Prescribed Drug Register (medications dispensed from all Swedish pharmacies) [Citation18], and the National Patient Register (hospital inpatient and outpatient diagnoses) [Citation19], with data collected until 16 December 2020. The study was approved by the Swedish Ethical Review Authority.
Cases of COVID-19 death and matched controls
We identified deaths from COVID-19 from entries in the Cause of Death Register for the International Classification of Diseases, version 10 (ICD-10) code U07.1 (virus verified) or code U07.2 (virus not verified) as the underlying or contributing cause of death. Each case was matched to 50 controls by region (to account for regional variation in COVID-19 prevalence), randomly sampled with replacement from the NPCR. Controls were required to be alive at the date of death of the case. The index date was the date of death for each case and his matched controls.
Exposure to ADT
Exposure to ADT was determined from filled prescriptions in the Swedish Prescribed Drug Register for the oral androgen receptor blocker bicalutamide (ATC code L02BB03 and L02AE51; flutamide L02BB01, the latter represented only 2% of men in this category), gonadotropin-releasing hormone (GnRH) agonists (L02AE), abiraterone (L02BX03), and enzalutamide (L02BB04) any time before the index date. We have previously demonstrated high adherence to ADT and that it is rare that a man prescribed GnRH agonists does not continue his medication [Citation20–24]. Men who in addition to GnRH agonist received 30 days of flare prophylaxis with bicalutamide were classified as exposed to GnRH agonist only. Bilateral orchidectomy was identified from procedure codes KFC10 or KFC15 registered in the National Patient Register. This method captured all men exposed to ADT, regardless of indication or line of treatment. However, to reduce confounding, we excluded men with a filled prescription for the androgen receptor targeting drugs abiraterone and enzalutamide in the 90 days before the index date due to the strong association between treatment with these and advanced stage prostate cancer. In 18 of the 21 Swedish regions (covering 91% of the Swedish population on the 31 December 2019), GnRH agonists were provided to patients through community pharmacies; however, in the three regions covering the remaining 9% of the Swedish population (Örebro, Värmland and Sörmland), GnRH agonists were provided to the patient directly from the hospital, and therefore were not captured in the Prescribed Drug Register. As a proxy for GnRH use in these three regions, we used a single filled prescription for bicalutamide under the assumption that bicalutamide had been used as flare protection.
Covariates
We obtained data on several variables to ascertain prostate cancer risk category at the time of diagnosis: cancer stage (TNM system [Citation25]), Gleason score, serum level of prostate-specific antigen (PSA), and history of radical prostatectomy or radical radiotherapy. Prostate cancer severity was also determined from the interval between diagnosis and the index date, and from fracture related to metastatic disease or low-energy fracture potentially related to general frailty, osteoporosis, or metastases. We used prescriptions for an opioid or a systemic corticosteroid up to six months before the index date as an indicator of advanced cancer (Supplemental eTable 1). We calculated the Charlson Comorbidity Index (CCI) from hospital discharge diagnoses registered in the National Patient Register during the 10-year period before the start of follow-up at the index date [Citation26,Citation27]. We calculated the Drug Comorbidity Index (DCI) based on prescriptions filled within 365 days before the index date, categorizing medications by chemical subgroup, that is, the first five positions of the ATC code [Citation28,Citation29]. The National Patient Register was also used to obtain data on myocardial infarction, chronic obstructive pulmonary disease (COPD) and diabetes in the 10-year period before the index date (Supplemental eTable 2), and data on recent healthcare use. We measured the latter in two ways: firstly, as the sum of all unique main or secondary diagnosis codes from all outpatient visits in the two years before the index date, and secondly, as the sum of days of in-hospital care for any reason in the two years before the index date.
Statistical analysis
Characteristics of cases and controls on the date of diagnosis of the prostate cancer and the index date for the study were described using frequency counts and percentages for categorical variables, and medians with interquartile range (IQR) for continuous variables. Adjusted odds ratios (ORs) with 95% confidence intervals (CIs) for COVID-19 death were estimated using conditional logistic regression, incrementally adding grouped confounders into the model but with no selection of covariates made based on results. Age, DCI, and the log PSA level at date of prostate cancer diagnosis were modeled as restricted cubic splines. Other numeric and count variables were stratified into categorical variables. The Gleason score was modeled as five categories (2–6, 7 [3 + 4], 7 [4 + 3], 8, 9–10). Five models were generated, each with increasing adjustment for confounders to assess the impact on the crude OR. Covariates with missing values were imputed using multiple imputation with chained equations as implemented in the R package Mice [Citation30]. Dichotomous variables were imputed with logistic regression, ordinal variables with ordinal regression, and numerical variables with predictive mean matching.
The main analysis was based on exposure to any ADT before the index date versus no ADT. In subgroup analyses, we evaluated exposure to bicalutamide monotherapy separately from GnRH agonists or orchidectomy (‘GnRH group’).
We performed several sensitivity analyses. Firstly, we restricted the analysis to cases with COVID-19 reported specifically as the underlying cause of death (rather than ‘underlying or contributing cause’). Secondly, we excluded cases from three regions, in which GnRH therapy is provided directly from the hospital. Lastly, we retained men who were prescribed abiraterone or enzalutamide in the analyses but adjusted for exposure to these medications in the analysis.
To quantify the potential level of residual confounding, we considered adjustment in our regression analysis as removing a certain proportion of the total amount of confounding, as proposed by Sawitz and Barón in relation to confounder misclassification [Citation31]. However, we enabled interpolation to take place on the scale of regression coefficients rather than on Mantel–Haenszel ORs. We considered this to be preferable because the regression coefficient scale is where a logistic regression model assumes linearity. We used the following notation for key estimates of association: crude, no adjustment (regression coefficient bc); partially adjusted, that is, the maximum possible adjustment with available measured covariates (regression coefficient bpa), and Completely adjusted, that is, the true causal effect, which would theoretically be obtained had all confounding been possible to remove (regression coefficient bca). The corresponding odds ratios are denoted ORc = exp(bc), ORpa = exp(bpa), and ORca = exp(bca). The relation between these is described by EquationEquation (1)(1)
(1) :
(1)
(1)
where α is the fraction of confounding that has been removed. This leads to the following relation between crude, partially adjusted and completely adjusted odds ratios, and α:
(2)
(2)
We plotted the relationship to illustrate the potential impact of residual confounding on the key estimate of association. The amount of residual confounding was expressed in terms of percent adjustment. Statistical analyses were performed using R version 4.0.3 (2020–10-10).
Results
We identified 474 men with prostate cancer and COVID-19 recorded as the underlying or contributing cause of death; the earliest death was recorded on 19 March 2020 and the last on 10 December 2020 (flowchart eFigure 1). For the majority of cases (89%), COVID-19 was recorded as the underlying cause of death (Supplemental eTable 3), and prostate cancer was the second most common underlying cause of death. Characteristics of the 474 cases and their controls are shown in . On average, cases were older and had more advanced cancer at the time of prostate cancer diagnosis than controls. Cases also had a greater number of comorbidities recorded before the index date, as well as more fractures potentially related to metastases or frailty, more healthcare visits and hospitalizations, and they more commonly received a prescription for opioids/systemic corticosteroids.
Table 1. Characteristics of men with prevalent prostate cancer who died from COVID-19 (cases), and their matched controls in PCBaSe RAPID 2019.
In crude analyses, ADT exposure was associated with an increased odds of death from COVID-19 (OR 5.05, 95% CI: 4.18–6.10) (); however, after adjustment for age, comorbidity, prostate cancer risk, and healthcare use, the association was substantially attenuated (adjusted OR 1.25, 95% CI: 0.95–1.65) (). The crude association was stronger for GnRH compared to antiandrogens, but this difference was similarly attenuated with increased control of confounders (). Results of sensitivity analyses were very similar to those seen in the main analyses (; Supplemental eTables 4 and 5).
Figure 1. Forest plot of associations between exposure to any ADT and COVID-19 mortality. Estimated odds ratios are represented by squares and 95% confidence intervals are represented by horizontal whiskers (variables adjusted for in each step are described in the methods section and in footnotes). GnRH: gonadotropin-releasing hormone
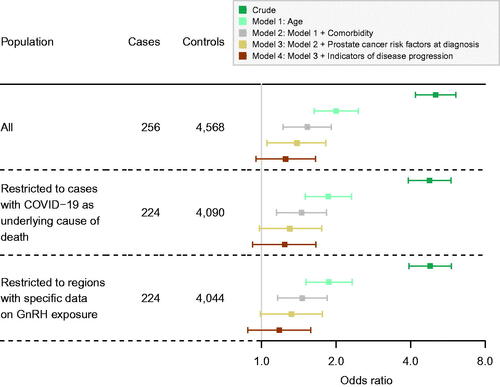
Table 2. Odds ratios (ORs) with 95% confidence intervals (CIs) for the association between ADT exposure and COVID-19 death (underlying or contributing cause) in men with prostate cancer in PCBaSe RAPID 2019.
Table 3. Sensitivity analyses: odds ratios (ORs) with 95% confidence intervals (CIs) for the association between ADT exposure and COVID-19 death in men with prostate cancer in PCBaSe RAPID 2019.
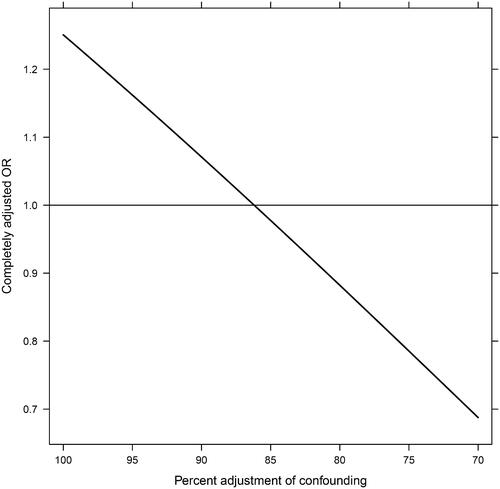
The potential impact of residual confounding (in terms of percent adjustment) is illustrated in , where the causal effect of ADT on the odds of death from COVID-19 relates to the magnitude of residual confounding. As an example, if adjustment has accounted for approximately 85% of confounding, then the true effect is expected to be a < 5% reduction of the odds for COVID-19 death.
Figure 2. Quantitative evaluation of impact of potential residual confounding. Interpolation of regression coefficients based on the change from the crude estimate to the maximally adjusted estimate. The curve describes the relation between percent adjustment of confounding and the resulting completely adjusted odds ratio (causal effect). The calculation is based on the maximally adjusted odds ratio from the main analysis (the intercept). OR: odds ratio
Discussion
In our large national population-based nested case–control study of men with prostate cancer in Sweden, we found no evidence to support the hypothesis that use of ADT is associated with a reduced risk of death from COVID-19. The strong increase in risk of COVID-19 death among men on ADT in unadjusted analyses was attenuated after controlling for age (the strongest confounder), prostate cancer risk category at diagnosis, comorbidity, healthcare use, and indicators of frailty and advanced cancer. In addition, quantitative bias analysis indicated that residual confounding would have to be substantial to hide a true beneficial effect of ADT that was of a clinically relevant magnitude.
Previous study results addressing this question have been mixed and have come from small studies with a low number of men exposed to ADT, and with limited control for confounding. For example, some small noninterventional studies have suggested that men on ADT had lower rates of hospitalization, requirement for supplemental oxygen and endotracheal intubation, and mortality [Citation9,Citation32]; whereas, other similar studies have failed to replicate these findings [Citation11,Citation12,Citation33,Citation34]. In a previous study using PCBaSe, we found no evidence that bicalutamide monotherapy or any ADT was associated with lower excess mortality among men with prostate cancer after comparing data from the first wave of the COVID-19 pandemic in March–June 2020 with corresponding months in 2015–2019 [Citation13]. Results of this study, focusing on COVID-19-specific mortality, are in line with our previous finding. While residual confounding can hide true associations, especially those of moderate strength and/or when confounding is present, our previous study [Citation13] indicated that any association between ADT and COVID-19 severity is unlikely to be strong. This contrasts with factors such as advanced cancer and severe comorbidity, which were expected to be strong confounders [Citation35,Citation36]. Androgen deprivation therapy is currently being tested in men without prostate cancer in randomized trials; it is possible the association between ADT and COVID-19 death is different in men free of prostate cancer. It should, however, be noted that the initial findings of a potential effect of ADT on COVID-19 came from a small single-center study of men with prostate cancer exposed to ADT [Citation9]. The ADT exposure in our study is also optimal in the sense that it was a pretreatment before the virus exposure, while ADT for treatment of COVID-19 is initiated after the initial virus exposure.
Our study population is highly representative of the prostate cancer population in Sweden. Other strengths of our study include the large size with sufficient number of events, complete follow-up to allow precise estimates of associations, information that enabled accurate identification of COVID-19 deaths and ADT exposure, and good control for important confounders. Although the PCBaSe does not contain information on some of the known risk factors for severe COVID-19, such as body mass index [Citation37] and smoking [Citation38], these are unlikely to be confounders because they are unlikely to influence the decision to initiate ADT. Furthermore, we believe our findings to be robust because our quantitative bias analysis demonstrated that any residual confounding would have to be substantial to have missed a clinically relevant beneficial effect of ADT. We acknowledge the possibility of residual confounding; for example, since information on prostate cancer stage and severity – both likely strong confounders – was incomplete.
In conclusion, in men with prevalent prostate cancer we found most notably age, but also comorbidity, and advanced cancer to be important risk factors for death from COVID-19. Our findings do not provide evidence that ADT reduces mortality from COVID-19.
Disclaimer
Rolf Gedeborg is also employed by the Medical Products Agency (MPA) in Sweden. The MPA is a Swedish Government Agency. The views expressed in this article may not represent the views of the MPA.
Consent to participate
The requirement for informed consent was waived by the Ethical Review Authority.
Ethics approval
Approved by the Swedish Ethical Review Authority: Dnr 2020-03889.
Supplemental Material
Download PDF (224.8 KB)Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chairman), Ingela Franck Lissbrant (co-chair), Camilla Thellenberg, Eva Johansson, Lennart Åström, Magnus Törnblom, Stefan Carlsson, Marie Hjälm Eriksson, David Robinson, Mats Andén, Ola Bratt, Jonas Hugosson, Maria Nyberg, Per Fransson, Fredrik Jäderling, Fredrik Sandin and Karin Hellström. We thank Susan Bromley, EpiMed Communications Ltd, Abingdon, Oxford, UK for editorial assistance funded by the University of Uppsala.
Disclosure statement
The public health-care administration for Region Uppsala in Sweden has, on behalf of the National Prostate Cancer Register, made agreements on subscriptions for quarterly reports from Patient-overview Prostate Cancer with Astellas, Sanofi, Janssen, and Bayer, as well as research projects with Astellas, Bayer, and Janssen. Johan Styrke is a co-PI of the Covidenza trial of Enzalutmide for Covid 19: ClinicalTrials.gov Identifier: NCT04475601. Johan Styrke report no financial interest in the present study or in the Covidenza trial. Stacy Loeb reports equity in Gilead. Region Uppsala has, on behalf of NPCR, made agreements on subscriptions for quarterly reports quarterly reports from Patient-overview Prostate Cancer with Astellas, Janssen, and Bayer, as well as research projects with Astellas, Bayer, and Janssen. All remaining authors have declared no conflicts of interest.
Data availability statement
Data used in the present study was extracted from the Prostate Cancer Database Sweden (PCBaSe), which is based on the National Prostate Cancer Register (NPCR) of Sweden and linkage to several national health-data registers. The data cannot be shared publicly because the individual-level data contain potentially identifying and sensitive patient information and cannot be published due to legislation and ethical approval (https://etikprovningsmyndigheten.se). Use of the data from national health-data registers is further restricted by the Swedish Board of Health and Welfare (https://www.socialstyrelsen.se/en/) and Statistics Sweden (https://www.scb.se/en/) which are Government Agencies providing access to the linked healthcare registers. The data will be shared on reasonable request in an application made to any of the steering groups of NPCR and PCBaSe. For detailed information, please see www.npcr.se/in-english, where registration forms, manuals, and annual reports from NPCR are available alongside a full list of publications from PCBaSe. The statistical program code used for the present study analyses can be provided on request (contact [email protected]).
Additional information
Funding
References
- Gupta S, Hayek SS, Wang W, STOP-COVID Investigators, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020;180(11):1436.
- Grasselli G, Greco M, Zanella A, COVID-19 Lombardy ICU Network, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345.
- Galloway JB, Norton S, Barker RD, et al. A clinical risk score to identify patients with COVID-19 at high risk of critical care admission or death: an observational cohort study. J Infect. 2020.
- de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the oxford royal college of general practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20(9):1034–1042.
- Takahashi T, Iwasaki A. Sex differences in immune responses. Science. 2021;371(6527):347–348.
- Scully EP, Haverfield J, Ursin RL, et al. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20(7):442–447. Jul
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280 e8. Apr 16
- Damodaran S, Lang JM, Jarrard DF. Targeting metastatic hormone sensitive prostate cancer: Chemohormonal therapy and new combinatorial approaches. J Urol. 2019;201(5):876–885.
- Montopoli M, Zumerle S, Vettor R, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol. 2020;31(8):1040–1045.
- ClinicalTrials.gov. The National Library of Medicine (NLM) at the National Institutes of Health (NIH); 2020. [29 November 2020].
- Caffo O, Zagonel V, Baldessari C, et al. On the relationship between androgen-deprivation therapy for prostate cancer and risk of infection by SARS-CoV-2. Ann Oncol. 2020;31(10):1415–1416.
- Koskinen M, Carpen O, Honkanen V, et al. Androgen deprivation and SARS-CoV-2 in men with prostate cancer. Ann Oncol. 2020;31(10):1417–1418.
- Gedeborg R, Styrke J, Loeb S, et al. Androgen deprivation therapy and excess mortality in men with prostate cancer during the initial phase of the COVID-19 pandemic. PLoS One. 2021;16(10):e0255966.
- Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort profile update: the national prostate cancer register of Sweden and prostate cancer data base-a refined prostate cancer trajectory. Int J Epidemiol. 2016;45(1):73–82.
- Hagel E, Garmo H, Bill-Axelson A, et al. PCBaSe Sweden: a register-based resource for prostate cancer research. Scand J Urol Nephrol. 2009;43(5):342–349. 2009/01/01
- Barlow L, Westergren K, Holmberg L, et al. The completeness of the Swedish cancer register: a sample survey for year 1998. Acta Oncol. 2009;48(1):27–33.
- Brooke HL, Talback M, Hornblad J, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765–773.
- Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish prescribed drug register - a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol. 2016;119(5):464–469.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
- George G, Garmo H, Rudman S, et al. Long-term adherence to GnRH agonists in men with prostate cancer. A nation-wide population-based study in prostate cancer data base Sweden. Scand J Urol. 2020;54(1):20–26.
- Grundmark B, Garmo H, Zethelius B, et al. Anti-androgen prescribing patterns, patient treatment adherence and influencing factors; results from the nationwide PCBaSe Sweden. Eur J Clin Pharmacol. 2012;68(12):1619–1630.
- Cindolo L, De Francesco P, Petragnani N, et al. Persistence and adherence to androgen deprivation therapy in men with prostate cancer: an administrative database study. Minerva Urol Nefrol. 2020;72(5):615–621.
- Lycken M, Drevin L, Garmo H, et al. Adherence to guidelines for androgen deprivation therapy after radical prostatectomy: Swedish population-based study. Scand J Urol. 2020;54(3):208–214.
- Suttmann H, Gleissner J, Huebner A, et al. Adherence measures for patients with metastatic castration-resistant prostate cancer treated with abiraterone acetate plus prednisone: results of a prospective, cluster-randomized trial. Cancers (Basel). 2020;12(9):2550.
- Paner GP, Stadler WM, Hansel DE, et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol. 2018;73(4):560–569. Apr
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. Nov
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Gedeborg R, Sund M, Lambe M, et al. An aggregated comorbidity measure based on the history of filled drug prescriptions – development and evaluation in two separate cohorts. Epidemiology. 2021;32(4):607–615.
- Gedeborg R, Garmo H, Robinson D, et al. Prescription-based prediction of baseline mortality risk among older men. PLoS One. 2020;15(10):e0241439.
- White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30(4):377–399.
- Savitz DA, Barón AE. Estimating and correcting for confounder misclassification. Am J Epidemiol. 1989; 129(5):1062–1071.
- Patel VG, Zhong X, Liaw B, et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol. 2020;31(10):1419–1420.
- Caffo O, Gasparro D, Di Lorenzo G, et al. Incidence and outcomes of severe acute respiratory syndrome coronavirus 2 infection in patients with metastatic castration-resistant prostate cancer. Eur J Cancer. 2020;140:140–146.
- Klein EA, Li J, Milinovich A, et al. Androgen deprivation therapy in men with prostate cancer does not affect risk of infection with SARS-CoV-2. J Urol. 2021;205(2):441–443.
- Meng Y, Lu W, Guo E, et al. Cancer history is an independent risk factor for mortality in hospitalized COVID-19 patients: a propensity score-matched analysis. J Hematol Oncol. 2020;13(1):75.
- Lee LYW, Cazier JB, Starkey T, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–1926.
- Pranata R, Lim MA, Yonas E, et al. Body mass index and outcome in patients with COVID-19: a dose-response Meta-analysis. Diabetes Metab. 2021;47(2):101178.
- Patanavanich R, Glantz SA. Smoking is associated with COVID-19 progression: a Meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656.
