Abstract
Objectives: To predict castration-resistant prostate cancer (CRPC) and prostate cancer (Pca) death by use of clinical variables at Pca diagnosis and PSA levels after start of gonadotropin-releasing hormone agonists (GnRH) in men with non-metastatic castration sensitive prostate cancer (nmCSPC).
Materials and Methods: PSA values for 1603 men with nmCSPC in the National Prostate Cancer Register of Sweden who received GnRH as primary treatment were retrieved from Uppsala-Örebro PSA Cohort and Stockholm PSA and Biopsy Register. All men had measured PSA before (pre-GnRH PSA) and 3–6 months after (post-GnRH PSA) date of start of GnRH. Unadjusted and adjusted Cox models were used to predict CRPC by PSA levels. PSA levels and ISUP grade were used to construct a risk score to stratify men by tertiles according to risk of CRPC and Pca death.
Results: 788 (49%) men reached CRPC and 456 (28%) died of Pca during follow-up. Post-GnRH PSA predicted CRPC regardless of pre-GnRH PSA. CRPC risk increased with higher post-GnRH PSA, HR 4.7 (95% CI: 3.4–6.7) for PSA > 16 ng/mL vs 0–0.25 ng/mL and with ISUP grade, HR 3.7 (95%: 2.5–5.4) for ISUP 5 vs ISUP 1. Risk of Pca death in men above top vs bellow bottom tertile of post-GnRH PSA and ISUP grade was HR 4.1 (95% CI: 3.0–5.5).
Conclusion: A risk score based on post-GnRH PSA and ISUP grade could be used for early identification of a target group for future clinical trials on additional therapy to GnRH.
Introduction
Androgen deprivation therapy (ADT) is standard treatment for men with advanced prostate cancer (Pca) and currently gonadotropin-releasing hormone agonists (GnRH) are the most common form of ADT [Citation1]. Following treatment with ADT, many men with castration sensitive prostate cancer (CSPC) will progress to castration-resistant prostate cancer (CRPC), which occurs after a median time of 18–24 months [Citation2,Citation3]. Median survival for men with metastatic CRPC (mCRPC) is 16 months [Citation4] and 60 months for men with non-metastatic CRPC (nmCRPC) [Citation5,Citation6]. Most studies on ADT and CRPC have focused on men with metastases and several prognostic factors for progression have been identified such as Gleason score (GS), presence of bone and visceral metastases, and performance status [Citation7,Citation8]. Increasing numbers of men are however diagnosed with nmCRPC and for those under high-risk of progression (PSA doubling time of less than 10 months), androgen-receptor target drugs have been shown to increase overall survival (OS) in large phase III randomized clinical trials [Citation5,Citation6,Citation9]. Identifying predictors of rapid progression to CRPC among men with non-metastatic castration sensitive prostate cancer (nmCSPC) can aid in the selection of men with poor prognosis who may be suitable for further treatment upfront.
The aim of this study was to investigate if PSA levels measured prior to treatment with GnRH along with clinical variables at date of Pca diagnosis combined with PSA levels 3–6 months after start of GnRH predict risk of CRPC and Pca death in men with nmCSPC. A risk score with the variables predictive of CRPC and Pca death will be then created.
Materials and methods
Setting
We retrieved data on PSA values for men in the Uppsala-Örebro PSA Cohort (UPSAC) [Citation10] and the Stockholm PSA and Biopsy Register (STHLM-0) [Citation11] in six regions (Uppsala, Örebro, Värmland, Dalarna, Gävleborg and Stockholm), which constitutes approximately 40% of the male population in Sweden [Citation12]. Data on PSA levels measured from January 2003 until December 2017 was available. From the National Prostate Cancer Register (NPCR) of Sweden [Citation13] we retrieved data on clinical variables at diagnosis: local clinical T stage, N stage, presence of metastases based on bone scintigraphy (M stage) and histological grading according to the International Society of Urological Pathology (ISUP grade). We defined nmCSPC as Pca without bone metastases (M0) on scintigraphy or bone metastases not assessed (Mx) as registered in NPCR according to the UICC 2002 classification. From the National Prescribed Drug Registry (NPDR) [Citation14] we retrieved data on fillings for GnRH (ATC code: L02AE01, L02AE02, L02AE03, L02AE04, L02AE05 and L02AE51) between January 2006 and December 2017. Men were defined as exposed to GnRH if they had been treated with GnRH more than 90 days defined by 90 Defined Daily Doses (DDD) during a 180-day calendar time period. Discharge diagnostic codes from the Swedish Patient Registry were used to calculate Charlson Comorbidity Index (CCI) [Citation15] and data on civil status and educational level was retrieved from Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA) [Citation16].
Study population
Men were included in the study if they were i) recorded in either UPSAC or STHLM-0, ii) registered with nmCSPC between 2006 and 2016 in NPCR and iii) received primary treatment with GnRH defined as initiation of GnRH within 6 months from diagnosis according to the National Prescribed Drug Registry. All men in the study had a PSA measurement before date of start for GnRH (pre-GnRH PSA) and a PSA measurement 3–6 months after GnRH initiation (post-GNRH PSA). None of the men in the study had received local therapy for Pca prior to start of GnRH.
Outcome assessment
We defined CRPC in men on GnRH as the first of (i) date of doubling of nadir PSA value with the latter value above 2 ng/mL or (ii) date of an absolute increase in PSA of 5 ng/mL or more or (iii) Pca death without CRPC date [Citation11]. Date and cause of death were retrieved from the Cause of Death Register [Citation17].
Follow-up
The baseline was set to the date of post-GnRH PSA. For the main outcome which was transition to CRPC, follow-up ended at date of CRPC or at last date with available data in the UPSAC and STHLM-0 cohorts, whichever came first. For the secondary outcome, Pca death, follow-up ended at the first of: date of Pca death, censoring for other causes of death, or at the end of the study. For the UPSAC cohort data was available until December 2014 and for the STHLM-0 cohort, data was available until December 2017.
Statistical analysis
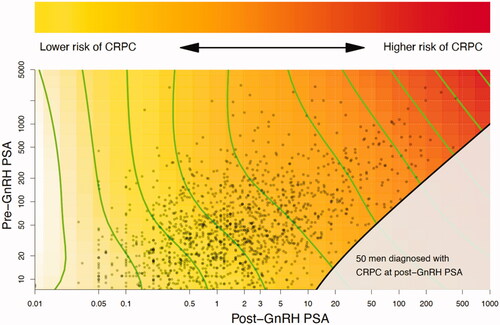
In a first analysis, a study of the combined effects of pre-GnRH PSA and post-GnRH PSA on the risk of CRPC was made and illustrated by a heat map which was based on a Cox proportional hazard model. The hazard function of both PSA values was represented by a restricted 2-dimensional cubic spline and estimated using the gam-function in the R-package mgcv [Citation18]. In the heat map, the hazard ratios form level curves in the plane defined by the pre- and post-GnRH PSA measurements. In our illustration the level curves are shown in green. If these green level curves are straight and parallel there is no interaction, and if they align horizontally or vertically then only one of the two PSA measurements may be sufficient to describe the risk of CRPC.
In a second analysis, univariable Cox models were used to calculate unadjusted hazard ratios (HR) of risk to CRPC for pre-GnRH PSA, other clinical variables at time of diagnosis (local clinical T stage, N stage, M stage and ISUP grade) and for the variables collected at start of follow-up (post-GnRH PSA, age, time from GnRH to post-GnRH PSA, civil status, educational level and CCI). In a third analysis, a multivariable analysis was performed including all variables above. C-index [Citation19] was calculated to assess the association with CRPC.
To create a simple risk score (denoted CRPCrisk-score), we assigned the numbers 1–5 to the ISUP grades and included this variable together with log (post-GnRH PSA) in a Cox proportional hazards model. The coefficients estimated from the model were used to define the CRPCrisk-score. A cross validation was performed by applying a model derived from the UPSAC data to the STHLM-0 data for validation, and vice versa. The cohort was divided in three groups by tertiles of the score: low-, intermediate- and high-risk for CRPC. Kaplan Meier analyses and non-adjusted Cox regression analyses were used to illustrate how well the risk groups can predict the risk of CRPC and of Pca death. In a sensitivity analysis, the added ISUP number was multiplied by 0.8, 1.2 and 1.4. Risk of Pca death for the cohort was assessed for men above increasingly higher percentiles of CRPCrisk-score and in a further sensitivity analysis, men with low- and intermediate-risk (local clinical T-stage <3, pre-GnRH PSA < 20 ng/mL and ISUP grade <4) nmCSPC were excluded, arguably these men might not have any benefit of GnRH at diagnosis.
Calibration plots for the score were created by splitting the UPSAC and STHLM-0 cohorts in five groups based on 20%-quantiles of the predicted 1, 2, and 3-year risk.
The statistical program package R, version 3.5.2 was used.
Ethical considerations
The Research Ethics Board in Uppsala approved of this study.
Results
Baseline characteristics for the 1603 men included in the study are presented in . Median follow-up was 2 years for the end-point CRPC, and 3 years for Pca death. 788 men reached CRPC, 101 men died of Pca without a prior CRPC date, 288 men died of other causes and 426 men were alive and had not reached CRPC at end of follow-up. Out of 1365 men with a registered ISUP grade, 363 men had died of Pca at end of follow up.
Table 1. Characteristics for men with non-metastatic castration sensitive prostate cancer in the Uppsala-Örebro PSA Cohort and in the Stockholm PSA and Biopsy Register with GnRH as primary treatment.
Median age at date of post-GnRH PSA was 79 years. 854 (53%) men had local clinical T stage 3 or higher, 763 (47%) had ISUP grade ≥ 4 and 1 113 (69%) pre-GnRH PSA > 20 ng/mL ().
The Cox proportional model combining the effects of PSA before and after GnRH initiation did not indicate the presence of an interaction between pre-GnRH PSA and post-GnRH PSA as illustrated by the straight parallel level curves in . Risk for CRPC increased with post-GnRH PSA regardless of pre-GnRH PSA ().
Figure 1. Heat map of the combined effects of pre-GnRH PSA and post-GnRH PSA on the risk of castration-resistant prostate cancer. The straight parallel level curves indicate that there is no interaction between pre-GnRH PSA and post-GnRH PSA. Since they align vertically, post-GnRH PSA is sufficient to describe the risk of CRPC. The red area reflects an increased risk of CRPC. Time to CRPC was not possible to assess for 50 men (grey area) since they already had reached CRPC at date of post-GnRH PSA.
The Cox proportional model for risk of CRPC and the following multivariable analysis showed that risk of CRPC increased with post-GnRH PSA, HR 4.73 (95% CI: 3.36–6.65) for PSA > 16.0 ng/mL vs 0–0.25 ng/mL. There was no relation between Time from GnRH to post-GnRH PSA and risk of CRPC, HR 0.90 (95% CI: 0.76–1.07) for day 146–180 vs day 90–110. The association between pre-GnRH PSA and risk of CRPC was substantially attenuated in the multivariable analysis, HR 1.38 (95% CI: 0.97–1.95) for 200 < PSA ≤ 500 ng/mL vs. PSA 0–10 ng/mL.
Risk of CRPC increased with ISUP grade, HR 3.65 (95% CI: 2.45–5.43) for ISUP 5 vs ISUP 1 ().
Figure 2. Risk of castration-resistant prostate cancer according to characteristics at date of pre-GnRH PSA and post-GnRH PSA. C-index and hazard ratios (HR) for univariable and multivariable Cox regression models. Multivariable models included the variables at date of pre-GnRH PSA and post-GnRH PSA. Abbreviations: C: C-index; M0: no metastases on bone scintigraphy; MX: bone metastases not assessed; ISUP: Pca grading according to the International Society of Urological Pathology; CCI: Charlson comorbidity index.
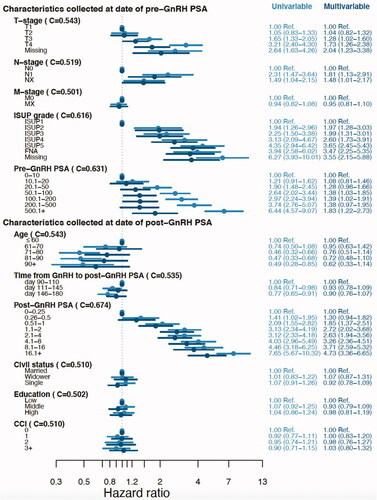
In our cohort, 50% of men had not undergone bone scintigraphy but their risk of CRPC was similar to men without metastases at imaging, HR 0.95 (95% CI: 0.81–1.10) for Mx vs M0.
CRPCrisk-score
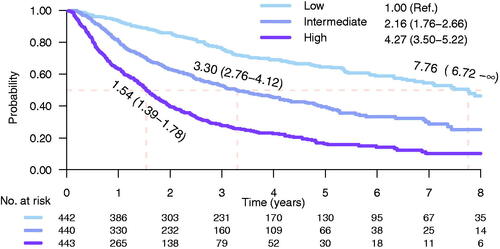
Following the extended analysis of PSA kinetics at start of GnRH treatment only post-GnRH PSA was considered predictive of CRPC ( and ). A risk score was created with the variables considered predictive of CRPC: post-GnRH PSA (C-index 0.67) and ISUP grade (C-index 0.62) (). The CRPCrisk-score was calculated by adding log (post-GnRH) to ISUP grade. A comparison of the coefficients in the CRPCrisk-score indicated that an increase in ISUP grade by one unit corresponded to an approximately doubling of post-GnRH PSA. Based on these observations, a CRPCrisk-score was created and further split in tertiles. Risk of CRPC was substantially higher for men above top vs bellow bottom tertile, HR 4.27 (95% CI: 3.50–5.22). Median time to CRPC was 1.5 years (95% CI: 1.39–1.78) for men above top tertile and 7.8 years (95% CI: 6.72–∞) in the low-risk group (). In a sensitivity analysis, C-index for CRPCrisk-score remained the same (0.70) when multiplying the added numeric ISUP grade by 0.8 (0.70), 1.2 (0.70) or 1.4 (0.70).
Figure 3. Kaplan-Meier estimates of time to castration-resistant prostate cancer for men in the low-, intermediate- and high-risk groups of CRPCrisk-score. Risk groups are defined by post-GnRH PSA and ISUP grade on diagnostic biopsy. Median time to CRPC was 1.5 years for men above the top tertile and 7.8 years for men bellow the bottom tertile. Shadowing represents 95% confidence interval.
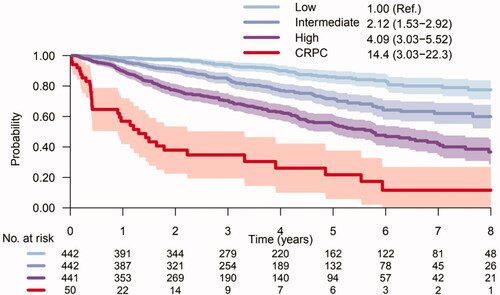
Our risk score showed similar results regarding risk of CRPC and Pca death ( and ). HR for risk of Pca death for men above top vs bellow bottom tertile was 4.09 (95% CI: 3.03–5.52). The 50 men who had reached CRPC already at date of post-GnRH PSA () had a particularly high risk of Pca death, HR 14.40 (95% CI: 3.03–22.30) vs men bellow bottom tertile ().
Figure 4. Risk of prostate cancer death for men in the low-, intermediate- and high-risk groups of CRPCrisk-score. Risk groups are defined by post-GnRH PSA and ISUP grade on diagnostic biopsy. Risk of Pca death for men that already had reached CRPC at date of post-GnRH PSA is given by the red curve. Shadowing represents 95% confidence interval.
Risk of Pca death increased gradually for the men above increasingly higher percentiles of the CRPCrisk-score (Supplementary Figure 1). A sensitivity analysis revealed that the model remained unaltered for risk of Pca death after excluding men with low- and intermediate-risk Pca (Supplementary Figure 2).
Validation of predictive ability
To limit overly optimistic estimation of predictive ability we derived cohort-specific parameter estimates for ISUP grade component of the score. The parameter estimate for ISUP-grade was 1.11 based on UPSAC and 1.16 based on STLHM-0. When applying a CRPCrisk-score derived from UPSAC on the STLHM-0 data, the C-index was 0.72. The corresponding C-index from applying the STLHM-0 model on UPSAC data was 0.68. These compare well to the overall C-index of 0.70.
Calibration of the score was assessed graphically for 1–3 years of follow-up and did not raise any major concern (Supplementary Figure 3).
Discussion
In this population-based register study, PSA 3–6 months after start of GnRH predicted CRPC regardless of pre-GnRH PSA. A risk score based on post-GnRH PSA and ISUP grade at biopsy identified men with high risk of rapid progression to CRPC and Pca death. Risk of CRPC and Pca death increased with four-fold for men above the top vs bellow bottom tertiles of CRPCrisk-score.
The predictive value of a drop in PSA levels after initiation of ADT for risk of progression to CRPC, development of metastases and Pca death has been demonstrated by several reports [Citation20,Citation21]. Most of these studies have used PSA nadir [Citation21–23]. For example, Keto et al. reported a five-fold increased risk of CRPC for men with PSA nadir 0.01–0.2 ng/mL compared with men with undetectable PSA nadir. The use of PSA nadir as a predictor has however limitations since the time point it occurs is variable. We aimed to find early predictors of rapid Pca progression and for that reason we focused PSA 3–6 months after start of GnRH which also reflects a time point commonly used in clinical praxis for assessment of the effect of ADT. Post-GnRH PSA was a strong predictor of CRPC regardless of pre-GnRH PSA which strikes the importance of a prompt PSA follow up upon GnRH initiation in order to ascertain the need of additional therapy. In contrast to some previous reports, we found no association between PSA before castration and risk of CRPC [Citation21,Citation23]. However, in these studies all men had been treated with either external beam radiotherapy or prostatectomy before ADT, whereas in our study no prior local therapy was given before castration.
Other clinical predictors have been associated with poorer Pca outcome (CRPC and Pca death) following ADT such as Gleason score ≥ 8, the presence of metastases at diagnosis and performance status 2–3 (7, 21, 23). One study found that after radical prostatectomy men with Gleason score 8–10 had a four-fold increased risk of CRPC compared to men with Gleason score 6 from biopsies and our results are in line with these findings [Citation21].
Limitations of our study include lack of data on circulating testosterone levels. As a proxy for castrate level we defined castration by use of a GnRH agonist for 90 days or more during a 180 day period and a prospective study has shown castrate testosterone levels in 100% of men following a single injection of GnRH [Citation24]. Another possible limitation is that 50% of the study men lacked information on M stage. However, risk of CRPC was similar for men with no bone metastases on imaging and men who had not undergone imaging. No information on additional therapy with docetaxel was available but it is unlikely that men in the analysis of risk of CRPC had received chemotherapy since Swedish guidelines do not recommend it for men with nmCSPC [Citation25]. Regarding risk of Pca death, it is possible that some men received docetaxel however, data from a population-based study in Sweden from 2013 showed that only 21% of men who died of Pca had received chemotherapy and among men above 80 years, only 5% [Citation26]. Lastly, it should be noted that some of the men in our cohort had low- or intermediate-risk Pca. However, excluding these men from analysis did not alter the risk estimates. Strengths of our study include that it was population-based and included a high number of study men with comprehensive high-quality data from a clinical cancer register and three national health care registries [Citation14,Citation17,Citation27]. In most studies on ADT and CRPC men have been treated with several lines of therapy prior to start of GnRH which complicates the analysis of factors affecting progression to CRPC whereas in our study all men received GnRH as primary treatment for Pca.
Conclusion
A combination PSA levels 3–6 months after start of GnRH with ISUP grade predicted time to CRPC and Pca death and could be used for early identification of a relevant target group for future studies on additional therapy to ADT.
Supplemental Material
Download Zip (160.1 KB)Acknowledgements
This project was made possible by the continuous work of the National Prostate Cancer Register of Sweden (NPCR) steering group: Pär Stattin (chair), Ingela Franck Lissbrant (deputy chair), Johan Styrke, Camilla Thellenberg Karlsson, Hampus Nugin, Stefan Carlsson, David Robinson, Mats Andén, Ola Bratt, Magnus Törnblom, Johan Stranne, Jonas Hugosson, Maria Nyberg, Olof Akre, Per Fransson, Eva Johansson, Gert Malmberg, Hans Joelsson, Fredrik Sandin, and Karin Hellström.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schröder F, Crawford ED, Axcrona K, et al. Androgen deprivation therapy: past, present and future. BJU International. 2012;109(s6):1–12.
- Hamberg P, Verhagen PC, de Wit R. When to start cytotoxic therapy in asymptomatic patients with hormone refractory prostate cancer? Eur J Cancer. 2008;44(9):1193–1197.
- Sternberg CN. Systemic chemotherapy and new experimental approaches in the treatment of metastatic prostate cancer. Ann Oncol. 2008;19(Suppl 7):vii91–vii95.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378(15):1408–1418.
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, Castration-Resistant prostate cancer. N Engl J Med. 2020;382(23):2197–2206.
- Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from southwest oncology group trial 9346 (INT-0162). J Clin Oncol. 2006;24(24):3984–3990.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–160.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383(11):1040–1049.
- Enblad AP, Bergengren O, Andrén O, et al. PSA testing patterns in a large Swedish cohort before the implementation of organized PSA testing. Scand J Urol. 2020;54(5):376–376.
- Aly M, Leval A, Schain F, et al. Survival in patients diagnosed with castration-resistant prostate cancer: a population-based observational study in Sweden. Scand J Urol. 2020;54(2):115–121.
- Statistics Sweden. Population 1 November by region, age and sex. Year 2002–2019; 2018. Available from: https://www.statistikdatabasen.scb.se/pxweb/sv/ssd/START__BE__BE0101__BE0101A/FolkmangdNov/.
- Van Hemelrijck M, Garmo H, Wigertz A, et al. Cohort profile update: The National Prostate Cancer Register of Sweden and Prostate Cancer data Base-a refined prostate cancer trajectory. Int J Epidemiol. 2016;45(1):73–82.
- Wallerstedt SM, Wettermark B, Hoffmann M. The first decade with the Swedish prescribed drug register – a systematic review of the output in the scientific literature. Basic Clin Pharmacol Toxicol. 2016;119(5):464–469.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450.
- Ludvigsson JF, Svedberg P, Olén O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437.
- Fall K, Strömberg F, Rosell J, et al. Reliability of death certificates in prostate cancer patients. Scand J Urol Nephrol. 2008;42(4):352–357.
- Mixed GAM computation vehicle with automatic smoothness estimation. Available from: https://CRAN.R-project.org/package=mgcv.
- Hosmer DL. Applied logistic regression. 2nd ed. New York: Wiley; 2000.
- Eisenberger M, Crawford E, McLeod D, et al. The prognostic significance of prostate specific antigen (PSA) in stage D2 prostate cancer (PC) interim evaluation of intergroup study 0105. J Clin Oncol. 1995;14:235a.
- Keto CJ, Aronson WJ, Terris MK, et al. Detectable prostate-specific antigen nadir during androgen-deprivation therapy predicts adverse prostate cancer-specific outcomes: results from the SEARCH database. Eur Urol. 2014;65(3):620–627.
- Benaim EA, Pace CM, Lam PM, et al. Nadir prostate-specific antigen as a predictor of progression to androgen-independent prostate cancer. Urology. 2002;59(1):73–78.
- Stewart AJ, Scher HI, Chen MH, et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol. 2005;23(27):6556–6560.
- Pettersson B, Varenhorst E, Petas A, et al. Duration of testosterone suppression after a 9.45 mg implant of the GnRH-analogue buserelin in patients with localised carcinoma of the prostate a 12-month follow-up study. Eur Urol. 2006;50(3):483–489.
- Samverkan RCi. Nationellt vårdprogram prostatacancer 2021. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/prostatacancer/vardprogram/.
- Lissbrant IF, Garmo H, Widmark A, et al. Population-based study on use of chemotherapy in men with castration resistant prostate cancer. Acta Oncol. 2013;52(8):1593–1601.
- Tomic K, Sandin F, Wigertz A, et al. Evaluation of data quality in the National Prostate Cancer Register of Sweden. Eur J Cancer. 2015;51(1):101–111.
