Abstract
Background
Urosepsis is a life-threatening condition that needs to be addressed without delay. Two critical issues in its management are: (1) Appropriate empirical antibiotic therapy, considering the patients general condition, comorbidity, and the pathogen expected; and (2) Timing of imaging to identify obstruction requiring decompression.
Objectives
To identify risk factors associated with 30-day mortality in patients with urosepsis.
Methods
From a cohort of 1,605 community-onset bloodstream infections (CO-BSI), 282 patients with urosepsis were identified in a Swedish county 2019–2020. Risk factors for mortality with crude and adjusted odds ratios were analysed using logistic regression.
Results
Urosepsis was found in 18% (n = 282) of all CO-BSIs. The 30-day all-cause mortality was 14% (n = 38). After multivariable analysis, radiologically detected urinary tract disorder was the predominant risk factor for mortality (OR = 4.63, 95% CI = 1.47–14.56), followed by microbiologically inappropriate empirical antibiotic therapy (OR = 4.19, 95% CI = 1.41–12.48). Time to radiological diagnosis and decompression of obstruction for source control were also important prognostic factors for survival. Interestingly, 15% of blood cultures showed gram-positive species associated with a high 30-day mortality rate of 33%.
Conclusion
The 30-day all-cause mortality from urosepsis was 14%. The two main risk factors for mortality were hydronephrosis caused by obstructive stone in the ureter and inappropriate empirical antibiotic therapy. Therefore, early detection of any urinary tract disorder by imaging followed by source control as required, and antibiotic coverage of both gram-negative pathogens and gram-positive species such as E. faecalis to optimise management, is likely to improve survival in patients with urosepsis.
Background
The overall incidence of sepsis worldwide is estimated to be 31.5 million cases per year, causing 5.3 million deaths. Detection and management of sepsis has become a main priority for many hospitals, and sepsis is recognised by the World Health Organisation as a serious problem [Citation1,Citation2]. It is crucial to differentiate between sepsis and septic shock because of the high mortality rate in the latter [Citation3–5].
Urinary tract infection (UTI) is the source in approximately 10–30% of all sepsis cases, with high morbidity and mortality [Citation1,Citation5–7]. Complicated UTI (cUTI) is the most common cause of urosepsis in adults over the age of 65 [Citation8]. It is essential to diagnose urosepsis rapidly and to provide time-sensitive antibiotic treatment, supportive therapy, and source control [Citation9]. Conditions that predispose to febrile UTI include any structural anatomic and/or functional abnormality that impedes urine flow [Citation10–13], and the main reason for uroseptic shock is urinary tract obstruction [Citation14]. Therefore, patients with urosepsis usually require early radiological evaluation to rule out any obstructive urinary tract disorder.
As a clinician facing a patient with suspected or proven urosepsis there are two critical issues. The first is the choice of appropriate empirical antibiotic treatment and dosage, taking into account the patient’s general condition, comorbidity, and the pathogen expected, especially in view of increasing antibiotic resistance amongst Enterobacteriaceae [Citation15–17]. The second is the timing of imaging for diagnosis and possible source control to rule out obstruction requiring decompression.
In this study on patients with community-onset bloodstream infection (CO-BSI) during 2019 and 2020, a well-defined retrospective cohort was selected to determine risk factors for urosepsis-related mortality within 30 days of the date when the first positive blood culture was taken.
Methods
Study design and setting
Patients were selected from an open cohort of all patients with culture-confirmed CO-BSI in a Swedish county between 1 January 2019 and 31 December 2020. The setting and criteria for assessment of bacteraemia have been published previously [Citation16]. The population of Östergötland County was 462,000 in 2019 and 467,000 in 2020, of a total Swedish population of approximately 10 million.
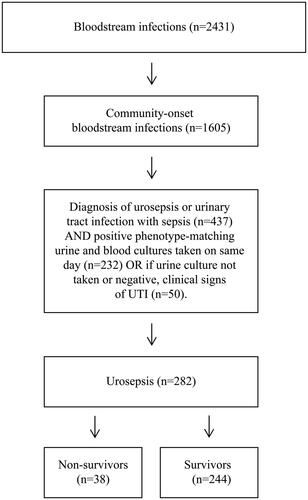
Inclusion criteria were adults ≥ 18 years of age resident in the County of Östergötland, treated at any time during the study period in one of the county’s hospitals. The patients were required to have: (1) Culture-confirmed CO-BSI with a significant pathogen; (2) ICD-10 diagnosis code for urosepsis or urinary tract infection with sepsis (A40-A40.3, A40.8–9, A41-A41.9, R65.1-2, R57.2, N30, N10.9, N12, N39.0) at discharge or death registration; and (3) Positive urine culture showing bacteria with phenotype matching blood sample cultures taken on the same day, or, if urine culture not taken or negative, clinical signs of UTI ().
Data collection
The following data were collected from the laboratory database: blood culture results; number of aerobic and anaerobic blood culture vials taken; site of puncture; species identification; and susceptibility pattern. The dataset was entered into a secondary database where it was linked to the patient-administration system providing the following data for all patients with a positive blood culture: sex; age; ICD-10 diagnosis codes; comorbidity (Supplementary Appendix: Table A1); admitting department; date of admission; date of discharge; and all-cause mortality.
All patients ≥ 18 years of age diagnosed with CO-BSI during 2019 and 2020 were extracted from the records and cross-matched with the patient-administration system for the diagnosis of urosepsis or urinary tract infection with sepsis (ICD-10 codes). These were then matched with data from the bacteriology laboratory register. Phenotype matching between microorganism in blood and urine samples (taken on the same day) were performed to confirm the presence of the same microorganism in the blood and urinary tract. Of the cohort of 282 patients, 38 died within and 244 survived 30-days.
All patient data were registered using a Case Report Form (CRF). The following data on admission were collected: limited life-sustaining treatment (LLST); vital signs; laboratory data; time in the emergency department (ED); time to fluid administration and antibiotic treatment; other sepsis treatment. Habitual-, admission-, and 24-hour SOFA scores were calculated (Supplementary Appendix: Table A2) giving sepsis on admission and sepsis at 24 h (sepsis defined as a life-threatening organ dysfunction, defined as 2 or more delta-SOFA (total maximum SOFA (Sequential Organ Failure Assessment) score minus habitual total SOFA score) due to the infection [Citation1]. CT-scan or ultrasound reports of a urinary tract disorder such as obstruction, renal abscess, urolithiasis, and hydronephrosis were registered. All diagnoses made within 24 months prior to the BSI were obtained from the patient-administration system, and Charlson Comorbidity Scores were then entered in the database.
Microbiology techniques
All isolated microorganisms were analysed at species level. Microorganism identification and susceptibility were determined at the regional clinical microbiology department. Matrix-assisted laser desorption ionisation time-of-flight mass-spectrometry (MALDI-TOF MS) was used for microbial identification.
Ethics approval
The Linköping Regional Ethics Committee (2017/300-31) approved the study.
Statistical analysis
Descriptive analysis included percentages, means, and medians. Numerical and categorical variables were analysed using Students T-test, Mann-Whitney U tests, Chi-square test, or Fisher’s exact test. A p-value < 0.05 was considered statistically significant. Covariates significant in crude analyses were used in a logistic regression model with mortality as dependent variable to investigate possible risk factors for 30-day mortality. Associations were expressed as odds ratio (OR) for mortality with 95% confidence intervals (CIs). All statistical analyses were performed with SPSS version 27.
Results
Demographics, comorbidity, and clinical characteristics
In this study, a total of 2,431 BSI episodes were identified during 2019 and 2020, of which 1,605 (66%) were CO-BSIs. From this group, a total of 282 (18%) patients were identified with urosepsis and included in the study (). Of those, 38 (14%) died within 30 days. The overall weighted Charlson Comorbidity score was 2.8 (SD = 2.5), and the most common comorbidities were congestive heart failure (23%), diabetes without chronic complications (18%), renal disease (18%), and malignancy (18%). The cohort was divided into non-survivors (n = 38) and survivors (n = 244) to determine mortality risk factors. No significant differences in LLST or comorbidity were seen between non-survivors and survivors prior to admission to hospital. However, non-survivors had higher SOFA scores compared to survivors on admission, with 95% (n = 36) of non-survivors and 91% (n = 223) of survivors fulfilling the Sepsis-3 criteria. Demographic, comorbidity, and clinical characteristics of the patients are provided in and , and Supplementary Appendix: Table A3.
Table 1. Demographics, comorbidity, and comparison of patients according to 30-day all-cause mortality in a cohort of 282 urosepsis patients.
Table 2. Comparison of clinical characteristics of non-survivors and survivors in community-onset urosepsis.
We observed a significant difference in time to diagnostic CT-scan or ultrasound (885 min in non-survivors vs 622 min in survivors, p < 0.01), and in the time thereafter to decompression (455 min vs 305 min, p < 0.01). There were significant differences between non-survivors and survivors regarding presence of a urinary tract disorder (58% vs 17%, p < 0.01), and adequate empirical antibiotic treatment based on bacterial culture (76% vs 92%, p < 0.01). There were no significant differences between non-survivors and survivors regarding the presence of urinary catheter before admission, choice of empirical antibiotics, correct choice of empirical antibiotics based on local recommendations, time to antibiotic treatment, time to ICU, or time to intravenous fluids (, Supplementary Appendix: Table A4). Urological intervention was performed on 19 non-survivors compared to 19 survivors. In total, 79% (n = 34) of patients with hydronephrosis were decompressed with nephrostomy or stent (JJ-catheter). Placement or replacement of an acute nephrostomy was performed in 29 patients (15 non-survivors vs 14 survivors), a JJ-catheter in five patients (2 vs 3), and four patients underwent drainage of abscess (2 vs 1) or nephrectomy (0 vs 1).
Microorganisms
A total of 308 microorganisms were obtained from 282 cultures, 272 (96%) of which were monomicrobial. In all, 238 (84%) of the primary microorganisms cultured were gram-negative with a 30-day all-cause mortality of 9.7%. The most common gram-negative bacterial species were Escherichia coli (E. coli) (62%), Klebsiella pneumoniae (5%), and Extended Spectrum Beta-Lactamase (ESBL) producing E. coli (4%). Forty-three (15%) of the isolates were gram-positive with a 30-day all-cause mortality of 33%. The most common gram-positive species were Enterococcus faecalis (E. faecalis) (6%) and Staphylococcus aureus (S. aureus) (3%). Furthermore, gram-negative bacteria where more common among survivors (214, 88%), compared to non-survivors (23, 61%; p < 0.01) and vice versa for gram-positive bacteria. E. coli (70%) were the most common bacteria among survivors, and E. faecalis (21%) among non-survivors ().
Table 3. Distribution of microorganisms in patients with community-onset urosepsis.
Crude analysis
Several factors associated with a significant increased risk for 30-day mortality were identified: (1) Severity of illness (In-SOFA and max 24-hour SOFA scores); (2) Time from admission to CT-scan or ultrasound; (3) Urinary tract disorder; (4) Time to decompression; and (5) Inadequate empirical antibiotic treatment based on bacterial culture. The predominant risk factors for 30-day mortality in the crude analysis were: urinary tract disorder (OR = 6.81, CI = 3.29–14.07); inadequate empirical antibiotic treatment based on bacterial culture (OR = 3.48, CI = 1.45–8.35); and in-SOFA score (OR = 2.49, CI = 1.60–3.88) ().
Table 4. Risk factors for 30-day mortality in patients with urosepsis.
Multivariable analysis
In the multiple logistic regression analysis of 30-day mortality, urinary tract disorder was the dominant risk factor (OR = 4.63, CI = 1.47–14.56) followed by inadequate empirical antibiotic treatment based on bacterial culture (OR = 4.19, CI = 1.41–12.48) and SOFA score at 24 h (OR = 1.98, CI = 1.29–3.05) (). In patients with a urinary tract disorder, time to CT-scan from admission (OR = 1.01, CI = 1.00–1.01) and time from CT-scan to decompression by nephrostomy or stent (OR = 1.01, CI = 1.00–1.02) were the most important factors associated with survival (Supplementary Appendix: Table A5). Furthermore, these patients had a significantly increased risk for inadequate empirical antibiotic treatment based on bacterial culture (Supplementary Appendix: Table A6).
Discussion
Several independent risk factors were associated with 30-day mortality in CO-BSI patients with urosepsis during the 2-year study period. In a multivariable logistic regression model, urinary tract disorder, inadequate empirical antibiotic treatment, and severity of illness (In-SOFA and 24-hour SOFA score) were associated with a significantly increased risk for 30-day all-cause mortality.
The covariate exhibiting the strongest association with 30-day mortality was urinary tract disorder, the most common ones being hydronephrosis caused by obstructive stone in the ureter, renal abscess, urological malignancy, and displaced nephrostomy. Clinically important associations between the presence of urinary tract disorders and urosepsis have been reported previously [Citation7,Citation10,Citation18], and several studies recommend radiology in patients at risk, or cases with poor response to initial treatment [Citation19–21]. In most previous studies, all patients with febrile UTI have been studied, not just those with urosepsis. In the present study on a selected retrospective cohort with urosepsis, a strong correlation was seen between urinary tract disorder and 30-day mortality, which clearly justifies early radiology to assess the urinary tract.
The second most important risk factor was inadequate empirical antibiotic treatment according to the pathogen cultured and its susceptibility pattern. It is crucial to recognise urosepsis as soon as possible and to initiate time-sensitive antibiotic treatment [Citation9]. The overall 30-day mortality rate was 14%, and of these 61% had a gram-negative urosepsis. The most common pathogen isolated was E. coli followed by other Enterobacteriaceae spp. There is growing concern regarding the worldwide increase in prevalence of ESBL-producing Enterobacteriaceae [Citation17,Citation22,Citation23]. In this study, 4% of cases were caused by ESBL E. coli causing 11% of deaths. An interesting finding was the high percentage of gram-positives (37%) related to 30-day mortality, in particular E. faecalis seen in 21% of deaths. This could possibly have been the consequence of lack of Enterococcal coverage in Swedish empirical treatment recommendations.
The third and fourth most important risk factors were severity of illness (In-SOFA and max 24-hour SOFA scores), as expressed by the SOFA score. This may possibly have been due to delay on the part of the patient or delay in prehospital care [Citation24]. Studies show that sepsis is difficult to diagnose in the early stages and this may explain the severity of illness of non-survivors on arrival at the ED [Citation25,Citation26], though more virulent pathogens or patient-specific factors could also have played a role.
Time to decompression by ureteric stenting or percutaneous nephrostomy were important factors for increased survival in urosepsis [Citation11]. In this study, significant delays in kidney decompression were seen in non-survivors. Delay may have been due to complexity of the procedure or the time of day when the need for decompression was discovered (limited access to staff and facilities for emergency decompression at night), or because seriously ill patients have an increased need for stabilisation and optimisation before intervention. Age is a well-known risk factor for BSI, sepsis, urosepsis, and mortality [Citation16], which was also the case in this study.
Previous studies have shown a significant association between number of comorbidities and mortality [Citation16,Citation27]. Diabetes, renal disease, and cancer were the comorbidities that had the greatest association with non-survival in this study. Diabetes has previously been shown to increase the risk for urinary tract disease [Citation10]. Inadequate empirical antibiotic treatment [Citation28], renal disease, and cancer may negate any patient-specific factors that would otherwise increase the chance of survival in BSI.
A limitation of this study is the small sample size and lack of a matching control group. This was a consequence of the strict inclusion criteria to reduce the risk of simple febrile UVI cases being included. This probably led to an under-estimation of the prevalence of urosepsis. Urosepsis, in this study, refers to the ICD-10 codes for urosepsis and not all patients have sepsis according to the Sepsis-3 criteria. However, 96% of all patients fulfilled the Sepsis-3 criteria within 24 h. There was no matching control group, but this probably did not affect our results since the distribution was 1:6. Furthermore, since this was a hospital-based study, we did not analyse prehospital management.
Another limitation is that radiology was not performed in all cases, leading to possible under-estimation of the number of urinary tract disorders. However, since the indication to perform radiology did not differ between patients, this probably had a minor influence on the results. Another limitation is that multiple foci of infection may be present, especially in cases of urosepsis caused by gram-positive bacteria. Furthermore, we only studied empiric antibiotic treatment, change of antibiotic treatment during admission for urosepsis was not considered. Since the prevalence of resistant bacteria in Sweden is low, this could only have had a minor influence on the results and both groups probably received adequate treatment by the time the blood culture results came.
In general, urosepsis has a higher survival rate than other forms of sepsis, possibly due to rapid identification of the source and achievement of source control by surgical intervention with minimal tissue damage, thus removing or reducing the focus of infection [Citation29]. Source control and adequate antibiotics are usually sufficient to combat urosepsis. Indeed, this study demonstrates the importance of early radiology and rapid decompression if indicated, and adequate empiric antibiotic treatment covering both gram-negative and gram-positives species such as Enterococcus faecalis, in elderly severely ill patients with comorbidity.
Conclusion
An obstructing urinary tract disorder was the main risk factor for 30-day all-cause mortality in this well-defined cohort of patients with urosepsis. In elderly individuals with comorbidity and severe urosepsis, early radiological evaluation to detect and control the source of infection is essential. This, together with appropriate empirical antibiotic treatment covering both gram-negative and gram-positive bacteria such as E. faecalis, is likely to increase survival.
Supplemental Material
Download MS Word (37.3 KB)Acknowledgements
Thanks to Peter Cox, consultant anaesthetist, for proof-reading the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All relevant data are within the manuscript and its supplementary files.
References
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–377.
- Global report on the epidemiology and burden of sepsis: current evidence, identifying gaps and future directions. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
- Bauer M, Gerlach H, Vogelmann T, et al. Mortality in sepsis and septic shock in Europe, North america and Australia between 2009 and 2019-results from a systematic review and meta-analysis. Crit Care. 2020;24(1):239.
- Vucelic V, Klobucar I, Duras-Cuculic B, et al. Sepsis and septic shock – an observational study of the incidence, management, and mortality predictors in a medical intensive care unit. Croat Med J. 2020;61(5):429–439.
- Levy MM, Artigas A, Phillips GS, et al. Outcomes of the surviving sepsis campaign in intensive care units in the USA and Europe: a prospective cohort study. Lancet Infect Dis. 2012;12(12):919–924.
- Wagenlehner FM, Weidner W, Naber KG. Optimal management of urosepsis from the urological perspective. Int J Antimicrob Agents. 2007;30(5):390–397.
- Bonkat G, Cai T, Veeratterapillay R, et al. Management of urosepsis in 2018. Eur Urol Focus. 2019;5(1):20–28.
- Kalra OP, Raizada A. Approach to a patient with urosepsis. J Glob Infect Dis. 2009;1(1):57–63.
- Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596.
- Sorensen SM, Schonheyder HC, Nielsen H. The role of imaging of the urinary tract in patients with urosepsis. Int J Infect Dis. 2013;17(5):e299-303–e303.
- Bonkat GP, Bartoletti R, et. al. Guidelines on urological infections. 2018. The Netherlands: European Association of UrologyArnhem.
- Serniak PS, Denisov VK, Guba GB, et al. The diagnosis of urosepsis. Urol Nefrol. 1990;1990(4):9–13.
- Wagenlehner FM, Pilatz A, Weidner W. Urosepsis–from the view of the urologist. Int J Antimicrob Agents. 2011;38(Suppl):51–57.
- Hofmann W. [Urosepsis and uroseptic shock]. Z Urol Nephrol. 1990;83(6):317–324.
- Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in enterobacteriaceae bacteraemia: a systematic review and Meta-analysis. J Antimicrob Chemother. 2007;60(5):913–920.
- Holmbom M, Giske CG, Fredrikson M, et al. 14-Year survey in a swedish county reveals a pronounced increase in bloodstream infections (BSI). Comorbidity – An independent risk factor for both BSI and mortality. PLoS One. 2016;11(11):e0166527.
- Holmbom M, Moller V, Nilsson LE, et al. Low incidence of antibiotic-resistant bacteria in South-east Sweden: an epidemiologic study on 9268 cases of bloodstream infection. PLoS One. 2020;15(3):e0230501.
- Wagenlehner FM, Pilatz A, Naber KG, et al. Therapeutic challenges of urosepsis. Eur J Clin Invest. 2008;38(Suppl 2):45–49.
- James JM, Testa HJ. Imaging techniques in the diagnosis of urinary tract infection. Curr Opin Nephrol Hypertens. 1994;3(6):660–664.
- Kaplan DM, Rosenfield AT, Smith RC. Advances in the imaging of renal infection. Helical CT and modern coordinated imaging. Infect Dis Clin North Am. 1997;11(3):681–705.
- Kawashima A, Sandler CM, Goldman SM. Imaging in acute renal infection. BJU Int. 2000;86(Suppl 1):70–79.
- European Center for Disease Prenvention and Control. Surveillance Atlas of Infectious Diseases. 2017. Available from: https://atlas.ecdc.europa.eu/public/index.aspx.
- Swedres-Svarm 2016. Consumption of antibiotics and occurrence of resistance in Sweden. Solna/Uppsala ISSN1650-6332.
- Whiles BB, Deis AS, Simpson SQ. Increased time to initial antimicrobial administration Is associated With progression to septic shock in severe sepsis patients. Crit Care Med. 2017;45(4):623–629.
- Sjosten O, Nilsson J, Herlitz J, et al. The prehospital assessment of patients with a final hospital diagnosis of sepsis: results of an observational study. Australas Emerg Care. 2019;22(3):187–192.
- Levy MM, Dellinger RP, Townsend SR, et al. The surviving sepsis campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Intensive Care Med. 2010;36(2):222–231.
- Hattori H, Maeda M, Nagatomo Y, et al. Epidemiology and risk factors for mortality in bloodstream infections: a single-center retrospective study in Japan. Am J Infect Control. 2018;46(12):e75–e79.
- Honda H, Higuchi N, Shintani K, et al. Inadequate empiric antimicrobial therapy and mortality in geriatric patients with bloodstream infection: a target for antimicrobial stewardship. J Infect Chemother. 2018;24(10):807–811.
- Wagenlehner FM, Lichtenstern C, Weigand MA, et al. [Urosepsis and treatment]. Urologe A. 2010;49(5):618–622.