Abstract
Objective
Guidelines support considering selected men with ISUP grade group (GG) 2 prostate cancer for active surveillance (AS). We assessed the association of clinical variables with unfavorable pathology at radical prostatectomy in low-volume GG 2 prostate cancer on biopsy in a retrospective cohort.
Materials and methods
This was a retrospective analysis of 378 men with low-volume (≤ 2 cores) GG 2 localized prostate cancer who underwent prostatectomy at a single tertiary cancer center. Multivariable logistic regression of unfavorable pathology, upgrading to ≥ T3, or GG ≥ 3 was performed in relation to clinical factors, common variables used in AS in GG 1 and percentage Gleason 4 at biopsy. We compared the performance of potential variables with commonly used combined AS restrictions in GG 1 prostate cancer.
Results
In total, 128/378 (34%) men had unfavorable pathology at radical prostatectomy. On multivariable analysis, > 5% Gleason pattern 4 was independently associated with an increased risk of GG ≥ 3. A maximum percentage core involvement > 50% was independently associated with an increased risk of pT-stage ≥ 3 and unfavorable pathology. Restriction to patients with ≤ 5% Gleason 4 decreased the upgrading of both unfavorable pathology (OR = 0.62, p = 0.041) and GG ≥ 3 (OR = 0.17, p = 0.0007) compared to the full cohort, while restriction to those with ≤ 50% of max core involvement did not.
Conclusion
In low-volume GG 2, the percentage of Gleason 4 of ≤ 5% was the strongest predictor in reducing upgrading at final pathology. This easily available pathological descriptor could be used to guide urologists and patients when considering AS in this setting.
Introduction
Active surveillance (AS) for prostate cancer is a well-established treatment alternative for men with localized low risk disease [Citation1–4]. Furthermore, AS is recommended in most guidelines as the primary modality for men with ISUP grade group 1 (GG 1; Gleason 3 + 3) disease [Citation5,Citation6]. There is increasing interest in AS for select patients with Gleason grade group 2 (GG 2; Gleason 3 + 4) prostate cancer due to their low risk of metastasis and prostate cancer specific death, though these remain elevated compared to GG 1 disease [Citation7,Citation8]. To date, only a few AS studies have included GG 2 patients. Criteria for selecting appropriate GG 2 patients who can be safely managed by AS remain unclear.
Studies assessing upgrading to unfavorable pathology have found that patients diagnosed with GG 2 disease on biopsy and ≤ 2 positive cores have a lower risk of adverse pathology on radical prostatectomy (RP) compared to those with > 2 cores [Citation9]. However, there was substantial heterogeneity within groups.
In this study, we aimed to evaluate the association of common clinical variables with the risk of upgrading at RP in patients with low-volume GG 2 prostate cancer (≤ 2 cores on biopsy of GG 2 disease). We hypothesized that there are subgroups of patients with GG 2 prostate cancer who are at lower risk of pathologic upgrading and may be good candidates for AS.
Methods
Patients at our tertiary cancer care center (Princess Margaret Cancer Center, Toronto, Canada) who underwent RP between January 2005 and October 2019, with a prior biopsy of GG 2 were identified and underwent retrospective chart review. Patients with one to two cores of GG 2 disease and any number of GG 1 cores were eligible for inclusion. Biopsies were taken with different protocols and templates during the study period, mainly taken transrectally.
The cohort consisted of patients with PSA < 20 ng/ml, clinical stage < T3 and N0/NX, M0/MX. Patients who had surgery performed later than 6 months after their biopsy were excluded to minimize the risk of grade or stage change from diagnosis to treatment [Citation10]. Men who had magnetic resonance imaging (MRI) of the prostate before biopsy were also excluded, as prebiopsy MRI was not standard practice in Canada and these patients might have a higher/different risk of grade inflation [Citation11].
Clinical factors such as age, cT-Stage, PSA, PSA density, percent positive cores, biopsy performed in an academic center, maximum percentage core involvement, as well as percentage Gleason pattern 4 were assessed for their association with the risk of upgrading at radical prostatectomy pathology. A cutoff for percentage Gleason 4 of 5% was selected a priori based on existing literature [Citation7]. Upgrading was defined as an upgrade in ISUP Grade group (GG ≥ 3), pathological stage (≥ pT3) or to unfavorable pathology at RP (any of: GG ≥ 3, ≥ pT3 and/or N1).
Potential predictors were assessed using multivariable logistic regression analyses. Sensitivity analysis was performed using multiple imputation for missing values (R ‘mice’ function using the random forest classifier). We also applied commonly-used GG 1 restrictions from the University of Toronto (T1c/T2a,PSA ≤ 10) [Citation4], Royal Marsden Hospital (T1/T2, PSA ≤ 15, ≤ 50% of total cores positive) [Citation12], Prostate Cancer Research International Active Surveillance (PRIAS) (T1c/T2, PSA ≤ 10, PSA density < 0.20, ≤ 2 cores positive) [Citation13] and Memorial Sloan Kettering Cancer Center (MSKCC) (T1/T2a, PSA ≤ 10, ≤ 3 cores positive, ≤ 50% of single core positive) [Citation14] on the cohort. These restrictions, and those generated from our data, were evaluated by comparing the odds of upgrading in each restricted cohort to that of the full baseline cohort using univariable logistic regression.
Results
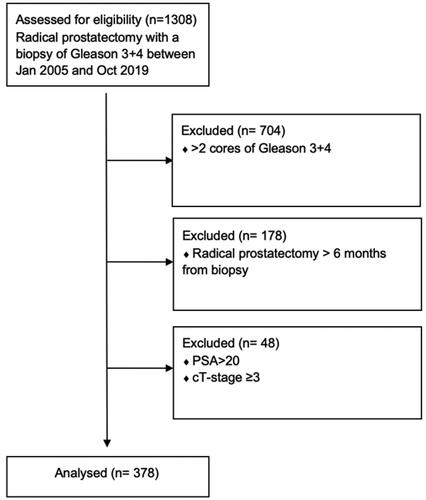
We identified 1,308 men who were diagnosed with GG 2 prostate cancer on biopsy between 2005–2019 and underwent RP (). Of these, 604 patients had a maximum of two GG 2 cores. After including men operated on within 6 months of their biopsy only and applying PSA and cT-stage criteria, 378 patients met the inclusion criteria for analysis.
The clinical and pathological characteristics of the patients are presented in . Of the 378 men, on final RP pathology, 53 (14%) patients had GG 1, 283 (75%) had GG 2 and 42 (12%) had ≥ GG 3 disease. One hundred and three (27%) men were upgraded to ≥ pT3 on RP and two (0.5%) men upgraded to N1. One hundred and twenty-eight (34%) men had unfavorable pathology.
Table 1. Clinical and pathological characteristics of patients (n = 378).
In a complete case multivariable analysis (), percent Gleason 4 at a threshold greater than 5% was associated with a statistically significant increase in the risk of upgrading at RP to GG ≥ 3 (Odds Ratio [OR] = 9.26, 95% Confidence Interval [CI] = 2.58–33.2, p < 0.001). A single core with > 50% cancer of any Gleason score increased the risk of pT-stage ≥ 3 (OR = 2.61, 95% CI = 1.46–4.69, p = 0.0013) and unfavorable pathology (OR = 1.85, 95% CI = 1.05–3.25, p = 0.033).
Table 2. Complete case multivariable analysis.
After multiple imputation of the missing values (), the association of maximum core percentage > 50% cancer with an increased risk of pT-stage ≥ 3 and unfavorable pathology at radical prostatectomy remained significant (OR = 2.53, 95% CI = 1.52–4.23, p < 0.001; and OR = 1.86, 95% CI = 1.14–3.05, p = 0.014, respectively). A biopsy with > 5% Gleason 4 also remained significant for predicting an upgrade to GG ≥ 3 (OR = 4.31, 95% CI = 1.48–12.5, p = 0.011).
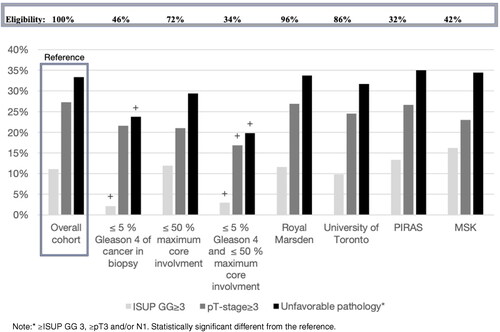
The variables were applied as restriction criteria to the full cohort to assess their effect on patient selection and risk of early upgrading. Patients eligibility in the cohort for each restriction was 139/301 (46%), 252/305 (72%) and 101/301 (34%) for the ≤ 5% Gleason 4, ≤ 50% maximum percentage core involvement and the two restrictions combined, respectively.
Restricting for ≤ 5% Gleason 4 patients alone led to a decrease in GG ≥ 3 upgrade from 42/378 (11%) to 3/139 (2%) cases. Restricting for ≤ 50% maximum cancer core involvement, there was a small decrease in pT-stage ≥ 3 upgrade from 103/378 (27%) to 53/252 (21%). The combined restriction of both ≤ 5% Gleason 4 and ≤ 50% cancer maximum core involvement resulted in a decrease in unfavorable pathology from 126/378 (33%) to 20/101 (20%) cases ( and Appendix 1).
An analysis of the restricted cohorts versus the baseline cohort are presented in , with further details in Appendix 1. Less than or equal to 5% Gleason 4 was associated with a significantly reduced risk of GG ≥ 3 upgrading on final pathology (OR = 0.17, 95% CI = 0.05–0.55, p = 0.0007) and a significantly reduced risk of unfavorable pathology (OR = 0.62, 95% CI = 0.40–0.97, p = 0.041). Maximal core involvement ≤ 50% cancer was not significant in reducing upgrading for any of the three outcomes.
The combination of ≤ 5% Gleason 4 with ≤ 50% maximal core involvement was significant in reducing pathological upgrading in all the outcomes (OR = 0.24, 95% CI = 0.07–0.81, p = 0.021; OR = 0.54, 95% CI = 0.31–0.95, p = 0.034; OR = 0.49, 95% CI = 0.29–0.84, p = 0.0096). None of the other tested restrictions could significantly reduce pathological upgrading in any of the three outcomes, as described in .
Discussion
This study demonstrated that, in men with low-volume GG 2 disease, ≤ 5% Gleason pattern 4 in the diagnostic biopsy specimen was strongly associated with a reduced risk of GG ≥ 3 disease and unfavorable pathology in the final RP pathology. A single core of ≤ 50% cancer (Gleason score 3 or 4) was initially predictive of a reduced risk of pT-stage upgrade in the multivariate analysis, but this was not maintained in the restricted cohort analyses. The performance of the combination of these two factors was mostly driven by the ≤ 5% Gleason 4.
With increasing evidence of the low risk of prostate cancer specific mortality in GG2 patients, as presented in the 29-year follow-up in the SPCG-4 study [Citation15], there is increased interest in expanding AS to these patients, also highlighted by the high prevalence of GG2 in tumors incidentally detected on autopsy [Citation16], suggesting that much of the GG 2 cancer will not present with symptoms increasing the need to select certain GG 2 men for AS.
A recent retrospective study by Carlsson et al. [Citation17] found that deferred treatment for GG 2 was a safe initial management strategy in the short-term. However, the risk of developing metastases is higher for patients with a Gleason 4 component [Citation18], and studies have shown a threefold increase in the risk of adverse pathology in intermediate compared to low risk prostate cancer [Citation19]. Furthermore, screening trials have shown a lower rate of failure-free survival for intermediate versus low-risk prostate cancer [Citation20]. As a result, GG 2 patients still present a clinical conundrum and most go directly onto treatment in clinical practice.
Our results show that patients in our cohort with low-volume GG 2 cancer at biopsy who exhibit ≤ 5% Gleason 4 have a very low risk of upgrading to GG ≥ 3 at surgical pathology. However, as seen in the risk of upgrading to a high risk PCa (GG ≥ 4) was very low (2%) in the entire cohort, indicating that this low volume pre-MRI GG 2 biopsy cohort has a low risk of harboring hidden ≥ GG4 disease.
The combination of adding ≤ 50% cancer in any core strengthened the results and reduced the risk of an adverse outcome but came at the cost of reducing potential patient eligibility from 46% to 34% in this cohort. This reduction in risk was driven through a reduction in upstaging to pT-stage ≥ 3.
Our results are in line with several published studies suggesting the critical impact of the percentage of Gleason pattern 4 on biopsy on the risk of upgrading/upstaging at RP [Citation21]. Cole et al. [Citation22] found that an incremental percentage of Gleason 4 predicted adverse pathology and biochemical recurrence across the entire range of percent Gleason 4 disease. Perlis et al. [Citation23] showed that, for each quantitative percent of Gleason pattern 4 increase, there were 2% higher odds of a pT3 or greater disease.
Our study is the first specifically evaluating men with low-volume GG 2 (one to two GG 2 cores) in order to identify those at low risk of upgrading to GG ≥ 3 at RP who therefore could be safely managed by AS.
Notably, while ≤ 5% Gleason 4 significantly decreased the odds of GG ≥ 3, no effect on pT-stage ≥ 3 was observed. This suggests that the combination of this criterion with a test or parameter that would reduce the risk of upstaging to pT-stage ≥ 3 might identify the ideal population with low-volume GG 2 disease suitable for AS. Although it could not be directly evaluated in this study, MRI has been shown to reduce extracapsular extension on surgical pathology [Citation24], and combination with MRI would likely reduce the risk of unfavorable pathology at RP substantially. To this end, MRI has been explored in the GG 2 setting with promising results [Citation25], but not in combination with the percentage of Gleason pattern 4.
The major limitation of our study is its retrospective nature with resulting selection, unmeasured confounders, and confounding biases in biopsied patients that went for surgery, radiation, active surveillance, and watchful waiting. However standard of practice during most of the study years have been active treatment in the GG 2 setting which should limit some of the unmeasured selection bias. Furthermore, biopsies and pathology reports were performed at multiple centers, increasing the risk of intra-observer variability and also resulted in some missing covariate data from outside centers.
A second major limitation is the exclusion patients who underwent MRI. MRI was not standard practice in Ontario, Canada, during the years of this study, and a targeted biopsy with multiple cores taken for a suspicious lesion of the prostate might have interfered with our initial low-volume criteria. Most patients still undergo a first systematic biopsy without the addition of an MRI – although this might be changing in the near future – making our results applicable for most primary diagnosed patients. This study can, therefore, help patients and doctors not routinely choose active treatment in a low-volume GG 2 setting but instead push toward AS, and in most places of the world an MRI would then be the next step.
Thirdly, the lack of long-term follow-up limits the interpretation on definitive correlations between our results and prostate cancer recurrence or prostate cancer specific death. There is also not a definitive correlation between histopathology and disease progression and subsequent treatments, as demonstrated by Kovac et al. [Citation26] in low-risk PCa. We also lack information extent of pT3 disease on final pathology, further limiting the prognostic interpretation.
These results strengthen previous results demonstrating that men with low-volume ≤ 5% Gleason pattern 4 on biopsy prostate cancer are at low risk of adverse pathology on RP. We believe that our results support including the percentage of Gleason 4 when discussing AS with patients with low-volume GG 2 prostate cancer. A combination with MRI will likely strengthen these results but requires further research.
Conclusion
In this retrospective cohort the restrictions of low-volume GG 2 both ≤ 50% any core length and ≤ 5% Gleason 4 in biopsy specimen were the strongest predictors regarding which patients would upgrade at subsequent radical prostatectomy. The restriction of ≤ 5% Gleason 4 providing the largest impact provided through fewer patients upgrading their final ISUP GG score compared to the biopsy.
Ethical approval
Ethical approval was granted from Regional ethics board University of Toronto, RIB number 08-0472.
Author contributions
Funding: Johan Björklund.
Study design: Johan Björklund, Douglas C. Cheung, Lisa J. Martin, Maria Komisarenko, Antonio Finelli.
Statistical analysis: Johan Björklund, Katharine Lajkosz, Douglas C. Cheung, Lisa J. Martin, Maria Komisarenko.
Manuscript draft: Johan Björklund, Douglas C. Cheung, Lisa J. Martin, Maria Komisarenko.
Critical review: Johan Björklund, Douglas C. Cheung, Lisa J. Martin, Maria Komisarenko, Robert J. Hamilton, Alexandre R. Zlotta, Antonio Finelli.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33(3):272–277.
- Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167(3):1231–1234.
- Komisarenko M, Martin LJ, Finelli A. Active surveillance review: contemporary selection criteria, follow-up, compliance and outcomes. Transl Androl Urol. 2018;7(2):243–255.
- Klotz LH, Choo R, Morton G, et al. Expectant management with selective delayed intervention for favorable-risk prostate cancer. Can J Urol. 2002;9(Suppl 1):2–7.
- Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243–262.
- Bekelman JE, Rumble RB, Freedland SJ. Clinically localized prostate cancer: ASCO clinical practice guideline endorsement of an AUA/ASTRO/SUO guideline summary. J Oncol Pract. 2018;14(10):618–624.
- Klotz L. Active surveillance in intermediate-risk prostate cancer. BJU Int. 2020;125(3):346–354.
- Preisser F, Cooperberg MR, Crook J, et al. Intermediate-risk prostate cancer: stratification and management. Eur Urol Oncol. 2020;3(3):270–280.
- Ploussard G, Isbarn H, Briganti A, et al. Can we expand active surveillance criteria to include biopsy gleason 3 + 4 prostate cancer? A multi-institutional study of 2,323 patients. Urol Oncol. 2015;33(2):71 e1–9.
- Patel P, Sun R, Shiff B, et al. The effect of time from biopsy to radical prostatectomy on adverse pathologic outcomes. Res Rep Urol. 2019;11:53–60.
- Vickers A, Carlsson SV, Cooperberg M. Routine use of magnetic resonance imaging for early detection of prostate cancer is not justified by the clinical trial evidence. Eur Urol. 2020;78(3):304–306.
- van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008;54(6):1297–1305.
- Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597–603.
- Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185(2):477–482.
- Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in prostate cancer - 29-Year follow-up. N Engl J Med. 2018;379(24):2319–2329.
- Zlotta AR, Egawa S, Pushkar D, et al. Prevalence of prostate cancer on autopsy: cross-sectional study on unscreened caucasian and asian men. J Natl Cancer Inst. 2013;105(14):1050–1058.
- Carlsson S, Benfante N, Alvim R, et al. Risk of metastasis in men with grade group 2 prostate cancer managed with active surveillance at a tertiary cancer center. J Urol. 2020;203(6):1117–1121.
- Yamamoto T, Musunuru HB, Vesprini D, et al. Metastatic prostate cancer in men initially treated with active surveillance. J Urol. 2016 May;195(5):1409–1414.
- Patel HD, Gupta M, Tosoian JJ, et al. Subtyping the risk of intermediate risk prostate cancer for active surveillance based on adverse pathology at radical prostatectomy. J Urol. 2018;200(5):1068–1074.
- Godtman RA, Holmberg E, Khatami A, et al. Long-term results of active surveillance in the goteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760–766.
- Huang CC, Kong MX, Zhou M, et al. Gleason score 3 + 4=7 prostate cancer with minimal quantity of gleason pattern 4 on needle biopsy is associated with low-risk tumor in radical prostatectomy specimen. Am J Surg Pathol. 2014;38(8):1096–1101.
- Cole AI, Morgan TM, Spratt DE, et al. Prognostic value of percent gleason grade 4 at prostate biopsy in predicting prostatectomy pathology and recurrence. J Urol. 2016;196(2):405–411.
- Perlis N, Sayyid R, Evans A, et al. Limitations in predicting organ confined prostate cancer in patients with gleason pattern 4 on biopsy: implications for active surveillance. J Urol. 2017;197(1):75–83.
- Zhang F, Liu CL, Chen Q, et al. Accuracy of multiparametric magnetic resonance imaging for detecting extracapsular extension in prostate cancer: a systematic review and meta-analysis. Br J Radiol. 2019;92(1104):20190480.
- Ploussard G, Beauval JB, Lesourd M, et al. Active surveillance eligibility of MRI-positive patients with grade group 2 prostate cancer: a pathological study. World J Urol. 2020;38(7):1735–1740.
- Kovac E, Vertosick EA, Sjoberg DD, et al. Effects of pathological upstaging or upgrading on metastasis and cancer-specific mortality in men with clinical low-risk prostate cancer. BJU Int. 2018;122(6):1003–1009.