ABSTRACT
The gingival epithelium acts as a physical barrier to separate the biofilm from the gingival tissue, providing the first line of defense against bacterial invasion in periodontal disease. Disruption of the gingival epithelial barrier, and the subsequent penetration of exogenous pathogens into the host tissues, triggers an inflammatory response, establishing chronic infection. Currently, more than 700 different bacterial species have been identified in the oral cavity, some of which are known to be periodontopathic. These bacteria contribute to epithelial barrier dysfunction in the gingiva by producing several virulence factors. However, some bacteria in the oral cavity appear to be beneficial, helping gingival epithelial cells maintain their integrity and barrier function. This review aims to discuss current findings regarding microorganism interactions and epithelial barrier function in the oral cavity, with reference to investigations in the gut, where this interaction has been extensively studied.
Introduction
Epithelial cells interface with the external environment at multiple sites, including airways, the oral cavity, and the gastrointestinal tract, functioning as a barrier against physical, chemical, and microbial insults.Citation1 Maintaining this barrier’s function is essential for health; disruptions constitute a risk factor for a variety of disorders and diseases.Citation2 Dysfunction of the intestinal epithelial barrier, also known as “leaky gut,” facilitates pathogenic agents access to the host, causing a variety of gastrointestinal disorders.Citation1 Inflammatory bowel disease (IBD), a chronic inflammation of the gastrointestinal tract, is closely associated with intestinal epithelial barrier dysfunction, implying that gut epithelial disruption due to microbes as IBD pathogenesis has been extensively studied.Citation3 Peridontitis, a chronic inflammatory disease in the oral cavity, is the most common oral condition in the human population and a major cause of tooth loss.Citation4 Acting as a structural barrier between the underlying tissue and the outside environment, the gingival epithelium provides the first line of defense against exogenous pathogens. A disruption of epithelial cell-to-cell adhesion, known as “leaky gum” condition, is associated with the initiation and progression of periodontal diseases.Citation5
The oral cavity houses the second-most diverse microbial community in the body, harboring over 700 species of bacteria that colonize on the surface of the teeth, gingival crevices, and buccal mucosa.Citation6,Citation7 The epithelial cells lining the gingival tissue are continuously exposed to large numbers of microorganisms. Also, the virulence factors of some periodontopathogens inflame gingival tissue by disrupting the epithelial barrier.Citation8 Some probiotic bacteria in the oral cavity have been shown to prevent periodontal diseases by maintaining and restoring the gingival epithelial barrier function.Citation9 However, the biological interaction between microbes and gingival epithelial cells in barrier function remains largely unknown.
In the present review, we discuss the positive and negative effects of several bacteria on gingival epithelial barrier function and consider possible mechanisms of how bacteria manipulate epithelial barrier function with our current findings.
Materials and methods
We identified studies throughout PUBMED Central electronic database with these following keywords: tissue barrier function AND oral bacteria AND periodontitis AND epithelial cell. Then, only full article papers written in English within the past 10 years were included, screened, analyzed, discussed, and summarized. The outcome of papers investigation that meets our interest were the name of bacterial species, regulated barrier junction markers/genes, and its mechanism (as summarized in ). Moreover, based on reported investigations, we propose plausible mechanism by which bacteria affect the barrier function and regulate the dysbiosis or homeostasis condition in the oral cavity ().
Table 1. Effects of pathogens on gingival epithelial barrier function.
Results and discussion
Components of epithelial barriers
Distinct from the oral epithelium, the gastrointestinal epithelium is composed of a simple layer of columnar epithelial cells. Goblet cells are a major secretory cellular lineage in the intestinal epithelium, synthesizing and secreting mucin into the intestinal lumen. Among other lineages of intestinal epithelial cells, enterocytes are involved in nutrient absorption and immunoglobulin secretion, and Paneth cells can synthesize and produce antimicrobial peptides.Citation23 These specialized epithelial cells are efficient physical and chemical barriers against invading microbes. Unlike the gut, the oral epithelium consists of a stratified squamous epithelium which can be subdivided into three components based on cell morphology: oral epithelium (OE), sulcular epithelium (SE), and junctional epithelium (JE).Citation24 The OE is a keratinizing form of epithelium, providing an effective physical barrier against microbial invasion of the underlying gingival connective tissue. In contrast, SE and JE are dominated by a non-keratinized epithelium,Citation25 which suggests that those epithelia are semipermeable and, thus, allow the transport of macro substances from the gingival sulcus into the underlying connective tissue.
The basal cell layers of all three types of gingival epithelia are composed of rapidly proliferating cells that migrate towards the tissue’s outer surface. In this process, epithelial cells are differentiated and matured. Epithelial cells are well connected through junction structures which reinforce effective barrier function against bacterial invasion.Citation8,Citation26 Certain bacteria and their products disrupt the epithelial barrier by degrading these cell-to-cell junction structures, resulting in epithelial barrier breakdown and subsequent induction of periodontal immune response and inflammation.Citation27 Therefore, the interaction between epithelial cells and pathogenic bacteria is critically involved in initiating periodontal disease.
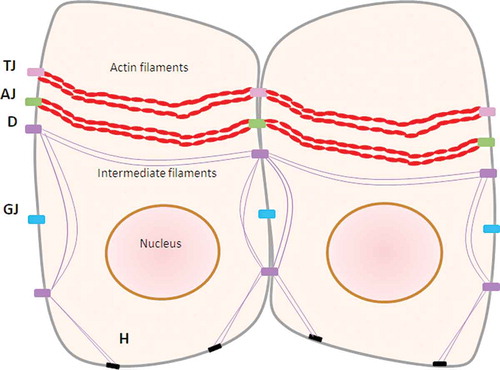
A primarily structural bond between epithelial cells is created by junctional molecules, including tight junctions, adherens junctions, and gap junctionsCitation8,Citation26 (). Tight junctions are responsible for paracellular transport of ions, water, and solutes due to their semipermeable structure.Citation28 Several proteins are found in the tight junctions, such as occludin,Citation29 claudins,Citation30 and zonula occludens (ZO) protein ZO-1, ZO-2, and ZO-3.Citation30–Citation32 Occludin has been detected in the gingival epithelium’s surface layer, whereas claudin-1 was found in the uppermost layer.Citation26 Claudins have barrier properties, which directly regulate gate function at paracellular tight junction channels.Citation30 Adherens junctions play a vital role in controlling the junctional complex activity.Citation8 Adherens junctions are cell-to-cell adhesion sites where the actin-based cytoskeleton and cytoplasmic components are constructed, also known as the classic cadherins function.Citation33 The intercellular communication in gap junctions is involved in homeostasis, regeneration, and developmental processes.Citation34 Furthermore, gap junctions regulate the reciprocal exchange of metabolites and ions of molecular weight ≤1 kDa, such as cyclic adenosine monophosphate and Ca,Citation35+ between adjacent cells. The form of this junction is a head-to-head docking of hexameric structures called connexons, and membrane proteins called connexins.Citation36
Barrier function in periodontal diseases
In addition to cell-to-cell junction structures as a physical barrier, saliva acts as a chemical barrier by containing secretory immunoglobulin A, mucins, and antimicrobial peptides in the oral cavity.Citation8 In the gingival sulcus, the cell turnover rate is remarkably high in both the JE (4–6 days) and SE (6–12 days).Citation37 This is advantageous in that it permits rapid replacement of cells and tissue components damaged by the microbial challenge. However, once a subgingival biofilm is formed and developed, the continuous interface between pathogens in the biofilm and the adjacent gingival epithelial cells leads initiates inflammatory responses in the gingiva. Microbes releases various metabolites, such as butyric and propionic acids, which are toxic to the tissues. Furthermore, microbes also release N-formyl-methionyl-leucyl-phenylalanine peptides, which are potent chemo-attractants for leukocytes. It is known that JE is more permeable than SE; thus neutrophils migrate chemotactically from the blood vessel in the connective tissue through intercellular spaces in the JE towards the biofilm.Citation38 These events lead to the early stages of gingivitis.
Gingivitis is reversible inflammation confined to the gingiva and, when left untreated, can develop into periodontitis.Citation4 This disease is initiated through the cleavage of the second or third layer of cells directly attached to the tooth in the most coronal part of the JE facing biofilms, forming a deep crevice and gingival pocket.Citation24 The accumulation of pathogens in the pocket is subsequently followed by chemotactic cytokines secretion by epithelial cells, depositing neutrophils in the JE. Consequently, the proteolytic enzymes released by the neutrophils disrupt the epithelial barrier.Citation24,Citation39 The disruption of the epithelium thereby allows pathogens and their products to penetrate deeper into the space between epithelial cells and lamina propria, triggering the release of pro-inflammatory cytokines and initiating tissue breakdown and bone resorption.Citation24 Factors which contribute to focal disintegration of the JE structure include an increased degree of gingival inflammation because of the emigration of PMNs, mononuclear leukocytes (e.g., T- and B-lymphocytes), and GCF passing through the intercellular spaces. T-cells are involved in the regulation of epithelial tight junctions, leading to homeostasis or disease direction. Intercellular spaces in the epithelium allow some bacterial products and antigens to enter the lamina propia. If these antigens are taken up by the antigen-presenting cells, such as dendritic cells which direct their differentiation to T helper 1 (Th1) or Th2 thereby activating IFNℽ, TNF, and IL-13, there is an increased flux across the tight junction leak pathway and inflammation.Citation1,Citation24
Aside from neutrophil-mediated epithelial barrier disruption, the strong contribution of periodontopathogens and their virulence factors to barrier disruption have been investigated. An in vitro study demonstrated that treatment with bacterial lipopolysaccharide (LPS) reduces claudin-1 expression in JE, reducing subsequent epithelial barrier disruption.Citation40 Other studies have demonstrated an outer membrane protein of Actinobacillus actinomycetemcomitans decreases connexin-43 levels.Citation17,Citation19,Citation41 Porphyromonas gingivalis and A. actinomycetemcomitans decrease E-cadherin expression in gingival epithelial cells.Citation10,Citation18 Most recently, the authors reported that a P. gingivalis virulence factor degrades gingival epithelial-derived E-cadherin protein, and thus disrupts epithelial barrier functions in vitro. Furthermore, the authors suggested that E-cadherin degradation is involved in the pathogenesis of periodontitis in an experimental mouse model.Citation35 Altogether, these findings suggest that the modulation of epithelial barrier function by microorganisms strongly contributes to the initiation and progression of periodontal diseases.
Interaction between microorganisms and epithelial cells in barrier functions
Accumulating studies have proposed direct and indirect mechanisms by which microbes affect epithelial barrier function. The direct effects are mediated primarily through manipulations of barrier function-related genes/proteins by the microbes or their products, and the indirect effects are mainly mediated by cellular immunoregulatory responses to bacteria.Citation1 For instance, pro-inflammatory cytokines and chemokines, released by epithelial cells in response to microbes, triggers the subsequent local secretion of inflammatory mediators by immune cells, such as IFN-γ and TNF-α; these mediators have been reported to influence epithelial barrier function.Citation42 Both antimicrobial peptides and secretory antibodies play a crucial role in maintaining microbiota homeostasis by reducing the growth of pathogenic organisms; this suggests that bacteria indirectly modify epithelial barrier function.Citation43 In the following sections, we briefly summarize the direct and indirect effects of several bacteria on the epithelial barrier function in both the oral cavity and gut.
In the oral cavity
Putative periodontal pathogens, such as P. gingivalis, Tannerella forsythia, and Treponema denticola, have negative impacts on the gingival barrier function as described below ().
P. gingivalis
P. gingivalis is an anaerobic Gram-negative bacteria described as a keystone pathogen in the pathogenesis of periodontitis, and possesses a variety of virulence factors such as gingipains, LPS, and fimbriae.Citation44 The involvement of P. gingivalis and its virulence factors in gingival epithelial barrier function has been studied extensively. For example, P. gingivalis ATCC 33277, one of the most well-characterized strains, decreases the transepithelial electrical resistance (TEER), which measures epithelial barrier function.Citation10 Another study by Katz and colleagues showed that gingipain, a trypsin-like cysteine proteinase produced by P. gingivalis, disrupted the adherens junctions (E-cadherin, occludin) in epithelial cells.Citation12 Additionally, barrier-related protein degradation is blocked by gingipain-specific inhibitors as well as a gingipain-deficient mutant of P. gingivalis; this suggests a major role of gingipain in epithelial barrier function.Citation8 Abe‐Yutori et al. demonstrated that P. gingivalis LPS decreases E-cadherin expression in gingival epithelial cells, acceleratig P. gingivalis LPS’ penetration of the monolayer.Citation13 Furthermore, Guo et al. demonstrated that LPS derived from P. gingivalis decreases occludin and claudin mRNA transcription,Citation14 indicating epithelial barrier disruption by P. gingivalis LPS. Fimbriae are thin, proteinaceous surface appendages that protrude from a bacterial cell’s outer membrane. Amano et al. reported that P. gingivalis fimbriae mediate adherence to oral epithelial cells, which enable bacteria to invade epithelial cells.Citation15 Given evidence that intracellular P. gingivalis with type II fimbriae degrades cell-adhesive protein,Citation16 P. gingivalis fimbriae appears to play a role in regulating the epithelial barrier.
A. actinomycetemcomitans
A. actinomycetemcomitans is a facultative Gram-negative bacterium, strongly associated with aggressive forms of periodontitis. Uchida et al. demonstrated that whole A. actinomycetemcomitans, especially its outer membrane protein 29, decreased gap junction protein connexin-43 expression in human gingival epithelial cells.Citation19 Furthermore, exposure of gingival epithelial cells to live A. actinomycetemcomitans decreased ZO-1 expression at both mRNA and protein levels.Citation17 Another study reported that the recombinant cyto-lethal distending toxin (Cdt), a putative virulence factor of A. actinomycetemcomitans, changed E-cadherin’s cytosolic distribution, which is accompanied by alteration in intracellular scaffolding proteins β-catenin and β-actin.Citation20 Taken together, these results suggest that A. actinomycetemcomitans substantially regulates adherens junctions, potentially impacting the gingival epithelium’s barrier function.
T. denticola
T. denticola is one of the major pathogens involved in chronic periodontitis along with P. gingivalis and T. forsythia. Uitto et al. reported that T. denticola-treated epithelial multilayers had loose cell contacts, collapsed intercellular spaces, and increased permeability.Citation21 Additionally, Chi et al. demonstrated that wild-type T. denticola degraded the ZO-1 protein, disrupting the epithelial barrier and substantially penetrating the epithelial layers.Citation22 They confirmed that dentilisin, an outer membrane-associated chymotrypsin-like protease of T. denticola, majorly impacts epithelial barrier impairment by comparing the wild-type and mutant strains.
Beneficial bacteria in the oral cavity
Lactobaccilus species
A clinical dentistry study has shown that some strains of Lactobacillus in the oral cavity, including Lactobacillus gasseri (L. gasseri), were associated with periodontal health.Citation45 More recently, a randomized placebo-controlled clinical study showed that oral administration of lozenges containing L. reuteri significantly improved some clinical parameters in periodontitis patients.Citation46 Also, an in vitro study showed that L. paracasei, L. plantarum, L. rhamnosus, and L. salivarius had the strongest antimicrobial ability against epithelial barrier-disrupting pathogens, such as A. actinomycetemcomitans, P. gingivalis, and P. intermedia.Citation45 Some specific strains of L. salivarius, a commensal bacterium in the oral cavity, can attenuate an H2O2-induced decrease in gut barrier function and disruption of tight junctions.Citation47
Bifidobacterium
Distribution of Bifidobacteria in the oral cavity is relatively low compared to Actinomyces, Streptococcus, and Veillonella.Citation48 Despite its relatively minor presence in the oral cavity, the number of Bifidobacterium adolescentis in the healthy control group’s saliva is higher than in the periodontitis group; this suggests that Bifidobacteria is involved in the pathogenesis of periodontal diseases.Citation48 In primary human keratinocyte cells treated with B. longum ATCC51870, the expression of tight junction proteins was mediated by the presence of toll-like receptor-2.Citation49
Streptococcus gordonii
S. gordonii has the Hsa sialic acid-binding protein that facilitates its binding to oral surfaces, thus promoting biofilm homeostasis.Citation50 Previous work has shown that early colonizer bacteria increased ZO-1 and ZO-2 expression in monolayered oral epithelial cells.Citation51 However, no significant changes were confirmed in transepithelial resistance challenged with S. gordonii in multilayered gingival epithelial cells.Citation52 This discrepancy in barrier function between monolayered and multilayered epithelial cells needs to be explored in future studies.
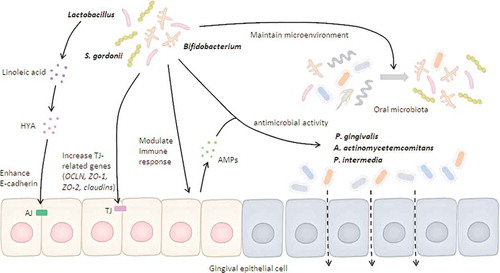
Herein, we proposed possible mechanisms of beneficial microbes in gingival epithelial barrier function, which are illustrated in .
Figure 2. Possible mechanisms of beneficial microbes on gingival epithelial barrier function.
Beneficial bacteria can either induce antimicrobial peptides (AMPs) through host immune response or express direct antimicrobial activity against barrier-disrupting pathogens. Beneficial bacteria and their derivatives (e.g., HYA) maintain the epithelial barrier by enhancing tight junction (TJ)-related gene expression. In addition, beneficial bacteria develop a favorable microenvironment that reduces the viability of barrier-disrupting pathogens. Altogether, both direct and indirect pathways regulated by beneficial bacteria are positively associated with maintenance of gingival epithelial barrier.
In the gut
Intestinal epithelium dysfunction can initiate pathophysiological processes leading to the development of some diseases (e.g., IBD, colitis, and gastrointestinal infection).Citation23 Escherichia coli (E. coli) challenge is the most well-known method to model gut-induced disease.Citation53,Citation54 In contrast, some microorganisms are considered beneficial bacteria because of their positive effects on gut epithelial barrier function. A number of studies have shown that Lactobacillus, Bifidobacterium, and Sreptococcus gordonii positively regulate the epithelial barrier function by direct and indirect mechanisms, as described below.
Lactobacillus species
Lactobacillus is a predominantly lactic acid-producing bacteria found in the human and animal gastrointestinal tract. It is commonly used as a probiotic. In an animal study, dextran sulfate-induced gut hyperpermeability was reduced by administration of a probiotic mixture containing Lactobacillus species.Citation55 In vitro studies using the intestinal epithelial cell line Caco-2 demonstrated that certain Lactobacillus species boosted tight junction formation-related genes (occludin, ZO-1, ZO-2, and cingulin) and inhibited TEER disturbance.Citation56,Citation57
Bifidobacterium species
Bifidobacteria are one of the most predominant bacterial groups detected in the human intestine.Citation48 An investigation using B. longum CCM7952 revealed a significant improvement in epithelial barrier function, especially on tight junction proteins in dextran sulfate sodium-induced colitis in a mouse model.Citation58 Consistent with previous studies, administration of B. longum HB55020 in an irritable bowel syndrome mouse model promoted expression of major tight junction proteins (claudin-1 and occudin) in colonic tissue.Citation59
Maintaining epithelial integrity and gingival barrier function
Several recent studies have demonstrated gingival epithelial barrier function maintenance using pharmaceutical products, nutrients, and metabolites. Irsogladine maleate is an anti-gastric ulcer agent which has prevented the periodontopathogen-induced disruption of E-cadherin and claudin-1 in gingival epithelial cells. This suggests that irsogladine maleate regulates epithelial barrier function.Citation60 Also, vitamins C and E show a positive result in recovering disrupted E-cadherin in human gingival epithelial cells challenged with P. gingivalis LPS.Citation13 In gingival keratinocyte cells infected with P. gingivalis, green tea polyphenols improved the expression of tight junction proteins, including occludin and ZO-1.Citation11 The authors recently reported that 10-Oxo-trans-11-octadecenoic acid (HYA), a metabolite generated from linoleic acid by Lactobacillus, protects against periodontopathic bacteria-induced gingival epithelial barrier impairment and, thus, contributes to the suppression of inflammatory responses in periodontitis.Citation35 Recent advances in metagenomic and metabolomic approaches may help us to discover unidentified probiotic bacteria and identify novel bioactive metabolites.Citation61,Citation62
Conclusion and perspectives
Future studies are required to improve our understanding of how gingival barrier dysfunction is caused, which can lead to new therapeutic strategies for periodontal disease. Ultimately, determining the function of specific oral bacteria in maintaining the gingival epithelial barrier will provide mechanistic insights and guide interventions for other systemic diseases associated with epithelia barrier dysfunction.
Competing interests
The authors declare no competing financial and/or non-financial interests in relation to the work described.
Author contributions statement
N.T wrote the manuscript with the help of M.Y and B.S. T.T collected and summarized the papers. K.T and K.Y supervised the manuscript.
Acknowledgments
We thank Prof. Jun Ogawa, Dr. Shigenobu Kishino, Ms. Nahoko Kitamura and Dr. Si-Bun Park (Kyoto University, Kyoto, Japan) for preparing bioactive fatty acids. We thank Dr. Shinya Murakami (Osaka University, Osaka, Japan) for sharing the Epi 4 cells. This work was financially supported by JSPS KAKENHI Grant Numbers 16K11827 (to N.T.) and 15H02578 and 18H04067 (to K.Y.).
Additional information
Funding
References
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi:10.1038/nri2653.
- König J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer RJ. Human intestinal barrier function in health and disease. Clin Transl Gastroenterol. 2016;7(10):e196. doi:10.1038/ctg.2016.54.
- Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072(1):242–252. doi:10.1196/annals.1326.017.
- Löe H, Theilade E, Jensen SB. Experimental Gingivitis in Man. J Periodontol. 1965;36(3):177–187. doi:10.1902/jop.1965.36.3.177.
- Ye P, Chapple CC, Kumar RK, Hunter N. Expression patterns of E-cadherin, involucrin, and connexin gap junction proteins in the lining epithelia of inflamed gingiva. J Pathol. 2000;192(1):58–66. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH740>3.0.CO;2-1.
- Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. Bacterial community variation in human body habitats across space and time. Science. 2009;326(5960):1694–1697. doi:10.1126/science.1177486.
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43(11):5721–5732. doi:10.1128/JCM.43.11.5721-5732.2005.
- Groeger SE, Meyle J. Epithelial barrier and oral bacterial infection. Periodontol. 2015;69(1):46–67. doi:10.1111/prd.12104.
- Chatterjee A, Bhattacharya H, Kandwal A. Probiotics in periodontal health and disease. J Indian Soc Periodontol. 2011;15(1):23–28. doi:10.4103/0972-124X.82260.
- Katz J, Sambandam V, Wu JH, Michalek SM, Balkovetz DF. Characterization of Porphyromonas gingivalis-induced degradation of epithelial cell junctional complexes. Infect Immun. 2000;68(3):1441–1449. doi:10.1128/IAI.68.3.1441-1449.2000.
- Lagha AB, Groeger S, Meyle J, Grenier D. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathog Dis. 2018;76(4):fty030. doi:10.1093/femspd/fty030.
- Katz J, Yang Q, Zhang P, Potempa J, Travis J, Michalek SM, Balkovetz DF. Hydrolysis of epithelial junctional proteins by porphyromonas gingivalis gingipains. Infect Immun. 2002;70(5):2512–2518. doi:10.1128/IAI.70.5.2512-2518.2002.
- Abe-Yutori M, Chikazawa T, Shibasaki K, Murakami S. Decreased expression of E-cadherin by Porphyromonas gingivalis-lipopolysaccharide attenuates epithelial barrier function. J Periodontal Res. 2017;52(1):42–50. doi:10.1111/jre.12367.
- Guo W, Wang P, Liu Z-H, Ye P. Analysis of differential expression of tight junction proteins in cultured oral epithelial cells altered by Porphyromonas gingivalis, Porphyromonas gingivalis lipopolysaccharide, and extracellular adenosine triphosphate. Int J Oral Sci. 2018;10(1):1–7. doi:10.1038/ijos.2017.51.
- Amano A. Disruption of epithelial barrier and impairment of cellular function by Porphyromonas gingivalis. Front Biosci. 2007;6:3965–3974. doi:10.2741/2363.
- Nakagawa I, Amano A, Inaba H, Kawai S, Hamada S. Inhibitory effects of Porphyromonas gingivalis fimbriae on interactions between extracellular matrix proteins and cellular integrins. Microbes Infect. 2005;7(2):157–163. doi:10.1016/j.micinf.2004.10.007.
- Fujita T, Ashikaga A, Shiba H, Uchida Y, Hirono C, Iwata T, Takeda K, Kishimoto A, Hirata R, Kawaguchi H, et al. Regulation of IL-8 by Irsogladine maleate is involved in abolishment of Actinobacillus actinomycetemcomitans-induced reduction of gap-junctional intercellular communication. Cytokine. 2006;34(5–6):271–277. doi:10.1016/j.cyto.2006.06.002.
- Noguchi T, Shiba H, Komatsuzawa H, Mizuno N, Uchida Y, Ouhara K, Asakawa R, Kudo S, Kawaguchi H, Sugai M, et al. Syntheses of prostaglandin E2and E-cadherin and gene expression of β-defensin-2 by human gingival epithelial cells in response to actinobacillus actinomycetemcomitans. Inflammation. 2003;27(6):341–349. doi:10.1023/B:IFLA.0000006702.27906.e9.
- Uchida Y, Shiba H, Komatsuzawa H, Hirono C, Ashikaga A, Fujita T, Kawaguchi H, Sugai M, Shiba Y, Kurihara H. Irsogladine maleate influences the response of gap junctional intercellular communication and IL-8 of human gingival epithelial cells following periodontopathogenic bacterial challenge. Biochem Biophys Res Commun. 2005;333(2):502–507. doi:10.1016/j.bbrc.2005.05.197.
- Damek-Poprawa M, Korostoff J, Gill R, Dirienzo JM. Cell junction remodeling in gingival tissue exposed to a microbial toxin. J Dent Res. 2013;92(6):518–523. doi:10.1177/0022034513486807.
- Uitto VJ, Pan YM, Leung WK, Larjava H, Ellen RP, Finlay BB, McBride BC. Cytopathic effects of Treponema denticola chymotrypsin-like proteinase on migrating and stratified epithelial cells. Infect Immun. 1995;63:3401–3410.
- Chi B, Qi M, Kuramitsu HK. Role of dentilisin in Treponema denticola epithelial cell layer penetration. Res Microbiol. 2003;154(9):637–643. doi:10.1016/j.resmic.2003.08.001.
- Kong S, Zhang YH, Zhang W. Regulation of intestinal epithelial cells properties and functions by amino acids. Biomed Res Int. 2018;2018.
- Bosshardt DD, Lang NP. The junctional epithelium : from health to disease. J Dent Res. 2005;84(1):9–20. doi:10.1177/154405910508400102.
- Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontol. 1997;13(1):91–120. doi:10.1111/prd.1997.13.issue-1.
- Hatakeyama S, Yaegashi T, Oikawa Y, Fujiwara H, Mikami T, Takeda Y, Satoh M. Expression pattern of adhesion molecules in junctional epithelium differs from that in other gingival epithelia. J Periodontal Res. 2006;41(4):322–328. doi:10.1111/jre.2006.41.issue-4.
- DiRienzo JM. Breaking the gingival epithelial barrier: role of the aggregatibacter actinomycetemcomitans cytolethal distending toxin in oral infectious disease. Cells. 2014;3(2):476–499. doi:10.3390/cells3020476.
- Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5(4):261–270. doi:10.1038/nrm1357.
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: A novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123(6 II):1777–1788. doi:10.1083/jcb.123.6.1333.
- Tsukita S, Furuse M. Overcoming barriers in the study of tight junction functions: from occludin to claudin. Genes to Cells. 1998;3:569–573.
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci U S A. 1991;88(8):3460–3464. doi:10.1073/pnas.88.8.3460.
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141(1):199–208. doi:10.1083/jcb.141.1.199.
- Provost E, Rimm DL. Controversies at the cytoplasmic face of the cadherin-based adhesion complex. Curr Opin Cell Biol. 1999;11(5):567–572. doi:10.1016/S0955-0674(99)00015-0.
- Gosselin F, Magloire H, Joffre A, Portier MM. Cytokeratins as molecular markers in the evaluation of the precise differentiation stage of human gingival epithelium reconstituted in vitro. Arch Oral Biol. 1990;35(SUPPL):S217–S221. doi:10.1016/0003-9969(90)90162-4.
- Yamada M, Takahashi N, Matsuda Y, Sato K, Yokoji M, Sulijaya B, Maekawa T, Ushiki T, Mikami Y, Hayatsu M, et al. A bacterial metabolite ameliorates periodontal pathogen-induced gingival epithelial barrier disruption via GPR40 signaling. Sci Rep. 2018;8(1):9008. doi:10.1038/s41598-018-27408-y.
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65(1):475–502. doi:10.1146/annurev.bi.65.070196.002355.
- Dabija-Wolter G, Bakken V, Cimpan MR, Johannessen AC, Costea DE. In vitro reconstruction of human junctional and sulcular epithelium. J Oral Pathol Med. 2013;42(5):396–404. doi:10.1111/jop.2013.42.issue-5.
- Kornman KS, Page RC, Tonetti S. The host response to the microbial challenge i n periodontitis : assembling the players. Periodontol. 1998;14:33–53. doi:10.1111/j.1600-0757.1997.tb00191.x.
- Fujita T, Yoshimoto T, Kajiya M, Ouhara K, Matsuda S, Takemura T, Akutagawa K, Takeda K, Mizuno N, Kurihara H. Regulation of defensive function on gingival epithelial cells can prevent periodontal disease. Jpn Dent Sci Rev. 2018;54(2):66–75. doi:10.1016/j.jdsr.2017.11.003.
- Fujita T, Firth JD, Kittaka M, Ekuni D, Kurihara H, Putnins EE. Loss of claudin-1 in lipopolysaccharide-treated periodontal epithelium. J Periodontal Res. 2012;47(2):222–227. doi:10.1111/j.1600-0765.2011.01424.x.
- Fujita T, Ashikaga A, Shiba H, Kajiya M, Kishimoto A, Hirata R, Tsunekuni N, Hirono C, Kawaguchi H, Shiba Y, et al. Irsogladine maleate counters the interleukin-1β-induced suppression in gap-junctional intercellular communication but does not affect the interleukin-1β-induced zonula occludens protein-1 levels in human gingival epithelial cells. J Periodontal Res. 2008;43(1):96–102.
- Miyamoto J, Mizukure T, Park S-B, Kishino S, Kimura I, Hirano K, Bergamo P, Rossi M, Suzuki T, Arita M, et al. 2014. A Gut Microbial Metabolite of Linoleic Acid, 10-Hydroxy-cis-12-octadecenoic Acid, Ameliorates Intestinal Epithelial Barrier Impairment Partially via GPR40-MEK-ERK Pathway *.
- Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, Wang Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front Microbiol. 2018;9:1–14.
- Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–725. doi:10.1038/nrmicro2873.
- Kõll-Klais P, Mändar R, Leibur E, Marcotte H, Hammarström L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20(6):354–361. doi:10.1111/j.1399-302X.2004.00192.x.
- Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J Clin Periodontol. 2013;40(11):1025–1035. doi:10.1111/jcpe.2013.40.issue-11.
- Miyauchi E, O’Callaghan J, Butto LF, Hurley G, Melgar S, Tanabe S, Shanahan F, Nally K, O’Toole PW. Mechanism of protection of transepithelial barrier function by Lactobacillus salivarius: strain dependence and attenuation by bacteriocin production. AJP Gastrointest Liver Physiol. 2012;303(9):G1029–G1041. doi:10.1152/ajpgi.00003.2012.
- Hojo K, Mizoguchi C, Takemoto N, Ohshima T, Gomi K, Arai T, Maeda N. Distribution of salivary lactobacillus and bifidobacterium species in periodontal health and disease. Biosci Biotechnol Biochem. 2007;71(1):152–157. doi:10.1271/bbb.60420.
- O’Neill CA, Sultana R, McBain AJ. Strain-dependent augmentation of tight-junction barrier function in human primary epidermal keratinocytes by lactobacillus and bifidobacterium lysates. Appl Environ Microbiol. 2013;79(16):4887–4894. doi:10.1128/AEM.00982-13.
- Kreth J, Merritt J, Qi F. Bacterial and host interactions of oral streptococci. DNA Cell Biol. 2009;28(8):397–403. doi:10.1089/dna.2008.0808.
- Ye P, Harty D, Commandeur Z, Hunter N. Binding of Streptococcus gordonii to oral epithelial monolayers increases paracellular barrier function. Microb Pathog. 2013;56:53–59. doi:10.1016/j.micpath.2012.11.004.
- Dickinson BC, Moffatt CE, Hagerty D, Whitmore SE, Brown TA, Graves DT, Lamont RJ. Interaction of oral bacteria with gingival epithelial cell multilayers. Mol Oral Microbiol. 2011;26(3):210–220. doi:10.1111/j.2041-1014.2011.00609.x.
- Ewaschuk JB, Murdoch GK, Johnson IRMadsen KL, Field CJ. Glutamine supplementation improves intestinal barrier function in a weaned piglet model of Escherichia coli infection.Br J Nutr. 2011 Sep;106(6):870–877. doi: 10.1017/S0007114511001152.
- Zhu HL, Liu YL, Xie XL, Huang JJ, Hou YQ. Effect of l-arginine on intestinal mucosal immune barrier function in weaned pigs after escherichia coli lps challenge. Innate Immun. 2013 Jun;19(3):242–252. doi:10.1177/1753425912456223.
- Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Liver Physiol. 2009;296:G1140–G1149.
- Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Liver Physiol. 2008;294:G1060–G1069.
- Anderson RC, Cookson AL, McNabb WC, Park Z, McCann MJ, Kelly WJ, Roy NC. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010;10(1):316. doi:10.1186/1471-2180-10-316.
- Srutkova D, Schwarzer M, Hudcovic T, Zakostelska Z, Drab V, Spanova A, Rittich B, Kozakova H, Schabussova I. Bifidobacterium longum CCM 7952 promotes epithelial barrier function and prevents acute dss-induced colitis in strictly strain-Specific manner. PLoS One. 2015;10(7):e0134050. doi:10.1371/journal.pone.0134050.
- Wang H, Gong J, Wang W, Long Y, Fu X, Fu Y, Qian W, Hou X. Are there any different effects of Bifidobacterium, Lactobacillus and Streptococcus on intestinal sensation, barrier function and intestinal immunity in PI-IBS mouse model? PLoS One. 2014;9(3):e90153. doi:10.1371/journal.pone.0090153.
- Fujita T, Kishimoto A, Shiba H, Hayashida K, Kajiya M, Uchida Y, Matsuda S, Takeda K, Ouhara K, Kawaguchi H, et al. Irsogladine maleate regulates neutrophil migration and E-cadherin expression in gingival epithelium stimulated by Aggregatibacter actinomycetemcomitans. Biochem Pharmacol. 2010;79(10):1496–1505. doi:10.1016/j.bcp.2010.01.017.
- Burczynska A, Dziewit L, Decewicz P, Struzycka I, Wroblewska M. Application of metagenomic analyses in dentistry as a novel strategy enabling complex insight into microbial diversity of the oral cavity. Polish J Microbiol. 2017;66(1):9–15. doi:10.5604/17331331.1234988.
- Belda-Ferre PAlcaraz LD, Cabrera-Rubio R, Romero H, Simón-Soro A, Pignatelli M, Mira A. Theoral metagenome in health and disease. Isme J. 2012;6(1):46–56. doi: 10.1038/ismej.2012.11.