ABSTRACT
Endothelial barrier dysfunction (EBD) is the hallmark of Acute Respiratory Distress Syndrome (ARDS), a potentially lethal respiratory disorder associated with the COVID-19 – related deaths. Herein, we employed a cecal ligation and puncture (CLP) murine model of sepsis, to evaluate the effects of sepsis-induced EBD in the expression of the never in mitosis A (NIMA)-related kinases (NEKs). Members of that family of kinases regulate the activity and expression of the tumor suppressor P53, previously shown to modulate the actin cytoskeleton remodeling. Our results introduce the induction of NEK2, NEK3, NEK4, NEK7, and NEK9 in a CLP model of sepsis. Hence, we suggest that NEKs are involved in inflammatory processes and are holding the potential to serve as novel therapeutic targets for pathologies related to EBD, including ARDS and sepsis. Further studies will delineate the underlying molecular events and their interrelations with P53.
Introduction
ARDS is an inflammatory lung disease associated with high mortality rates, as reflected in the severe outcomes of the current pandemic (COVID-19).Citation1 Lung endothelial hyper-permeability to fluid, proteins, neutrophils, and red blood cells contributes to the deposition of edematous fluid into the alveolar air space, leading to severe respiratory complications.Citation2,Citation3
The family of never in mitosis A (NIMA)-related kinases (NEKs) includes eleven members (NEK1-11) involved in several cellular functions including intracellular traffickingCitation4 and actin remodeling.Citation5,Citation6 NEK9 regulates RhoA activation via Rho guanine nucleotide exchange factor 2 phosphorylation,Citation7 which in turn promotes the myosin light chain 2 (MLC2) phosphorylation. Under hypoxic conditions, the promoter of NEK1 binds to the hypoxia-inducible factor 2-α, inducing hyperpermeability responses.Citation8 NEK7 is an important component of the NLRP3 (NLR family pyrin domain containing 3) inflammasome,Citation9 which enhances the lipopolysaccharides (LPS)-mediated stimulation of toll-like receptor 4/nuclear factor-kappa B (NF-κB) signaling.Citation10 The expression of that serine/threonine protein kinase (NEK7) is also associated with the hyperpermeability of the blood-brain barrier (BBB) and cerebral edema.Citation11
The previously mentioned observations suggest the strong involvement of certain NEKs in EBD. However, the effects of sepsis on the expression of NEKs are unknown. Hence, we employed a cecal ligation and puncture (CLP) model of murine sepsis to evaluate the effects of sepsis in the pulmonary expression of NEK2, NEK3, NEK4, NEK7, and NEK9. This experimental model (CLP) replicates the clinical picture of sepsis-inflicted pulmonary edema, a principal cause of ARDS. Our results indicate for the first time that the expression of NEKs in the lungs is elevated in murine sepsis. Thus, we suggest that NEKs may serve as a potential therapeutic target in sepsis-induced ARDS. However, further studies are needed to advance our understanding toward those events.
Materials and methods
Reagents
RIPA buffer (AAJ63306-AP), sheep anti-mouse IgG HRP-linked polyclonal antibody (95,017–554), donkey anti-rabbit IgG HRP-linked polyclonal antibody (95,017–556), EZBlock™ protease inhibitor cocktail (75,837–938), and nitrocellulose membranes (10,063–173) were obtained from VWR (Radnor, PA). The NEK2 (sc-55,601), NEK3 (sc-390,872), NEK4 (sc-81,332), NEK7 (sc-393,539), and NEK9 (sc-100,401) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The phospho-ERK1/2 (9101S) and ERK1/2 (9102S) antibodies were obtained from Cell Signaling (Danvers, MA). The β-actin (A5441) antibody was purchased from Sigma-Aldrich (St Louis, MO).
Animals
Six-week-old male C57BL/6 mice (Envigo, Indianapolis, IN) were used in this study. They were maintained in a 12:12-h light/dark cycle, in pathogen-free conditions. All animal experiments were conducted in accordance with the approved protocol by the University of Louisiana Monroe Institutional Animal Care and Use Committee (IACUC) and were in line with the principles of humane animal care adopted by the American Physiological Society.
CLP model of sepsis
Mice were anesthetized with a subcutaneous injection of ketamine and xylazine (9:1) solution. A midline laparotomy was performed under sterile conditions. The cecum was exposed and was ligated below the ileocecal valve without disturbing the intestinal continuity. The ligated cecum was punctured twice with a 20-gauge needle. Small amount of intestinal content was extruded through the punctured holes before returning the ligated cecum to the peritoneal cavity. The abdominal and skin incision were sealed using a 3–0 silk running suture and 7 mm reflex wound clips, respectively. In the sham-operated mice, the cecum was exposed and returned to the abdominal cavity without ligation and puncture. The animals were resuscitated by subcutaneous administration of 1 ml of 0.9% NaCl solution immediately after surgery. The mice were sacrificed after 24 hours of CLP operation, and the lungs were collected and homogenized for protein analysis.
Western blot analysis
The protein expression was determined by western blots, as previously described.Citation12 Briefly, proteins were isolated from mice lung tissues using RIPA buffer and equal amounts (40 µg) of protein samples were separated onto sodium dodecyl sulfate polyacrylamide gel electrophoresis. Wet transfer was used to transfer the proteins onto the nitrocellulose membranes. After blocking with 5% nonfat dry milk at room temperature, the blots were exposed to appropriate primary and secondary antibodies to detect protein signals. The β-actin was used as a loading control unless indicated otherwise.
Densitometry and statistical analysis
The densitometry of the immunoblots was performed by using Image J software (NIH). All values are expressed as the mean ± SEM (standard error of the mean). Graphpad Prism (version 5.01) was used to analyze the data. Student’s t-tests were performed to determine the statistical differences among groups.
Results and discussion
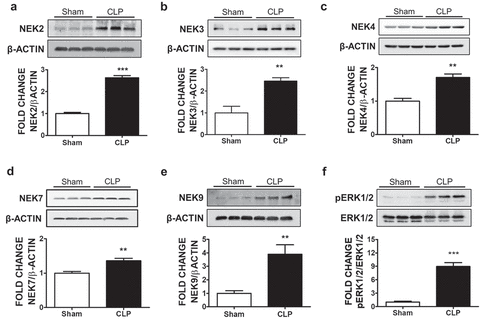
To evaluate the effects of sepsis in the expression of NEKs in the lungs, we subjected the mice to the CLP operation. Our results indicate that the expression of NEK2 (), NEK3 (), NEK4 (), NEK7 (), and NEK9 () in the lungs of the septic mice were increased as compared to the sham group. The increase in the expression levels of NEK9 appear to be higher than NEK7, and that may be due to the fact that NEK7 is a downstream target of NEK9.Citation13 Moreover, the septic lungs expressed more phospho-ERK1/2 (), as previously reported.Citation14 Kinases (e.g. AMPK, MLCK, PKC, FAK, PAK, ROCK) are well-known inflammatory modulators of endothelial permeability.Citation15 To the best of our knowledge, this is the first study to report the induction of NEKs in sepsis.
Figure 1. Effects of CLP-induced sepsis on NEK kinases expression in mice lungs. C57BL/6 mice were subjected to either sham surgery or CLP. 24 H after the surgery, mice were sacrificed and lung tissues were collected for the western blot analysis of NEK2 and β-actin (a), NEK3 and β-actin (b), NEK4 and β-actin (c), NEK7 and β-actin (d), NEK9 and β-actin (e), phospho-ERK1/2 (pERK1/2) and ERK1/2 (f). The signal intensity of the bands was analyzed by densitometry. Protein levels were normalized to β-actin unless indicated otherwise in the graph of densitometry. **P <.01, ***P <.001 vs sham, N = 3. Means ± SEM
Tight junctions serve as the main regulators of fluid and ion exchange, and are involved in the regulation and maintenance of endothelial barrier function. Several tight junction proteins (TJs) have been identified in the lungs (e.g. occludins, claudins and zonula occludens),Citation16 and at least 14 different mRNAs encode for claudins in the lung epithelium. Claudin-3, claudin-4 and claudin-18 are the most abundant.Citation17 Indeed, claudin-4 is associated with fluid clearance from the alveolar airspace, and exerts a protective role against ventilator-induced lung injury (VILI).Citation17 Claudin-4 was also shown to induce alveolar barrier function, since its augmentation substantially increased transepithelial resistance.Citation18 In a CLP-induced model of sepsis, the expression of both claudin-4 and claudin-18 were reduced, suggesting the important role of those proteins in the maintenance of normal respiratory functions.Citation19
Previous studies have associated certain members of the NEK family of kinases with the mediation of inflammatory responses. NEK2 activated the NF-кB signaling pathway and increased the expression of heparanase, an endoglycosidase responsible for bone destruction.Citation20 This kinase was reported to induce the interleukin-8 expression and potentiate angiogenesis.Citation21 It was also suggested that NEK6 targets the signal transducer and activator of transcription 3,Citation22 and induces the hypoxia-inducible factor 1α.Citation23 In lung cancers it was shown that NEK9 is recruited to the microtubules, and it is a target of EML4-ALK which in turn accelerates the NEK7-dependent migration.Citation24
In recent studies NEK7 has been reported to activate the NLRP3 inflammasome by binding to its leucine-rich repeat domain.Citation25,Citation26 The inflammasome is a multiprotein complex which activates caspase-1 and it is involved in the secretion of IL-1β and IL-18. This occurs in response to different stimuli, including multiple microbial products, endogenous molecules and particulate matter.Citation26 NEK7-induced NLRP3 activation is associated with various diseases such as inflammatory bowel disease (IBD),Citation10 atherosclerosis, and the development of neuroinflammation in post-traumatic brain injury (TBI).Citation27 In our study, we also observed the significant induction of NEK7 in CLP-induced sepsis.
NEKs have been associated with P53, a tumor suppressor and anti-inflammatory protein, which exerts protective activities in the lung microvasculature.Citation28,Citation29 P53 increases the endothelial barrier integrity by deactivating cofilin; and by suppressing the formation of filamentous actin. Moreover, this endothelial defender (P53) mediates the barrier enhancing effects of heat shock protein 90 (Hsp90) inhibitorsCitation30,Citation31 and growth hormone releasing hormone antagonistsCitation28,Citation32 in the lungs and the blood-brain barrier.Citation33,Citation34 Indeed, the hydrogen peroxide-induced BBB hyperpermeability was counteracted by those unfolded protein response inducers.Citation35
P53 is subjected to post-translational modifications which regulate its intracellular abundance and activity,Citation36 since P53 phosphorylation accelerates its degradation.Citation37,Citation38 In bovine pulmonary artery endothelial cells, LPS, an endotoxin of the Gram-negative bacteria, reduced P53 via phosphorylation at Ser. 6, Ser. 15, Ser. 33, and Ser. 392.Citation39 Furthermore, lipoteichoic acid, an endotoxin of Gram-positive bacteria, induced P53 phosphorylation in Ser. 15, Ser. 33, Ser. 46, and Ser. 392.Citation40 The Hsp90 inhibitors 17-AAG, 17-DMAG, and AUY-922 counteracted those phosphorylations, to increase P53 expression.Citation41
The NIMA family proteins can also phosphorylate P53. Polo-like kinase 1 (Plk1), an upstream activator of NEK6/7/9, inhibits the function of P53 by physical interaction and phosphorylation. Moreover, it shares common phosphorylation-site motif with NEK6, NEK7 and NEK9.Citation13 Interestingly, NEK2 and NEK10 reduce P53 by Ser315 and Y327 phosphorylation.Citation42 Hence, we speculate that the interrelations of P53 and NEKs in the context of the pulmonary microvasculature may dictate the severity and outcomes of the sepsis-induced cytokine storm in the lung context.Citation43 Further studies in endothelial specific knock-out mice that do not express NEKs and/or P53 will test our hypothesis.
To the best of our knowledge, the present study introduces the upregulation of NEKs in the lungs of septic mice. Hence, it is highly probable that certain NEKs (NEK2, NEK3, NEK4, NEK7, and NEK9) may serve as a potential therapeutic target in sepsis and other pathologies related to EBD.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Barabutis N. Unfolded protein response in the COVID-19 context. Aging Health Res. 2021;1(1):1. doi:https://doi.org/10.1016/j.ahr.2020.100001.
- Linfield DT, Raduka A, Aghapour M, Rezaee F. Airway tight junctions as targets of viral infections. Tissue Barriers. 2021;9(2):1883965. doi:https://doi.org/10.1080/21688370.2021.1883965.
- Gorman E, Millar J, McAuley D, O’Kane C. Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential. Expert Rev Respir Med. 2021;15(3):301–6. doi:https://doi.org/10.1080/17476348.2021.1848555.
- Joseph BB, Wang Y, Edeen P, Lažetić V, Grant BD, Fay DS. Control of clathrin-mediated endocytosis by NIMA family kinases. PLoS Genet. 2020;16(2):e1008633. doi:https://doi.org/10.1371/journal.pgen.1008633.
- Vasileva E, Citi S. The role of microtubules in the regulation of epithelial junctions. Tissue Barriers. 2018;6(3):1539596. doi:https://doi.org/10.1080/21688370.2018.1539596.
- Lazetic V, Joseph BB, Bernazzani SM, Fay DS. Actin organization and endocytic trafficking are controlled by a network linking NIMA-related kinases to the CDC-42-SID-3/ACK1 pathway. PLoS Genet. 2018;14(4):e1007313. doi:https://doi.org/10.1371/journal.pgen.1007313.
- Lu G, Tian S, Sun Y, Dong J, Wang N, Zeng J, Nie Y, Wu K, Han Y, Feng B, et al. NEK9, a novel effector of IL-6/STAT3, regulates metastasis of gastric cancer by targeting ARHGEF2 phosphorylation. Theranostics. 2021;11(5):2460–2474. doi:https://doi.org/10.7150/thno.53169.
- Chen G, Zhou J, Chen J, Zhu J, Liu S-C, Ding X-F, Zhang Q. VHL regulates NEK1 via both HIF-2α pathway and ubiquitin-proteasome pathway in renal cancer cell. Biochem Biophys Res Commun. 2019;509(3):797–802. doi:https://doi.org/10.1016/j.bbrc.2019.01.001.
- He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530(7590):354–357. doi:https://doi.org/10.1038/nature16959.
- Chen X, Liu G, Yuan Y, Wu G, Wang S, Yuan L. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 2019;10(12):906. doi:https://doi.org/10.1038/s41419-019-2157-1.
- Li G, Dong Y, Liu D, Zou Z, Hao G, Gao X, Pan P, Liang G. NEK7 coordinates rapid neuroinflammation after subarachnoid hemorrhage in mice. Front Neurol. 2020;11:551. doi:https://doi.org/10.3389/fneur.2020.00551.
- Uddin MA, Akhter MS, Kubra K-T, Barabutis N. P53 deficiency potentiates LPS-induced acute lung injury in vivo. Curr Res Physiol. 2020;3:30–33. doi:https://doi.org/10.1016/j.crphys.2020.07.001.
- Van De Kooij B, Creixell P, Van Vlimmeren A, Joughin BA, Miller CJ, Haider N, Simpson CD, Linding R, Stambolic V, Turk BE, et al. Comprehensive substrate specificity profiling of the human Nek kinome reveals unexpected signaling outputs. Elife. 2019;8. doi:https://doi.org/10.7554/eLife.44635
- Smith JA, Mayeux PR, Schnellmann RG. Delayed mitogen-activated protein kinase/extracellular signal–regulated kinase inhibition by trametinib attenuates systemic inflammatory responses and multiple organ injury in murine sepsis*. Crit Care Med. 2016;44(8):e711–20. doi:https://doi.org/10.1097/CCM.0000000000001672.
- Barabutis N, Verin A, Catravas JD. Regulation of pulmonary endothelial barrier function by kinases. Am J Physiol Lung Cell Mol Physiol. 2016;311(5):L832–L845. doi:https://doi.org/10.1152/ajplung.00233.2016.
- Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi:https://doi.org/10.1038/s41572-019-0069-0.
- Koval M. Claudin heterogeneity and control of lung tight junctions. Annu Rev Physiol. 2013;75(1):551–567. doi:https://doi.org/10.1146/annurev-physiol-030212-183809.
- Mitchell LA, Overgaard CE, Ward C, Margulies SS, Koval M. Differential effects of claudin-3 and claudin-4 on alveolar epithelial barrier function. Am J Physiol Lung Cell Mol Physiol. 2011;301(1):L40–9. doi:https://doi.org/10.1152/ajplung.00299.2010.
- Cohen TS, Gray Lawrence G, Margulies SS, Morty RE. Cultured alveolar epithelial cells from septic rats mimic in vivo septic lung. PLoS One. 2010;5(6):e11322. doi:https://doi.org/10.1371/journal.pone.0011322.
- Franqui-Machin R, Hao M, Bai H, Gu Z, Zhan X, Habelhah H, Jethava Y, Qiu L, Frech I, Tricot G, et al. Destabilizing NEK2 overcomes resistance to proteasome inhibition in multiple myeloma. J Clin Invest. 2018;128(7):2877–2893. doi:https://doi.org/10.1172/JCI98765.
- Wu SM, Lin S-L, Lee K-Y, Chuang H-C, Feng P-H, Cheng W-L, Liao C-J, Chi H-C, Lin Y-H, Tsai C-Y, et al. Hepatoma cell functions modulated by NEK2 are associated with liver cancer progression. Int J Cancer. 2017;140(7):1581–1596. doi:https://doi.org/10.1002/ijc.30559.
- Hong J, Won M, Ro H. The molecular and pathophysiological functions of members of the LNX/PDZRN E3 ubiquitin ligase family. Molecules. 2020;25(24):5938. doi: https://doi.org/10.3390/molecules25245938. PMID: 33333989; PMCID: PMC7765395.
- Donato MD, Fanelli M, Mariani M, Raspaglio G, Pandya D, He S, Fiedler P, Petrillo M, Scambia G, Ferlini C, et al. Nek6 and Hif-1alpha cooperate with the cytoskeletal gateway of drug resistance to drive outcome in serous ovarian cancer. Am J Cancer Res. 2015;5(6):1862–1877.
- O'Regan L, Barone G, Adib R, Woo CG, Jeong HJ, Richardson EL, et al. EML4-ALK V3 oncogenic fusion proteins promote microtubule stabilization and accelerated migration through NEK9 and NEK7. J Cell Sci. 2020;133(9):jcs241505. doi:https://doi.org/10.1242/jcs.241505. PMID: 32184261; PMCID: PMC7240300.
- Sharif H, Wang L, Wang WL, Magupalli VG, Andreeva L, Qiao Q, Hauenstein AV, Wu Z, Núñez G, Mao Y, et al. Structural mechanism for NEK7-licensed activation of NLRP3 inflammasome. Nature. 2019;570(7761):338–343. doi:https://doi.org/10.1038/s41586-019-1295-z.
- Sun Z, Gong W, Zhang Y, Jia Z. Physiological and pathological roles of mammalian NEK7. Front Physiol. 2020;11:606996. doi:https://doi.org/10.3389/fphys.2020.606996.
- Chen Y, Meng J, Bi F, Li H, Chang C, Ji C, Liu W. NEK7 regulates NLRP3 inflammasome activation and neuroinflammation post-traumatic brain injury. Front Mol Neurosci. 2019;12:202. doi:https://doi.org/10.3389/fnmol.2019.00202.
- Barabutis N. Growth hormone releasing hormone in endothelial barrier function. Trends in Endocrinology & Metabolism. 2021;32(6):338–340. doi:https://doi.org/10.1016/j.tem.2021.03.001.
- Barabutis N. Regulation of lung endothelial permeability by NEK kinases. IUBMB Life. 2020;72(4):801–804. doi:https://doi.org/10.1002/iub.2251.
- Uddin MA, Barabutis N. P53: the endothelium defender. J Cell Biochem. 2019;120(7):10952–10955. doi:https://doi.org/10.1002/jcb.28511.
- Barabutis N, Dimitropoulou C, Gregory B, Catravas JD. Wild-type p53 enhances endothelial barrier function by mediating RAC1 signalling and RhoA inhibition. J Cell Mol Med. 2018;22(3):1792–1804. doi:https://doi.org/10.1111/jcmm.13460.
- Uddin MA, Akhter MS, Singh SS, Kubra K-T, Schally AV, Jois S, Barabutis N. GHRH antagonists support lung endothelial barrier function. Tissue Barriers. 2019;7(4):1669989. doi:https://doi.org/10.1080/21688370.2019.1669989.
- Uddin MA, Akhter MS, Kubra K-T, Whitaker KE, Shipley SL, Smith LM, Barabutis N. Hsp90 inhibition protects the brain microvascular endothelium against oxidative stress. Brain Disord. 2021;1:100001. doi:https://doi.org/10.1016/j.dscb.2020.100001.
- Barabutis N, Akhter MS, Uddin MA, Kubra K-T, Schally AV. GHRH antagonists protect against hydrogen peroxide-induced breakdown of brain microvascular endothelium integrity. Horm Metab Res. 2020;52(5):336–339. doi:https://doi.org/10.1055/a-1149-9347.
- Barabutis N. Unfolded protein response: a regulator of the endothelial barrier. Endocrine and Metabolic Science. 2021;3:100092. doi:https://doi.org/10.1016/j.endmts.2021.100092.
- Chen L, Liu S, Tao Y. Regulating tumor suppressor genes: post-translational modifications. Signal Transduct Target Ther. 2020;5:90.
- Gully CP, Velazquez-Torres G, Shin J-H, Fuentes-Mattei E, Wang E, Carlock C, Chen J, Rothenberg D, Adams HP, Choi HH, et al. Aurora B kinase phosphorylates and instigates degradation of p53. Proc Natl Acad Sci U S A. 2012;109(24):E1513–22. doi:https://doi.org/10.1073/pnas.1110287109.
- Xia Y, Padre RC, De Mendoza TH, Bottero V, Tergaonkar VB, Verma IM. Phosphorylation of p53 by IκB kinase 2 promotes its degradation by β-TrCP. Proc Natl Acad Sci U S A. 2009;106(8):2629–2634. doi:https://doi.org/10.1073/pnas.0812256106.
- Barabutis N, Uddin MA, Catravas JD. Hsp90 inhibitors suppress P53 phosphorylation in LPS - induced endothelial inflammation. Cytokine. 2019;113:427–432. doi:https://doi.org/10.1016/j.cyto.2018.10.020.
- Kubra KT, Uddin MA, Akhter MS, Barabutis N., et al. 2020. P53 is subjected to lipoteichoic acid-induced phosphorylation in the lungs. TH Open.
- Barabutis N. P53 in acute respiratory distress syndrome. Cell Mol Life Sci. 2020;32(6):4725–4727. doi:https://doi.org/10.1007/s00018-020-03629-1.
- Haider N, Dutt P, Van De Kooij B, Ho J, Palomero L, Pujana MA, Yaffe M, Stambolic V. NEK10 tyrosine phosphorylates p53 and controls its transcriptional activity. Oncogene. 2020;39(30):5252–5266. doi:https://doi.org/10.1038/s41388-020-1361-x.
- Mangalmurti N, Hunter CA. Cytokine storms: understanding COVID-19. Immunity. 2020;53(1):19–25. doi:https://doi.org/10.1016/j.immuni.2020.06.017.
