ABSTRACT
Delivery of cargo to cells through the use of cell-penetrating peptide (CPP) sequences is an area of rich investigation for targeted therapeutics. Specific to the endothelium, the layer of cells that cover every blood vessel in the body, the loss or alteration of a key enzyme, endothelial nitric oxide synthase (eNOS), is known to contribute to endothelial health during severe, infectious challenge. While the beneficial effects of eNOS are often thought to be mediated through the generation of nitric oxide, some protection is theorized to be through eNOS binding to regulatory pathways via a pentabasic RRKRK motif. We hypothesized that delivery of the eNOS-RRKRK peptide sequence using common CPPs would allow protection against gram-negative lipopolysaccharide (LPS). Combination of the eNOS-RRKRK sequence to the CPP antennapedia (AP) reduced the impact of LPS-induced permeability in cultured human microvascular endothelial cells (HMVECs) as measured by transendothelial electrical resistance (TEER). There was also a modest reduction in cytokine production, however it was observed that AP alone significantly impaired LPS-induced endothelial permeability and cytokine production. In comparison, the CPP trans-activator of transcription (TAT) did not significantly alter endothelial inflammation by itself. When TAT was coupled to the eNOS-RRKRK sequence, protection against LPS-induced permeability was still demonstrated, however cytokine production was not reduced. These data demonstrate that the RRKRK sequence of eNOS can offer some NO-independent protection against LPS-mediated endothelial inflammation, however the degree of protection is highly dependent on the type of CPP utilized for cargo delivery.
Introduction
The delivery of compounds to targeted sites of interest remains at the forefront of pharmacologic interventions. While some sites are in the extracellular environment making them more accessible to intervention, many diseases modify intracellular structures and proteins which are protected by the plasma membrane and therefore less targetable. Coupling desirable compounds to cell penetrating peptides (CPPs) is one method that can be employed to enhance the cellular uptake to modulate intracellular targets.Citation1 Several of the most commonly utilized CPPs incorporate the cationic amino acid arginine and have been generally shown to have a desirable safety profile compared to other methods used to deploy intracellular compounds.Citation1,Citation2 Though the mechanism of action regarding CPPs remains relatively obscure and controversial, they continue to be thoroughly investigated as drug delivery tools in a variety of diseases, including diseases of inflammation.Citation3
Among diseases of inflammation, sepsis, which is an exaggerated, systemic inflammatory host response to a pathogenic challenge, is a major contributor of morbidity and mortality worldwide.Citation4 Though many different pathogens and cellular responses participate in the observed inflammation, the dysfunction within the endothelium, the layer of cells that cover the blood vessels of the body, lead to the characteristic phenotypes of sepsis such as cytokinemia, capillary leak and circulatory shock.Citation5 The processes of enhanced cytokine production, altered vasomotor tone, and increase monolayer permeability are deliberate mechanisms intended for allowing the host’s immune system to be recruited and target the source of infection. Yet, dysregulation of these processes has negative consequences to the host and prevent the restoration of cellular homeostasis necessary for survival. Endothelial nitric oxide synthase (eNOS) is a key molecule that regulates the homeostatic function of the endothelium and is perturbed during sepsis, contributing to many of the clinical findings.Citation6 Loss of eNOS increases endothelial cytokine production during infectious challenge and worsens vasomotor responsiveness to pharmacological agents.Citation7 Further, it potentiates the loss of barrier integrity, allowing hydrostatic pressure to drive intravascular fluid into the tissues, worsening organ function.Citation5,Citation8 Though the primary function of eNOS is to generate the biologically active gas, nitric oxide (NO), eNOS also exhibits NO-independent functions in regulating inflammation.Citation9 The postulated mechanism of such functions is through an arginine (R) and lysine (K) rich pentabasic docking motif, RRKRK, which has been demonstrated to allow for docking between eNOS and various mitogen activated protein kinases (MAPKs) that express a reciprocal common docking (CD) domain.Citation10 Beyond interactions with MAPKs, the eNOS-RRKRK sequence has also been suggested to modulate mitochondria function through interactions with voltage-dependent anion channel 1 (VDAC1). VDAC1, which is also known as porin, is the most abundantly expressed protein on the mitochondrial outer membrane and allows for ATP transport out of the mitochondria.Citation11 Thus, given the potential protective impact that modulation of eNOS-mediated protein–protein interactions could have during infectious challenge, we postulated that the eNOS-RRKRK sequence coupled to common CPPs would reduce the inflammatory effect of gram-negative derived lipopolysaccharide (LPS) on endothelial cells.
Materials and methods
Cells and culture
Pooled neonatal dermal human microvascular endothelial cells (HMVECs) were purchased from Lonza (Basel, Switzerland). HMVECs were grown in Endothelial Growth Media-2 (Lonza) supplemented with 5% fetal bovine serum (FBS). Cells were plated at a density of approximately 30,000 cells/cm2 and grown to confluence. Experiments were conducted between the second and fifth passages. Media was exchanged at least every 3 days.
Peptides and reagents
The following reagents were used in experiments: 1–25 μM AP (Genscript USA, Piscataway, NJ, USA), 25–50 μM TAT (AnaSpec Inc, Fremont, CA, USA) and 100 ng/ml Ultra-Pure LPS (List Biological Laboratories, Campbell, CA, USA). Conjugated peptides synthesized with AP were generated by Genscript USA (Piscataway, NJ, USA) and those utilizing TAT were generated by Lifetein, LLC (Somerset, NJ, USA). provides the sequences of all peptides tested under experimental conditions. Agonists, peptides, and vehicle controls were provided to cells in the presence of fresh culture media. Peptides were freshly resuspended prior to cell exposure to reduce the impact of denaturing. Doses of peptides were based on prior published data as well as preliminary data regarding peptide concentration to interleukin (IL)-6 response after LPS challenge (Supplemental ).
Figure 1. Peptides utilized in experiments. Amino acid sequences of CPPs and cargo peptides (underlined) as well as conjugated cargo-CPP fusion peptides used in experiments. The associated abbreviations of the peptides are shown next to the displayed sequence. The RRKRK pentabasic docking site on eNOS is bolded.
Transendothelial electrical resistance (TEER) assay
TEER analysis was performed utilizing the CellZScope2 (nanoAnalytics GmbH, Münster, Germany). Experiments were performed as previously reported.Citation8 Briefly, HMVECs were plated on ThinWell Cell Culture inserts (0.4 μm pore diameter, Greiner Bio-One, Monroe, NC, USA) coated with gelatin (40x), then washed with D-PBS. Afterward, cells were plated at 40,000 cells/transwell and allowed to grow over 24 h until resistance stabilized. Then, cells were exposed to the respective peptides or control (no peptide) for 30 min followed by exposure to LPS (100 ng/ml) in fresh media. TEER was measured continuously in 1 h increments. Prior to analysis, data were normalized to resistance prior to peptide exposure. The area under the curve (AUC) was then calculated relative to pre-peptide resistance. Averages for AUC with 95% CI were then calculated and compared per statistical analysis methods.
Cytokine production
Supernatants from cell cultures were collected at 6 h post-LPS exposure. Collected supernatants were stored at −80°C until analyzed. Supernatant IL-6 (eBioScience, San Diego, CA, USA) and angiopoietin-2 (R&D Systems, Minneapolis, MN, USA) concentrations were assessed using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer’s specifications.
siRNA transfection
HMVECs were treated with siRNA (scrambled siControl, sieNOS) according to the manufacturer’s recommendations. In brief, siRNA was procured from Dharmacon (Lafayette, CO, USA). siRNA (25 nmol/L) was incubated with Dharmafect (Dharmacon) in serum-free medium for 20 min. The resultant complex of siRNA-Dharmafect was added to the cells in 5% FBS media without antibiotics for 6 h. Afterward, the transfection media was replaced with complete media including antibiotics for another 66 h for a total of 72 h siRNA incubation time prior to imaging or peptide addition.
Proximity ligation assay (PLA)
PLAs were performed using a commercially available kit according to the manufacturer’s instructions (Duolink PLA, MilliporeSigma, St. Louis, MO, USA). In brief, cells were plated in 96-well glass bottom plates. If indicated, HMVECs were exposed to siRNA for 72 h total incubation time as previously described. Following the indicated times, cells were washed with PBS and exposed to 4% formaldehyde fixative in PBS for 15 min then permeabilized with 0.1% TritonX-100 in PBS for 15 min. Following solution removal, 40 μL of Duolink blocking solution (MilliporeSigma) was placed in each well and incubated at 37°C for 1 h. The blocking solution was then replaced with Duolink antibody diluent containing the following primary antibodies at a 1:100 dilution: eNOS rabbit mAb (clone #D9A5L, Cell Signaling Technology, Danvers, MA, USA), p38 mouse mAb (clone #9F12, Novus Biologicals, Littleton, CO, USA), and VDAC1 mouse mAb (clone # S152B-23, Novus Biologicals). Plates were then sealed and incubated overnight at 4°C. The next day, the primary antibodies were removed, and the cells were washed with Duolink Wash Buffer. Duolink PLA probes (anti-rabbit secondary antibody with plus oligonucleotides and anti-mouse secondary antibody with minus oligonucleotides) were diluted in a 1:5 ratio in Duolink Antibody Diluent and 40 μL of probe containing solution was added to each well for 1 h at 37°C. Kit ligase was added at a 1:40 dilution to Duolink Ligation Buffer, which contained pre-mixed concentrations of bridging oligonucleotides, and added to each well for 30 min at 37°C. Next, cells were washed and kit-provided polymerase was added in a 1:80 dilution in Duolink Amplification Buffer and applied to each well for 100 min at 37°C. Duolink Orange fluorescent probes (ex 554/em 576) were present in the amplification buffer. Cells were then washed and Duolink Wash Buffer containing 1 drop NucBlue (ex 360/em 460, Thermo Fischer Scientific, Waltham, MA, USA) per mL of buffer was added to each well at room temperature for 10 min. The wash buffer was then removed and replaced with 120 μL of Live Cell Imaging Solution (Thermo Fischer Scientific) and imaged at the appropriate wavelength using an inverted confocal microscope (Zeiss 780, Carl Zeiss AG, Oberkochen, Germany). Fluorescent reactions and cell number were counted by averaging two random fields per well using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
For cell culture experiments, data are represented as means ± SD of multiple, individual experiments. Comparisons of treatment groups, controls, and other conditions were done via unpaired t-test for single comparisons and one-way ANOVA, with Bonferroni correction, for multiple-group comparisons. All analyses were done using GraphPad Prism 9 statistical software (GraphPad Software Inc., La Jolla, CA, USA). A p-value cutoff of <0.05 was used for statistical significance.
Results
Application of AP to a truncated eNOS:RRKRK peptide sequence reduces endothelial permeability
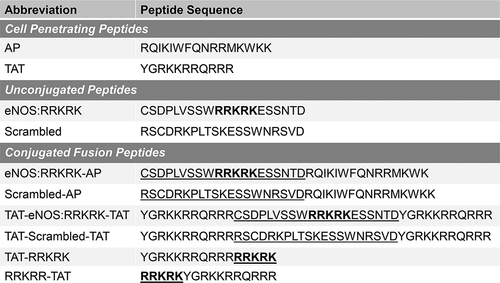
Based on prior knowledge regarding the potential NO-independent activities of the RRKRK docking motif on eNOS, our first goal was to determine if a truncated sequence of eNOS that incorporated the RRKRK domain would provide any benefit to endothelial barrier function. For this initial experiment, we utilized the CPP antennapedia (AP), due to its prior use as a CPP to target endothelial cells and eNOS-mediated responses.Citation12 The sequence of the eNOS:RRKRK-AP peptide is shown in . In addition, we tested cells without any CPP or with AP alone for comparison. As shown in , the application of 25 μM of AP with or without the eNOS:RRKRK sequence significantly improved dynamic barrier function compared to no peptide, as measured by trans-endothelial electrical resistance (TEER). This was observed for endothelial barrier function either in the presence or absence of LPS. Given the dynamic nature of endothelial barrier function over time, resistance curves were normalized to their respective baseline resistance prior to peptide treatment and an area under the curve (AUC) was then generated as the total positive and negative change relative to the baseline. As shown in , the eNOS:RRKRK-AP peptide did enhance endothelial barrier resistance compared to AP alone, both of which significantly improved resistance to no peptide. In the presence of LPS, the effect of the eNOS:RRKRK-AP peptide was not significant compared to AP alone due to some outliers, however again, both peptides greatly enhanced resistance compared to no peptide. Despite the arginine-rich nature of the RRKRK sequence, an eNOS:RRKRK peptide without a conjugated CPP had no effect on TEER compared to no peptide at all (data not shown). These results suggest that while eNOS:RRKRK does appear to enhance baseline endothelial barrier function, the application of AP alone provided the most benefit to the endothelial layer.
Figure 2. eNOS:RRKRK-AP improves endothelial resistance. a) Transendothelial electrical resistance (TEER) of HMVECs treated with either eNOS:RRKRK-AP or AP 25 μM (or no peptide) 30 minutes prior to the addition of LPS (100 ng/ml) or vehicle control. All data is normalized to baseline resistance per condition prior to the addition of peptide treatments and recorded every hour. Arrows indicate the time of peptide and LPS (or control) given. b) Area under the curve (AUC) relative to the change from baseline resistance over time calculated for all conditions in the absence (left) or presence (right) of LPS addition for the entire 24 hours of data. * = p < .05 between designated groups. † = p< .05 between indicated group and no peptide.
AP and TAT differentially effect endothelial permeability and cytokine signaling
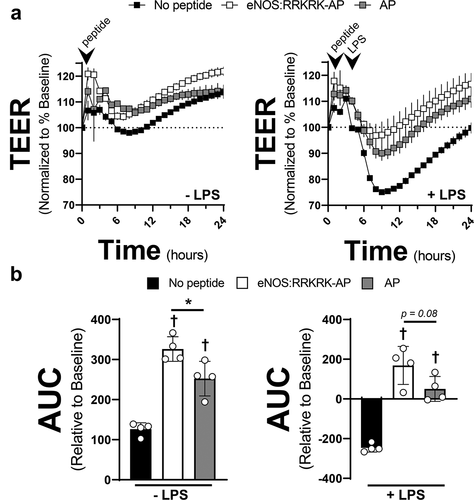
Given the somewhat unexpected effects of AP on endothelial barrier function, we next opted to test AP against a different, but also commonly utilized CPP, trans-activator of transcription (TAT). In addition, to ensure that it was the RRKRK sequence that was causing the relative, albeit small, difference in permeability and not the addition of basic charged residues on the peptide, we added an additional peptide that combined AP with a scrambled eNOS peptide sequence (scrambled-AP). Scrambling as opposed to substitution was done so as not to add changes in peptide charge as a potential confounder of the data collected. As shown in , the eNOS:RRKRK-AP peptide improved endothelial resistance in both unstimulated conditions and after LPS compared to AP alone, similar to previous experiments. In comparison, the AP-scrambled also had some improved resistance compared to AP alone, though not quite to the same degree as eNOS:RRKRK-AP. Strikingly though, the TAT peptide provided no protection to endothelial barrier function and appeared similar to no peptide treatments. Because endothelial inflammation results not only in the loss of barrier integrity, but also the production of pro-inflammatory cytokines, we tested if IL-6 production after LPS stimulation would be differentially regulated by any of the CPPs or conjugated peptides. After LPS, we found that both endothelial cells exposed to 6 hours of LPS in the absence of any peptide or in the presence of TAT had similar outputs of IL-6 production. In contrast, endothelial cells exposed to AP then challenged with LPS had significantly reduced IL-6 levels. Further, the scrambled-AP peptide had higher levels of IL-6 than eNOS:RRKRK-AP or AP alone, though this difference was small and AP versus eNOS:RRKRK-AP were not statistically different from one another.
Figure 3. AP protects against endothelial permeability and IL-6 production compared to TAT. a) TEER of HMVECs treated with 25 μM of eNOS:RRKRK-AP, scrambled-AP, or AP compared to 25 μM TAT (or no peptide) 30 minutes prior to the addition of LPS (100 ng/ml, right) or vehicle control (left). All data is normalized to baseline resistance per condition prior to the addition of peptide treatments and recorded every hour. Arrows indicate time of peptide and LPS (or control) given. b) Supernatant levels of IL-6 from peptide groups in A after 6 hours of LPS exposure (100 ng/ml). * = p < .05 between designated groups. † = p< .05 between indicated group and no peptide.
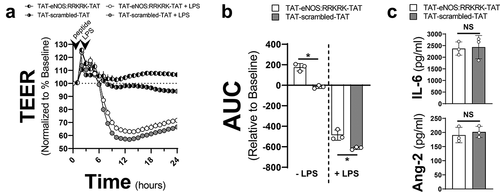
TAT Linked to the eNOS:RRKRK sequence improves barrier function without an impact on cytokine production
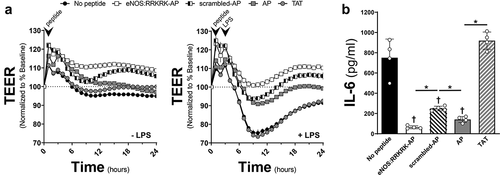
The two prior experiments demonstrated that the conjugation of the AP CPP to any peptide sequence dramatically altered the cellular response to LPS, making it challenging to interpret the relevance of RRKRK. We therefore changed the conjugated CPP solely to TAT to reduce this issue. In addition, we attached TAT to both the C and N termini based on prior data that showed flanking a protein with TAT improves cell penetration as opposed to single terminus conjugation.Citation13 As shown in , the application of a TAT-eNOS:RRKRK-TAT improved endothelial barrier function compared to TAT-scrambled-TAT peptide either in the presence or absence of LPS. However, when examining whether this improved barrier function corresponded to reduced cytokine production, there was no demonstratable difference between the eNOS:RRKRK or scrambled peptides with regard to IL-6 or angiopoietin-2 (Ang-2) after LPS exposure (). In addition, we confirmed that attaching TAT to either the C or N terminus to just the RRKRK sequence alone had no substantial impact on endothelial barrier function, confirming that some secondary structure is necessary for the protective effects of the eNOS:RRKRK peptides (Supplemental ).
Figure 4. TAT-eNOS:RRKRK-TAT improves endothelial resistance without effects on cytokine production. a) TEER of HMVECs treated with either 25 μM TAT-eNOS:RRKRK-TAT or TAT-scrambled-TAT 30 minutes prior to the addition of LPS (100 ng/ml) or vehicle control. Data is normalized to baseline resistance per condition prior to the addition of peptide treatments and recorded every hour. Arrows indicate the time of peptide and LPS (or control) given. b) Area under the curve (AUC) of panel A relative to the change from baseline resistance over time calculated for all conditions in the absence or presence of LPS addition for the entire 24 hours of data. c) Supernatant levels of IL-6 and angiopoietin-2 (Ang-2) from peptide groups after 6 hours of LPS exposure (100 ng/ml). * = p < .05 between designated groups.
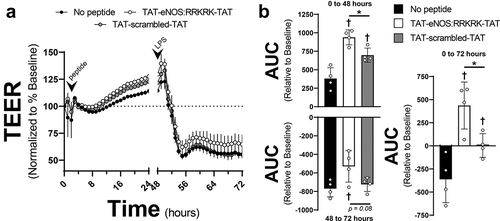
Application of an eNOS:RRKRK peptide leads to sustained improvements in endothelial barrier function
Though there appeared some variable protection of the eNOS:RRKRK peptide 30 minutes prior to LPS exposure, the application of the eNOS:RRKRK peptide consistently improved barrier function compared to the scrambled peptide under unstimulated conditions. However, prior experiments were conducted at only 30 minutes prior to LPS exposure. Given the sustained effects of the eNOS:RRKRK peptide as well as the dynamic physiology of endothelial barrier function, we tested whether an initial exposure to the eNOS:RRKRK peptide could provide sustained protection. As shown in , application of the TAT-eNOS:RRKRK-TAT peptide improved the endothelial monolayer resistance compared to TAT-scrambled-TAT or no peptide for up to 48 hours after its initial application. At 48 hours, the challenge of endothelial cells with LPS was blunted in those previously exposed to the eNOS:RRKRK sequence, as opposed to those that were not. This is demonstrated further in through AUCs for the resistance relative to the peptide application pre- and post-LPS, as well as the total resistance. Overall, the data show that the application of the eNOS:RRKRK peptide can provide sustained endothelial barrier function and reduce the impact of LPS treatment for up to 48 hours after the initial peptide exposure.
Figure 5. Prolonged TAT-eNOS:RRKRK-TAT exposure enhances endothelial resistance to late LPS. a) TEER of HMVECs treated with either 25 μM TAT-eNOS:RRKRK-TAT or TAT-scrambled-TAT 48 hours prior to the addition of LPS (100 ng/ml), then followed 24 hours after LPS exposure. Data is normalized to baseline resistance per condition prior to the addition of peptide treatments and recorded every hour. Arrows indicate the time of peptide and LPS given. b) Area under the curve relative to the change from baseline (prior to peptide exposure) over time calculated for all conditions in the first 48 hours after peptide exposure (top), 24 hours after LPS exposure (bottom) and duration of the entire 72 hours (right). * = p < .05 between designated groups. † = p< .05 between indicated group and no peptide.
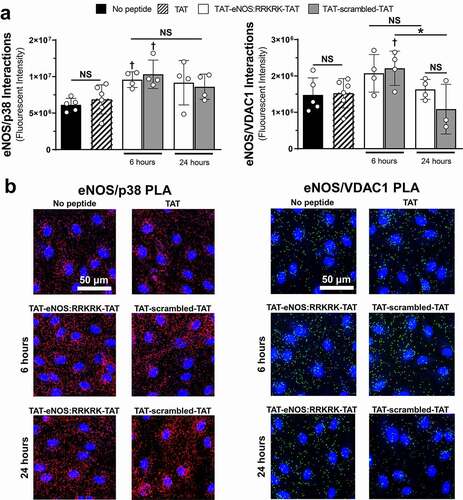
eNOS protein–protein interactions with p38 and VDAC are not significantly impacted by an eNOS:RRKRK peptide application
As we observed consistent enhancement of the endothelial barrier resistance after the application of the eNOS:RRKRK peptide, we wanted to examine if the peptide altered the binding of eNOS with two specific proteins known to regulate endothelial inflammation and interact with the RRKRK domain of eNOS: the MAPK p38, and the mitochondrial ATP-channel VDAC1. Using proximity ligation assays (PLA), we confirmed that under unstimulated conditions (no peptide), eNOS interacted with p38 and VDAC1, the former interaction being the more abundant of the two (). Confirmation of the specificity of these interactions was done through siRNA knockdown of eNOS, with concomitant reductions in eNOS-p38 and eNOS-VDAC1 PLA signals (Supplemental ). The addition of 50 μM TAT peptide for 6 hours had no effect on either eNOS-p38 or eNOS-VDAC1 interactions. Alternatively, 6 hour exposures of either eNOS:RRKRK or scrambled peptide increased eNOS-p38 interactions compared to TAT alone, while only the scrambled peptide increased eNOS-VDAC1 interactions. Extending the duration of exposure to 24 hours, both eNOS:RRKRK and scrambled peptides moved toward unstimulated conditions with regards to eNOS-p38 and eNOS-VDAC1 interactions, with 24 hours of scrambled peptide being significantly reduced compared to 6 hours for eNOS-VDAC1 interactions only. This data shows that despite prior evidence of the functional relationship of the RRKRK sequence with eNOS binding partners, the application of the eNOS:RRKRK peptide did not significantly alter eNOS interactions in relation to changes in endothelial barrier function.
Figure 6. TAT-eNOS:RRKRK-TAT and TAT-scrambled-TAT peptides do not significantly alter eNOS protein–protein interactions compared to one another. a) Total fluorescent signal (intensity) using proximity ligation assays (PLA) for cells exposed to control (no peptide), 50 μM TAT for 6 hours or 25 μM TAT-eNOS:RRKRK-TAT or TAT-scrambled-TAT for 6 and 24 hours. PLAs were performed to assess eNOS/p38 interactions (left) and eNOS/VDAC1 interactions (right). b) Representative images of eNOS/p38 PLA signal (red, left) or eNOS/VDAC1 PLA signal (green, right) with nuclear stain (blue) for the different PLA treatment groups. Scale bar indicates 50 μm. * = p < .05 between designated groups. † = p< .05 between indicated group and TAT, NS = non-significant.
Discussion
The targeting and modification of intracellular processes that contribute to human pathophysiology remains an area ripe for discovery. This is no less true for diseases related to acute, systemic inflammation, such as sepsis, where targeted therapies have remained elusive.Citation14 However, some of that difficulty can be attributed to the heterogenous and dynamic nature of sepsis, where therapies need to be given early and proactively to a cohort to impact outcomes. Within these cohorts are temporal alterations in nitric oxide producing enzymes that are critical for the regulation of vascular homeostasis. Nonspecific blockade of NO producing enzymes was demonstrated to be harmful in sepsis patients, clarifying their important role in the pathophysiology of human sepsis.Citation6 Thus, providing a more specific or targeted approach to nitric oxide synthase pathways continues to be sought as a potential therapeutic avenue in sepsis.
In this study, we tested the hypothesis that given the potential NO-independent role of eNOS through protein–protein interactions, a peptide containing the RRKRK docking motif coupled to a CPP would provide benefit to endothelial cells undergoing LPS challenge. We were able to confirm that peptides containing the RRKRK motif consistently improved endothelial barrier function as measured by TEER. In addition, the RRKRK sequence also provided some protection against LPS-induced endothelial permeability as well as cytokine production. This pentabasic sequence shares homology with other proteins within the mitogen activated protein kinase (MAPK) signaling pathway and has been deemed a MAPK-docking site. These sites are comprised of positively charged residues surrounded by hydrophobic amino acids that bind to reciprocal negatively charged residues on MAPKs called common docking (CD) domains.Citation15 While the eNOS RRKRK motif is not flanked by hydrophobic residues, it nonetheless has been shown to bind to MAPKs and has been suggested to exert some physiological function through its interaction with MAPKs and in particular, p38.Citation9,Citation10 This sequence has also been demonstrated to allow for interactions between eNOS and voltage-dependent anion channel 1 (VDAC1).Citation16
VDAC1, also known as porin, is the most abundantly expressed protein on the outer mitochondrial membrane and the main channel through which ATP, produced through oxidative phosphorylation, is able to leave the mitochondria and enter the cytosol.Citation17 In one study, the authors used a similar peptide with RRKRK and found that it induced mitochondrial reactive oxygen species production in pulmonary endothelial cells and increased the vasoconstriction of isolated pulmonary arteries.Citation11 However, the authors also showed that the peptide induced apoptosis of endothelial cells, a finding inconsistent with our observations of increased resistance across the monolayer. Whether these differences are a result of the peptide sequence, the CPPs employed or the type of endothelial cell, it is not clear, but there is likely some overlap in the observations since both vasomotor tone and permeability can be regulated by alterations in myosin light chain kinase activity.Citation18,Citation19 Yet, despite these prior observations, the application of the eNOS:RRKRK peptide did not appear to have significant effects on the interactions between eNOS and either p38 or VDAC1. Somewhat unexpectedly, the scrambled and eNOS:RRKRK peptides had similar effects on interactions of eNOS with its postulated binding partners, suggesting that from a protein-specific standpoint, interactions with VDAC1 or p38 were not the primary drivers of the observed phenotype regarding endothelial barrier function. Instead, the peptide provided protection via another, unclear mechanism. What this mechanism could be remains unknown and theoretically, could be any number of potential reciprocal proteins, as both the RRKRK and CD domain sequences fall under the category of small linear motifs. These short three to ten amino acid long sequences form the structural basis of protein–protein interactions and therefore, from a specific physiologic standpoint, can be challenging to identify given their ubiquitous and promiscuous nature in cellular biology.Citation20 Along these lines, the most obvious remaining mechanisms promoting endothelial barrier integrity by the eNOS:RRKRK peptide would be other MAPKs, such as extracellularly regulated kinases (ERKs) or c-Jun N-terminal kinase (JNK), which share similar CD domains to p38. While p38-regulated endothelial barrier dysfunction is the most studied, both JNK and ERKs have been implicated in regulating barrier integrity.Citation21,Citation22 These MAPKs have both been demonstrated to regulate tight junction stability, including via claudins and occludins, and it is certainly possible that despite their less studied role, they could mechanistically be involved in the protective role of RRKRK-mediated permeability.Citation23,Citation24
Specific to the impact of the peptides on cytokine production, we observed from our data that protection seemed dependent on the type of CPP utilized. Indeed, beyond the role of RRKRK impacting the endothelial barrier, one striking outcome of this study was the impact of CPPs in isolation in changing the physiologic output to bacterial toxin challenge. The specific reasons for this remain unknown, especially in light of the fact that both are protein-derived, cationic CPPs that are suggested to theoretically follow similar mechanisms of cargo delivery.Citation25 However, it is possible that the secondary structures of AP versus TAT account for the different responses between the two CPPs tested. Certainly, it has been demonstrated that changes to the secondary structure of AP can alter its activity compared to that of TAT.Citation26 We were concerned about the potential of AP binding to the lipid-rich LPS as a potential modifier of the response, hence why we challenged the cells with LPS without AP in the media, however it is possible that residual AP could have a different binding to LPS compared to TAT. An additional possibility is the nuanced differences in the mechanisms through which AP and TAT enter the cell. The endocytic processes of cell entry between AP and TAT differ with regard to interactions with glycosaminoglycan species and given the importance of endocytosis for LPS signaling in endothelial cells, it is possible that mechanisms that account for their cell penetration lead to differential responses to bacterial toxins.Citation27–29 It also has been demonstrated that TAT can induce a pro-inflammatory response in and of itself, specifically with regards to cytokine production, which may explain the differences in cytokine protection when the eNOS sequence was linked to AP versus TAT.Citation30,Citation31 A more straightforward explanation for the observed differences could be that given the overlap of CPPs with antimicrobial peptides (AMPs), AP simply acts more like a AMP that can act as a negative regulator of endothelial inflammation, while TAT elicits more of a pro-inflammatory response in endothelial cells, possibly through integrin signaling, which is known to promote endothelial activation.Citation32,Citation33 Further investigation is warranted regarding these differences due to the common use of these CPPs as postulated therapeutic avenues and their potential off-target effects, especially within the context of endothelial cell inflammation and bacterial challenge.
In conclusion, we herein demonstrate that peptides containing the pentabasic sequence, RRKRK, of eNOS enhance endothelial barrier resistance. Additionally, the application of peptides containing this sequence offer some protection against LPS-mediated endothelial inflammation as seen by reduced monolayer permeability and minor reductions in cytokine production. However, many of these effects are heavily influenced by the type of CPP employed, with TAT being quite inert with regard to endothelial biology. On the other hand, AP has dramatic effects on both endothelial barrier function and cytokine production. While the specific reasons of this remain unclear, this data highlights the importance of weighing in the biological effects of CPPs alone when analyzing for the potential physiological effects of the cargos that are being delivered.
Author contributions
SRK performed the experiments and acquired and interpreted data. RJS designed and performed experiments, interpreted data, wrote the manuscript, and provided overall guidance of the project.
Supplemental Material
Download PDF (795.5 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website
Additional information
Funding
References
- Derakhshankhah H, Jafari S. Cell penetrating peptides: a concise review with emphasis on biomedical applications. Biomed Pharmacother. 2018;108:1–12. doi:10.1016/j.biopha.2018.09.097.
- Schmidt N, Mishra A, Lai GH, Wong GC. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584(9):1806–1813. doi:10.1016/j.febslet.2009.11.046.
- Wang YF, Xu X, Fan X, Zhang C, Wei Q, Wang X, Guo W, Xing W, Yu J, Yan J-L, et al. A cell-penetrating peptide suppresses inflammation by inhibiting NF-kappaB signaling. Mol Ther. 2011;19(10):1849–1857. doi:10.1038/mt.2011.82.
- Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, Colombara DV, Ikuta KS, Kissoon N, Finfer S, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7.
- Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med. 2020;202(3):361–370. doi:10.1164/rccm.201910-1911TR.
- Lambden S. Bench to bedside review: therapeutic modulation of nitric oxide in sepsis-an update. Intensive Care Med Exp. 2019;7(1):64. doi:10.1186/s40635-019-0274-x.
- Coletta C, Modis K, Olah G, Brunyanszki A, Herzig DS, Sherwood ER, Ungvári Z, Szabo C, et al. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Crit Care. 2014;18(5):511. doi:10.1186/s13054-014-0511-3.
- Koch SR, Choi H, Mace EH, Stark RJ. Toll-like receptor 3-mediated inflammation by p38 is enhanced by endothelial nitric oxide synthase knockdown. Cell Commun Signal. 2019;17(1):33. doi:10.1186/s12964-019-0345-3.
- Stark RJ, Koch SR, Choi H, Mace EH, Dikalov SI, Sherwood ER, Lamb FS. Endothelial nitric oxide synthase modulates Toll-like receptor 4–mediated IL-6 production and permeability via nitric oxide-independent signaling. FASEB J. 2018;32(2):945–956. doi:10.1096/fj.201700410R.
- Chrestensen CA, McMurry JL, Salerno JC. MAP kinases bind endothelial nitric oxide synthase. FEBS Open Bio. 2012;2(1):51–55. doi:10.1016/j.fob.2012.02.002.
- Konduri GG, Afolayan AJ, Eis A, Pritchard KA Jr., Teng RJ. Interaction of endothelial nitric oxide synthase with mitochondria regulates oxidative stress and function in fetal pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2015;309(9):L1009–17. doi:10.1152/ajplung.00386.2014.
- Bernatchez P, Sharma A, Bauer PM, Marin E, Sessa WC. A noninhibitory mutant of the caveolin-1 scaffolding domain enhances eNOS-derived NO synthesis and vasodilation in mice. J Clin Invest. 2011;121(9):3747–3755. doi:10.1172/JCI44778.
- Ryu J, Han K, Park J, Choi SY. Enhanced uptake of a heterologous protein with an HIV-1 Tat protein transduction domains (PTD) at both termini. Mol Cells. 2003;16:385–391.
- Vignon P, Laterre PF, Daix T, Francois B. New agents in development for sepsis: any reason for hope? Drugs. 2020;80(17):1751–1761. doi:10.1007/s40265-020-01402-z.
- Tanoue T, Nishida E. Molecular recognitions in the MAP kinase cascades. Cell Signal. 2003;15(5):455–462. doi:10.1016/S0898-6568(02)00112-2.
- Gao S, Chen J, Brodsky SV, Huang H, Adler S, Lee JH, Dhadwal N, Cohen-Gould L, Gross SS, Goligorsky MS, et al. Docking of endothelial nitric oxide synthase (eNOS) to the mitochondrial outer membrane: a pentabasic amino acid sequence in the autoinhibitory domain of eNOS targets a proteinase K-cleavable peptide on the cytoplasmic face of mitochondria. J Biol Chem. 2004;279(16):15968–15974. doi:10.1074/jbc.M308504200.
- Camara AKS, Zhou Y, Wen PC, Tajkhorshid E, Kwok WM. Mitochondrial VDAC1: a key gatekeeper as potential therapeutic target. Front Physiol. 2017;8:460. doi:10.3389/fphys.2017.00460.
- Han YJ, Hu WY, Piano M, de Lanerolle P. Regulation of myosin light chain kinase expression by angiotensin II in hypertension. Am J Hypertens. 2008;21(8):860–865. doi:10.1038/ajh.2008.199.
- Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res. 2010;87(2):272–280. doi:10.1093/cvr/cvq144.
- Van Roey K, Uyar B, Weatheritt RJ, Dinkel H, Seiler M, Budd A, Gibson TJ, Davey NE, et al. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem Rev. 2014;114(13):6733–6778. doi:10.1021/cr400585q.
- Ricard N, Scott RP, Booth CJ, Velazquez H, Cilfone NA, Baylon JL, Gulcher JR, Quaggin SE, Chittenden TW, Simons M, et al. Endothelial ERK1/2 signaling maintains integrity of the quiescent endothelium. J Exp Med. 2019;216(8):1874–1890. doi:10.1084/jem.20182151.
- Miller MR, Koch SR, Choi H, Lamb FS, Stark RJ. Apoptosis signal-regulating kinase 1 (ASK1) inhibition reduces endothelial cytokine production without improving permeability after toll-like receptor 4 (TLR4) challenge. Transl Res. 2021;235:115–128. doi:10.1016/j.trsl.2021.04.001.
- Zheng Y, Zhang M, Zhao Y, Chen J, Li B, Cai W. JNK inhibitor SP600125 protects against lipopolysaccharide-induced acute lung injury via upregulation of claudin-4. Exp Ther Med. 2014;8(1):153–158. doi:10.3892/etm.2014.1684.
- Zhai Z, Ni X, Jin C, Ren W, Li J, Deng J, Deng B, Yin Y. Cecropin A modulates tight junction-related protein expression and enhances the barrier function of porcine intestinal epithelial cells by suppressing the MEK/ERK pathway. Int J Mol Sci. 2018;19(7):1941. doi:10.3390/ijms19071941.
- Bechara C, Sagan S. Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 2013;587(12):1693–1702. doi:10.1016/j.febslet.2013.04.031.
- Caesar CE, Esbjorner EK, Lincoln P, Norden B. Membrane interactions of cell-penetrating peptides probed by tryptophan fluorescence and dichroism techniques: correlations of structure to cellular uptake. Biochemistry. 2006;45(24):7682–7692. doi:10.1021/bi052095t.
- Nakase I, Niwa M, Takeuchi T, Sonomura K, Kawabata N, Koike Y, Takehashi M, Tanaka S, Ueda K, Simpson JC, et al. Cellular uptake of arginine-rich peptides: roles for macropinocytosis and actin rearrangement. Mol Ther. 2004;10(6):1011–1022. doi:10.1016/j.ymthe.2004.08.010.
- Stark R, Choi H, Koch S, Lamb F, Sherwood E. Monophosphoryl lipid A inhibits the cytokine response of endothelial cells challenged with LPS. Innate Immun. 2015;21(6):565–574. doi:10.1177/1753425914564172.
- Console S, Marty C, Garcia-Echeverria C, Schwendener R, Ballmer-Hofer K. Antennapedia and HIV transactivator of transcription (TAT) “protein transduction domains” promote endocytosis of high molecular weight cargo upon binding to cell surface glycosaminoglycans. J Biol Chem. 2003;278(37):35109–35114. doi:10.1074/jbc.M301726200.
- Nookala AR, Kumar A. Molecular mechanisms involved in HIV-1 Tat-mediated induction of IL-6 and IL-8 in astrocytes. J Neuroinflammation. 2014;11(1):214. doi:10.1186/s12974-014-0214-3.
- Ben Haij N, Planes R, Leghmari K, Serrero M, Delobel P, Izopet J, BenMohamed L, Bahraoui E. HIV-1 tat protein induces production of proinflammatory cytokines by human dendritic cells and Monocytes/Macrophages through engagement of TLR4-MD2-CD14 complex and activation of NF-kappaB pathway. PLoS One. 2015;10(6):e0129425. doi:10.1371/journal.pone.0129425.
- Splith K, Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J. 2011;40(4):387–397. doi:10.1007/s00249-011-0682-7.
- Rusnati M, Presta M. HIV-1 Tat protein and endothelium: from protein/cell interaction to AIDS-associated pathologies. Angiogenesis. 2002;5(3):141–151. doi:10.1023/A:1023892223074.
