 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The intestinal epithelial barrier is susceptible to injury from insults, such as ischemia or infectious disease. The epithelium’s ability to repair wounded regions is critical to maintaining barrier integrity. Mechanisms of intestinal epithelial repair can be studied with models that recapitulate the in vivo environment. This review focuses on in vitro injury models and intestinal cell lines utilized in such systems. The formation of artificial wounds in a controlled environment allows for the exploration of reparative physiology in cell lines modeling diverse aspects of intestinal physiology. Specifically, the use of intestinal cell lines, IPEC-J2, Caco-2, T-84, HT-29, and IEC-6, to model intestinal epithelium is discussed. Understanding the unique systems available for creating intestinal injury and the differences in monolayers used for in vitro work is essential for designing studies that properly capture relevant physiology for the study of intestinal wound repair.
Introduction
The intestinal mucosa is lined by a single layer of epithelium that serves as a crucial barrier between the gut lumen and the body. Numerous insults are known to disrupt the epithelial barrier. For example, SARS-CoV2 (also known as COVID-19) has profound effects on the intestinal epithelium. This is believed to be in part due to the affinity SARS-CoV2 S for the angiotensin converting enzyme 2 receptors (ACE2) which is extensively expressed on the apical surface of small intestinal epithelium. While the exact mechanism is not entirely clear, the ability of SARS-CoV2 to damage the intestinal mucosa and result in increased permeability may result in the onset of diarrhea and malabsorption.Citation1 Numerous other infectious diseases, such as Rotavirus, also disrupt epithelial barrier function, and disease processes such as ischemia/reperfusion are known to result in epithelial lifting from the basement membrane, and loss of epithelial monolayer integrity.Citation2–6 Therefore, a crucial component of epithelial barrier function is its ability to rapidly repair using a mechanism called restitution. This involves cell spreading and extension of lamellipodia in order to bridge the wound that results from sloughing of epitheliumCitation7 Reductionist models of restitution have been employed to develop a mechanistic understanding of restitution, including in vitro cell models. However, restitution remains incompletely understood, and this is partly related to the anatomical and physiological complexity of the intestinal mucosa and the limitations of cell models and methods of this reductionist barrier modeling. The wounding process itself can also present a technical dilemma. For instance, wounding typically involves ‘scratching’ in a mechanical fashion, but this differs from in vivo wounding more typically attributable to inflammation from infectious disease or hypoxia and inflammation induced by ischemia/reperfusion. Therefore, this review outlines different models of epithelial wounding and the characteristics of the cell lines used to aid investigators in designing optimal epithelial wounding models for the study of barrier restitution.
The intestinal barrier
The epithelial barrier functions as a physical boundary between luminal contents and the remainder of the body while also serving as a filter for crucial nutrients, ions, and water. Effective barrier function is therefore essential to maintaining intestinal homeostasis and this should be recapitulated in in vitro intestinal modeling systems. Tight junctional proteins bridge across the paracellular space of subjacent epithelial cells and are largely responsible for controlling the flow of ions and macromolecules between cells and create measurable differences in transepithelial electrical resistance (TEER) and macromolecular flux. However, tight junctional proteins retain their own unique size-selective properties which are classified as either pore or leak pathways. Pore pathways are defined as those permeable to molecules with a radius of ~4 Å or less, whereas leak pathways are defined as allowing the passage of large non-charged solutes.Citation8 To accurately assess the integrity of these pathways, methods to assess TEER have been developed for use in cells. One pitfall of using TEER to assess wound healing is that both transcellular and paracellular transport contributes to this measurement, whereas macromolecular fluxes of labeled macromolecules, such as FITC-dextran, are more indicative of changes to the paracellular space.Citation9 Therefore, to avoid confusion as to whether cellular crawling or closure of tight junctions is responsible for differences in barrier function, restitution studies continue to largely rely on the area of epithelial wound coverage.
Mechanisms of barrier repair
In the small intestine, following barrier injury, there are three phases of wound healing: villus contraction, cell migration (restitution), and paracellular space closure.Citation7 Given that cell migration is a major component of the reparative response to injury, different wound models have been developed to closely examine this process during the restitution of epithelial barrier defects. Epithelial restitution utilizes the ability of epithelial cells adjacent to injured portions of mucosa to flatten and migrate over the intact basement membrane following epithelial loss attributable to insults, such as infectious disease, or ischemia.Citation10 This process is accomplished when cell–cell and cell-matrix adhesions are temporarily remodeled, allowing for cells to extend forward and cover denuded surfaces and can occur rapidly before the onset of increased enterocyte proliferation to ultimately replace lost epithelial cells.
Originally, investigators focused on the mechanisms that initiate restitution. One pivotal study in epithelial restitution performed by Nusrat et al. evaluated cellular migration, while cell monolayers recovered from scratch wounding in vitro. The study ultimately concluded that cell flattening, spreading, and formation of lamellipodia-like protrusions were among the first steps involved in restitution.Citation11 However, a greater understanding of the mechanisms of restitution is needed to screen new potential therapeutics and extend the findings to the clinic. One study utilized IEC-6 cells in order to evaluate the effects of various cytokines on wound closure, and found enhanced epithelial restitution when cells were exposed to TGF-α, EGF, and IL-1β, whereas there was no change in migration of cells surrounding wounds in the presence of IL-6, TNF-α, PDGF, and lipopolysaccharide.Citation12 Other studies have focussed on the role of hypoxic conditions at the site of mucosal injury. Activation of hypoxia-inducible factor-1α (HIF-1α) was found to initiate protection of the mucosal barrier to allow for epithelial wound repair.Citation13–15 Specifically, one study found an increase in expression of fibroblast integrin β1 (ITGB1) through transcriptional mechanisms dependent on HIF, which is critical to epithelial wound restitution.Citation16 In contrast to previous studies, cytokines such as IFN-γ have been studied in relation to integrin-mediated restitution and were found to have an inhibitory effect on restitution as a result of dysfunction in the F-actin lamellipodia on T84 cells at the edge of epithelial wounds, raising further questions about the implications of inflammatory cytokines and precursors on restitution.Citation17 While these are only some areas of study surrounding restitution mechanics, much remains still unknown. Therefore, the restitution process continues to be studied extensively using scratch wounding models, cell exclusion assays, neutrophil-induced epithelial wounding models, and electrical wound-healing assays to study healing events, and the mechanisms that regulate cell crawling.
Following restitution, wound healing is completed by resealing of tight junctions. Select tight junction proteins have been studied to refine our understanding of the reparative mechanisms of tight junctions. For example, claudins have been investigated and found to have seemingly complementary functions in barrier function, with some claudins acting as “leaky” or pore-forming claudins, and others as “tight” sealing claudins.Citation18 Works evaluating the ClC-2 pathway in ischemic-injured murine jejunum have shown a critical role for claudin-1 and occludin in resealing tight junctions following epithelial restitution.Citation19 Additionally, an in vitro study using Caco-2 cells overexpressing ClC-2 resulted in a decrease of the pore-forming claudin-2 protein while maintaining claudin-1 and claudin-4 expression.Citation20 Other tight junction proteins have been studied as it appears their reassembly plays a role in epithelial repair; however further investigation is needed to fully understand the extent of their role in healing.
InVitro injury models
A number of in vitro cellular wounding and repair models have been created to assess epithelial restitution, all of which have strengths and weaknesses when attempting to recapitulate intestinal wound repair in clinical patients (). In addition, models to explore the more complex effects of multiple cell types on wound repair have also been studied, particularly the role of epithelial neutrophil transmigration, in order to provide more physiologically relevant models.
Table 1. Comparison of in vitro injury models.
Scratch assay
Scratch wounding is one of the most frequently utilized methods to study barrier restitution in vitro. It offers a simple procedure to induce cell crawling similar to that which happens in vivo post injury. Due to its simplicity, it can be used on any cell line or primary culture that can successfully be grown into a monolayer. This method involves growing confluent monolayers, scratch wounding, and wound measurement. Scratch processes differ by lab, but the two most popular methods are scratches formed by razor blades and those created by pipette tips.Citation21–31 Typically, razor blades are carefully pressed into the confluent monolayer of cells, while pipette tips are dragged across it (). More advanced methods of scratching have been developed to standardize the scratch. One technique resembles a multichannel pipette but has guiding rails on the outside to line up with the plate. This method scratches eight wells at once and has adjustable hex screws to set varying angles.Citation32 Another method does not use any pipette tips, but instead uses a 96 floating-pin array. The operator sets the device on top of the plate, engages the pins, and drags the device toward one corner of the plate scratching 96 wells at once.Citation33 These advancements have helped to standardize scratches within experiments.
Figure 1 Diagrammatic representation of the use of epithelial monolayers for studies of injury and repair. a. Scratch wounding is accomplished with a pipette tip. b. The cell wound exclusion assay involves creation of a ‘wound’ by growing cells in the presence of a silicone stopper.
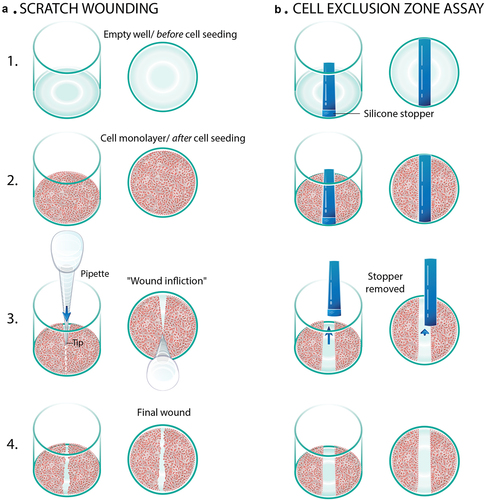
Initially, cells are grown to confluence, after which monolayers are either serum deprived or serum reduced from anywhere between 18 and 48 hours before the wound is inflicted. The level of serum present depends on the type of cell. Transformed cell lines can accommodate complete serum deprivation, while primary cells continue to need small amounts of serum to function properly.Citation34 The intention of serum deprivation is to coerce the cells into a quiescent state, so that cell proliferation is not mistaken for cell migration. Although serum deprivation is a standard step in many scratch wound assays, serum deprivation is somewhat controversial. In some cell lines, it can cause unpredictable effects such as phenotypic changes or varying responses to certain stimuli.Citation35 Given this controversy, researchers need to decide whether serum deprivation or reduction is a necessary component of their scratch wound assay in order to optimally study restitution. In addition, proliferation assays like nuclear labeling with Edu incorporation may be used to ensure rapid proliferation into the wound bed is not being misinterpreted as purely cellular migration.Citation36 Edu labeling has been utilized in place of BrdU as it uses a simple chemical reaction instead of DNA denaturation in order to analyze cellular proliferation, population homeostasis, and cell marking procedures.Citation37
Once the monolayers are injured, the wells can be washed to ensure there are no remaining cells in the scratched area. Immediately following this, the cells and the scratch are imaged to assess the size of the wound. The wells are then imaged at different time intervals to record the migration process. Quantification of wound closure can also vary; some researchers choose to do a simple count of nucleated cells across the wound margins,Citation24,Citation25,Citation27,Citation28 while others use image analysis software to compare the area of the scratch before, during, and after cell migration.Citation7,Citation22,Citation26,Citation38,Citation39 Overall, the process of scratch assays is relatively straightforward and does not require advanced technology.
Due to the simplicity of this process, it has been used for many animal cell line studies to determine whether different growth factors,Citation23,Citation25,Citation38,Citation40 cytokines,Citation27 mRNA proteins,Citation26 nucleic acids,Citation28,Citation39 or supplementationsCitation22,Citation24 improve the epithelial restitution by enhancing cell migration. For example, when TGF-β was added to medium, although it is known for the inhibition of proliferation, it actually sped up the migration process post scratching.Citation25
Although scratch wounding is both quick and simple, it does have its drawbacks. In order for comparison among studies and among samples to be made, the scratches need to be of similar length, width, and depth. There is extensive variability between scratching tools and techniques. Even within the same procedure, variation occurs due to varying operator dexterity patterns (pressure, angle, length) between each scratch. However, additional tools previously mentioned have been developed to minimize these discrepancies. Another problem with scratch assays is that the scratching instrument disrupts more than just the cells. Specifically, most experiments require culture surfaces to be coated with components of the extracellular matrix (ECM). This matrix is often also damaged during wound infliction. This damage can affect the migration of the cells and adds yet another variable impacting restitution.Citation41 In order for scratch assays to be compared, standard procedures including scratch methods and coating materials should be established.
Cell exclusion zone assay
Given its injurious nature to epithelial cells, the scratch wounding method has a multitude of limitations that may influence restitution such as an influx of intracellular fluid or ECM damage.Citation42 Therefore, the cell exclusion zone assay was created to analyze cell crawling in response solely to neighboring barren space, thereby eliminating some of the confounding factors generated by physically wounding a monolayer. Cell exclusion models use patterned cell seeding, created by molds that are placed on the surface before the cells are seeded (). Before this assay was even named, the concept was first used in 1984 to study cytoskeletal regulation in aortic endothelial cells.Citation43 In that experiment, a Teflon fence was used to create the cell barren areas. The experiment that coined the name ‘Cell Exclusion Zone Assay’ used a microfabricated Polydimethylsiloxane (PDMS) mold with rectangular holes in it.Citation42 The mold was placed on ECM coated plastic, and cells were seeded into the holes. The cells were grown to confluence, and the molds were peeled off. This resulted in geometrically perfect ‘wounds’ into which the cells began crawling. The migration rate or area coverage was captured microscopically and then analyzed. This method has been utilized amongst a wide variety of epithelial cell crawling studies involving kidney cells,Citation42 esophageal cells,Citation44 and bronchial cells.Citation45 Due to the fast-healing effects of the intestine, this assay was also optimized in the intestine. In this study, researchers employed the cell exclusion zone model to study cell crawling of fetal small intestine (FHs-74 int) cells.Citation46 Silicone stoppers were used to create the cell barren spaces, after which cell crawling was microscopically examined. This model was compared to the standard scratch wounding assays, with the key finding that the cell exclusion zone assay created a more uniform wound with no damage to the underlying ECM components or leakage of intracellular contents as would occur during scratching, making the quantification of cell crawling easier and more accurate.Citation46
However, this model does not perfectly recreate in vivo wound healing. Researchers have been hesitant to call this cell excluded ‘wound’ an actual wound because of the non-injurious way that it is created. Nevertheless, this model is still thought to be relevant because cell migration is one of the biggest components of epithelial restitution, and this assay has helped to narrow down mechanisms of cell crawling. For example, researchers using a cell exclusion zone assay with canine kidney cells verified that the cells did not need to be damaged in order to migrate. They found that exposing the cells to barren space was enough to trigger their movement. This finding supports the hypothesis that there is a mechanical type of communication between cells and exposed matrix, which induces migration.Citation42
Neutrophil wounding models
Intestinal disease processes, such as ischemia/reperfusion injury, inflammatory bowel disease, and infectious disease that damages intestinal epithelium, are all known to attract significant numbers of neutrophils to the sites of epithelial injury.Citation6,Citation47 This is in part due to neutrophilic chemoattractants accumulating in the area when oxidants are released by the damaged tissue. For example, LTB4 is generated from oxidation of lipid membranes, and is a potent neutrophil chemoattractant. As neutrophils are chemoattracted to the basal side of epithelium via chemoattractant gradients, they continue to migrate through paracellular pathways, disrupting interepithelial junctional structures, ultimately entering the intestinal lumen. This process is regulated in part by CD44v6 and CD55 as well as ICAM-1.Citation48 As the number of neutrophils migrating across the epithelium increases, paracellular spaces are initially widened, followed by lifting and loss of epithelium, with wound formation.Citation49
To model this process in vitro, advances in existing cell motility models have occurred to observe neutrophilic migration across epithelial monolayers. Notably, Brazil et al. developed an advanced model for investigating mediators associated with increased neutrophil transmigration and luminal migration. To achieve this, T84 monolayers were grown on collagen-coated transwells, and a chemotactic gradient was created by adding 100 nM formyl-methionyl-leucyl-phenylalanine to the media on the apical side of a cell monolayer in order to establish a basolateral to apical direction of migration. Next, 1 × 106 polymorphonuclear leukocytes (PMN; (neutrophils) were added to the upper chambers of transwells (in order to achieve technical simplicity and accuracy, application of neutrophils to the apical surface was adopted), and transmigrated PMNs were measured by a colorimetric enzyme activity assay specific for the PMN azurophilic marker myeloperoxidase (MPO). The resulting round wound was observable by using the epithelial desmosomal marker, desmoglein.Citation50 This technique has been further employed to investigate the role of interepithelial junctional complexes in signaling and guiding neutrophil transmigration. For example, a study evaluating junctional-adhesion molecule like protein (JAML) found that binding of JAML to epithelial coxsackie and adenovirus receptor (CAR) plays a role in the regulation of neutrophil transmigration. The bound JAML/CAR fusion proteins specifically inhibited neutrophil migration when introduced into PMN-rich T84 monolayers. While this model can be used to represent wounding following transmigration of PMNs, the restitution that follows is markedly different from that described in other monolayer wound assays. In particular, the wound develops a rim of actinomyosin that appears to help close the wound by contraction, rather than relying solely on epithelial migration.Citation51
Electric cell-substrate impedance sensing
Modern advances have sought to alleviate the problems of inconsistency noted in scratch wounding systems of modeling. This has led to the development of in vitro systems that are able to monitor monolayer behavior after wounding the epithelial cells via high electrical current pulses. The electric cell-substrate impedance sensing (ECIS) system utilizes gold film electrodes that are inserted into the bottom of cell culture plates.Citation52 Cell monolayers are then established and grown on the surface of this gold film electrode, with a larger counter electrode inserted into the culture media in order to create an electrical circuit. In addition to wounding the cell monolayer, the electrode is then able to quantify and chart changes in impedance resulting from cellular behavior as they migrate to heal the wound.Citation53 This technique allows for the investigator to manipulate the wound severity by altering the duration and current strength to fit study parameters.
One study seeking to understand the connection between integrins and epithelial injury in the intestine employed ECIS to help better understand the role of epithelial CD98 in colonic inflammation. This group injured Caco2-BBE cell monolayers apically with application of 3% Dextran Sodium Sulfate (DSS) treatment prior to ECIS with voltage pulses of 40 kHz and 4.5 V for 30s and then allowed the wound to heal. The resulting examination of CD98 during wound infliction and restitution revealed that the upregulation of epithelial CD98 mediated by INF-γ during intestinal inflammation in DSS-treated monolayers hampered cellular restitution in this model.Citation54 Another study performed with Caco2-BBE cell monolayers explored the role of ADAM-15 in intestinal wound healing. Monolayers with overexpressed ADAM-15 and control groups were injured with a voltage pulse of 40 Hz and 4.5-V amplitude for 30s to cause cell death. Ultimately, cells with upregulated ADAM-15 did not recover as well as the control group, which was able to completely heal the wound after 19 hrs.Citation55
Where many of the existing models fail to provide consistent injury that can be modified to exacerbate or change the level of injury, ECIS produces a consistent injury via gold film electrodes while also allowing investigators to track restitution in real-time via impedance measurements. However, the heat created from the high current can alter the viability of the surrounding cells and cause additional damage. In addition, the density of cell monolayers and the extent to which interepithelial junctions are intact can influence impedance measurements and make the interpretation of study results challenging.
Organoid models
Recent advances in 3-D organoid models of the intestine have created a unique opportunity to evaluate epithelial injury and repair. Organoids display architecture and physiologic similarity to in vivo tissues including crypt and villus regions and homeostasis of stem cell populations.Citation56–58 One study subjected small intestinal organoids to γ-radiation to support previous reports indicating hepatocyte nuclear factor 4α as an upstream regulator of repair.Citation59 Another investigated injury-responsive stem cell populations to identify VPA and EPZ6438 as features critical to regeneration after epithelial damage.Citation60 However, organoids pose a challenge for investigators evaluating physical damage to the epithelial monolayer. Due to the closed nature of the enteroid, accessing the apical surface is extremely difficult without inadvertently disrupting the barrier of the organoid. This model does still provide a means of studying important questions regarding the reparative process of intestinal epithelium in a physiologically relevant 3-D system.
Cell lines
In addition to knowledge of the characteristics of epithelial wound models, an important component of any study is the epithelial cell type. There is significant utility in highly reductionist model systems that allow precise study of biological mechanisms. Although these models do not fully recapitulate the complexity of the intestinal mucosa, they do enable the selective study of epithelial wound repair, which is arguably the most vital component of the intestinal mucosal barrier.Citation7,Citation21,Citation61 Although transformed epithelial cell lines have been developed for convenient usage in the lab, primary cell culture techniques have also undergone major advances to not only be more convenient for lab use, but also represent the multiple cell lineages that make up the epithelial barrier. While primary cultured epithelium more closely represents the in vivo environment, they have drawbacks related to passage time, confluence abilities, and susceptibility to experimental variation. These disadvantages can prevent investigators from understanding mechanisms of restitution, and therefore must be fully understood before embarking on further studies. In this section, some of the more commonly used cell lines that can be used for the study of restitution are described.
IEC-6
Quaroni et al. were one of the first labs to characterize an epithelial cell line that could be passaged multiple times when they developed IEC-6 cells.Citation62 IEC-6 cells are a non-transformed cell line derived from rat small intestinal crypt cells that mimic small intestinal epithelium in vitro. They are polygonal in shape with large nuclei and have microvilli present on one side of the cell demonstrating polarization.Citation62 They also express tight junction proteins, such as occludin, claudin-6, and ZO-1, as well as adherens junction proteins such as E-cadherin that are all crucial to adhere cells together into a uniform barrier.Citation63 In addition, their average peak TEER is ~30 Ω·cm2 after 6 days of seeding,Citation64 which mimics the ‘leakier’ TEER of small intestine. Originally, serial passages were made for ~6 months. From these passages, other researchers have been able to use these cells for various barrier function studies involving iron absorption,Citation65 mitogen activated protein (MAP) kinases’ role in apoptosis,Citation66 and enteric protozoan’s effect on TEER.Citation64 It must be noted that these cells mimic undifferentiated small intestinal crypt cells and therefore need additional coercion to differentiate. Fortunately, certain cell-medium additions such as transforming growth factor beta (TGF-β),Citation67 interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α),Citation68 and gastrinCitation69 have been found to successfully differentiate IEC-6 cells. Although differentiated IEC-6 cells seem to have only been used in one scratch wounding study investigating the role of polyamines and myosin II in cell motility,Citation70their undifferentiated counterparts have been widely used for different types of in vitro restitution studies. Most commonly, scratch wound models have utilized IEC-6 monolayers to investigate how various treatments like polyamine depletion,Citation71 cytokine modulation,Citation12 and Astragalus polysaccharidesCitation72 can affect cell migration. These cells have also been used in in vitro hypoxia studies investigating how hypoxia affects barrier functionCitation73 and different ways to protect against these hypoxic injuries.Citation74
Caco-2
Researchers have found multiple tumor-based cell lines that have the ability to differentiate. One example is the Caco-2 cell line which was derived from a human colon adenocarcinoma and was developed in part due to the inability of researchers to passage and culture differentiated primary epithelial cells.Citation9 This transformed cell line spontaneously differentiates, and forms monolayers connected by tight junctions. These cells are absorptive, polarized, and have distinct apical, lateral, and basal cell membrane characteristics.Citation9 On their apical side, they have microvilli similar to both enterocytes and colonocytes.Citation75 Furthermore, Caco-2 cells do exhibit mature enterocytic properties, such as disaccharidase and peptidase expression, more typical of small intestinal epithelium.Citation76 However, Caco- 2 cells are a transformed cell line, and have been shown to have gene mutations in p53, APC, ββ-catenin, and Smad4, which are uncharacteristic of intestinal epithelial cells.Citation77 Even with these mutations, Caco-2 cells are still widely used for intestinal studies focused on barrier functions, such as barrier maintenance in the absence of glutamine,Citation78 tight junction modulation,Citation79 and permeability response to short-chain fatty acids.Citation80 They have also been a part of multiple restitution studies involving varying model types. Caco-2 scratch assays have been performed to investigate how certain actions or factors enhance wound healing like the activation of protease activated receptor-2.Citation81 Caco- 2 cells were also used to optimize the cell exclusion zone assayCitation46 as well as investigate how the loss of lipolysis-stimulated lipoprotein receptors affect barrier integrity.Citation82 In addition, these monolayers have undergone hypoxic injury to better understand the effect on barrier integrity.Citation83,Citation84
There also exists a Caco-2 derived cell line, C2BBe1, that is more similar to the colon due to its brush border enzyme (BBe) expression. The BB of this cell line contains proteins such as villin, fimbrin, BB myosin I, fodrin, myosin II, and sucrase-isomaltase, all of which are found in the BB of colonocytes.Citation85 Given these unique properties, C2BBe1 has been used in a variety of intestinal studies. For example, C2BBe1 was used to study the effects of essential oils on inflammatory diseasesCitation86 and recently used to investigate how SARS-Cov-2 affects the gastrointestinal tract.Citation5 However, C2BBe1 cells have not been used for restitution studies involving the models previously stated.
HT29
Another cell line originating from a human colon adenocarcinoma is HT29 cells. HT29 cells are more versatile than Caco-2 cells because they can accurately represent multiple cell lineages in vitro. In the presence of glucose concentrated media, these cells remain undifferentiated. When glucose is replaced by galactose, they differentiate into enterocyte-like cells.Citation76 In media highly concentrated with glucose and methotrexate (MTX), they begin producing large amounts of mucin, mimicking goblet cells.Citation75 This led to the subcell line called HT29-MTX. This cell line has been used to study drug transportCitation87 and protein digestion.Citation88 This cell line has also been used in conjunction with Caco-2 cells to study drug absorptionCitation89 and toxicity.Citation90 In these co-culture studies, HT29-MTX cells are cultured with Caco-2 cells on a transwell at a biologically informed ratio of 1:9, respectively. In regard to wound studies, the HT29 cell line has been used to analyze how different biological components like MUC2,Citation91 beta-Adrenoreceptor antagonists,Citation92 and protease activated receptor 2 agonistsCitation93 influence restitution given a cell barren space either caused by scratch wound or cell exclusion zone assay. In addition, they have also been used to study epithelial integrity following hypoxic injury and the mechanisms whereby calcium channel blockade following this injury affects barrier integrity.Citation94
T84
With similar origins to Caco-2 and HT29 cells, T84 cells are also derived from human colon adenocarcinoma; however, these cells originated from a metastatic lung tumor. T84 cells possess similarities to Caco-2 cells, such as their absorptive properties, spontaneous differentiation post confluence, and columnar cell physiology with apical microvilli.Citation95 These similarities have led researchers to use both cell lines interchangeably as a monolayer model of intestinal epithelial cells. However, some studies have shown that T84 cells act more like undifferentiated crypt cells and colonocytes. T84 cells, like many intestinal cell lines, also exhibit atypically high measures of barrier resistance (). Madara et al. indicated that the T84 cell Cl− secretory response was more similar to crypt cells’ secretory response than mature absorptive epithelial cells.Citation103 In 2017, Devriese et al. built on this argument by showing the difference between T84 models and Caco-2 models.Citation95 More specifically, this study showed that T84 cells had 3-fold shorter microvilli than Caco-2 cells, which is a characteristic of colon epithelial cells. In addition, the colonic marker, monocarboxylate transporter 1 (MCT1), was upregulated in differentiated T84 cells, while enterocyte brush border enzymes were not.Citation95 Based on these findings, and the fact that Cl− secretion plays a large role in the regulation of diarrhea and constipation, T84 cells have been widely used as models for electrogenic Cl− secretion in the colon.Citation96,Citation104–107 This cell line has also been used to model restitution following injury. Scratch assays have been conducted to see how factors such as galectinsCitation108 or microbiota-derived butyrateCitation109 affect re-epithelization. In addition, T84 cells have been used in conjunction with neutrophils or PMNs, to better understand their role following intestinal injury. One study coupled a scratch wound assay with a PMN migration assay and found that PMN adhesion to epithelial cells post-migration enhances cell proliferation thereby aiding in wound healing. They believed that although transmigrating PMNs do cause additional damage to the tissue, once they adhere to epithelial cells, ICAM-1, a luminal epithelial surface receptor, is engaged, and this initiates Akt and β-catenin signaling, both of which are important signaling pathways for cell proliferation.Citation97 In addition, as previously mentioned, Nusrat et al. utilized T84 monolayers to model a wound caused by PMN infiltration. From this model, they found that wound closure methods vary with regard to wound size. Lamellipodia extensions are the main method of closure for larger wounds, while smaller wounds tend to form and contract an actinomyosin cytoskeletal ring.Citation98
Table 2. Comparison of intestinal cell lines.
IPEC-J2
The IPEC-J2 cell line is a non-transformed line derived from jejunal crypts of a neonatal pig, developed in 1989.Citation99 Historically, these cells have been used for a variety of functional studies due to their similarity to small intestinal epithelia. Specifically, IPEC-J2 cells contain several tight junctional proteins, are capable of secreting mucins (specifically Muc1 and Muc3), and cytokines such as TNF-α, and express a number of toll like receptors (TLR-1, −2, −3, −4, −5, −6, −8, −9, and −10).Citation100,Citation101 However, one of the largest limitations of the IPEC-J2 line grown conventionally in 5–10% Fetal Bovine Serum has been its unusually high barrier resistance, with TEER values averaging upwards of 1000 .Citation102 Previous studies performed by Zakrewski et al. showed a dramatic decrease in TEER values of IPEC-J2 cells when grown in 10% Pig Serum, with average TEER being 200–400 Ω·cm2after 14 days.Citation102 Recently, studies in our laboratory evaluating the effects of media containing Wnt3a, R-spondin, Noggin (WRN) on IPEC-J2 cells were performed demonstrating decreased TEER values in IPEC-J2 cells grown in WRN-conditioned media. TEER values in these studies were 50.3 ± 2.3 Ω·cm2 over 12 days as opposed to IPEC-J2s grown in conventional media which exceeded 2000 Ω·cm2.Citation110 This suggests potential for IPEC-J2 cells to be manipulated using media typically used for organoid culture, and may represent a more physiologically relevant model of barrier function in vitro in the future.Citation110 IPEC-J2 cells have also been used when studying restitution mechanisms. For example, one study utilized scratch wounded IPEC-J2 cell monolayers to observe restitution during tryptophan supplementation and its effects on the CaSR/Rac1/PLC-γ1 signaling pathway.Citation111 Additionally, IPEC-J2 cells have been employed to explore the positive effect of sodium butyrate and identify it as an agent capable of recovering intestinal tissues after assault or injury.Citation112
Conclusion
The in vitro models used to study gastrointestinal pathophysiology are an integral component of mechanistic research. For example, the HT-29 and IPEC-J2 cell lines have contributed heavily to research in restitution mechanisms by performing scratch wounding assays and cell exclusion models. Similarly, IEC-6 cells have been utilized in neutrophil exclusion studies. The T84 cell line has been utilized to better understand neutrophilic injury during the reparative process. Caco-2 cells have also been invaluable in studies seeking to understand restitution through ECIS and its novel system for modeling injury through electrical pulses. However, the success of these models lies in the ability of researchers to properly choose the cell line and model best suited for their study.
Future work in optimizing these modeling systems to create increasingly relevant models of the in vivo environment is necessary to speed the pace toward clinical application. In addition, the field of in vitro modeling systems of intestinal epithelium is expanding rapidly and new systems which rely on primary cells derived from individual patients and grown in monolayers or 3-D organoids are becoming commonplace. These systems will inevitably require alternative or adapted methods of in vitro injury and repair to assess their viability properly and accurately as a model of restitution after insult.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):164–178. doi:10.1053/j.gastro.2020.02.054.
- Jacobi SK,Moeser AJ, Blikslager AT, Rhoads JM, Corl BA, Harrell RJ, Odle J. Acute effects of rotavirus and malnutrition on intestinal barrier function in neonatal piglets. World J Gastroenterol. 2013;19(31):5094–5102. doi:10.3748/wjg.v19.i31.5094.
- Crawford SE,Ramani S, Tate JE, Parashar UD, Svensson L, Hagbom M, Franco MA. Rotavirus infection. Nat Rev Dis Primers. 2017;3:17083. doi:10.1038/nrdp.2017.83.
- Jung K, Saif LJ, Wang Q. Porcine epidemic diarrhea virus (PEDV): an update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020;286:198045. doi:10.1016/j.virusres.2020.198045.
- Lee E,Sandgren K, Duette G, Stylianou VV, Khanna R, Eden JS, Blyth E, Gottlieb D, Cunningham AL, Palmer S. Identification of SARS-CoV-2 nucleocapsid and spike T-cell epitopes for assessing T-cell immunity. J Virol. 2020;94(3). doi:10.1128/JVI.01270-19.
- Leoni G, Neumann PA, Sumagin R, Denning TL, Nusrat A. Wound repair: role of immune-epithelial interactions. Mucosal Immunol. 2015;8(5):959–968. doi:10.1038/mi.2015.63.
- Blikslager AT, Moeser AJ, Gookin JL, Jones SL, Odle J. Restoration of barrier function in injured intestinal mucosa. Physiol Rev. 2007;87(2):545–564. doi:10.1152/physrev.00012.2006.
- Shen L, Weber CR, Raleigh DR, Yu D, Turner JR. Tight junction pore and leak pathways: a dynamic duo. Annu Rev Physiol. 2011;73(1):283–309. doi:10.1146/annurev-physiol-012110-142150.
- Sambuy Y,De Angelis I, Ranaldi G, Scarino ML, Stammati A, Zucco F. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol Toxicol. 2005;21(1):1–26. doi:10.1007/s10565-005-0085-6.
- Blikslager A, Gonzalez L. Equine intestinal mucosal pathobiology. Annu Rev Anim Biosci. 2018;6:157–175. doi:10.1146/annurev-animal-030117-014748.
- Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89(5):1501–1511. doi:10.1172/JCI115741.
- Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993;105(5):1323–1332. doi:10.1016/0016-5085(93)90136-Z.
- Furuta GT, et al. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193(9):1027–1034. doi:10.1084/jem.193.9.1027.
- Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med (Berl). 2007;85(12):1295–1300. doi:10.1007/s00109-007-0277-z.
- Louis NA, et al. Selective induction of mucin-3 by hypoxia in intestinal epithelia. J Cell Biochem. 2006;99(6):1616–1627. doi:10.1002/jcb.20947.
- Keely S, et al. Selective induction of integrin beta1 by hypoxia-inducible factor: implications for wound healing. FASEB J. 2009;23(5):1338–1346. doi:10.1096/fj.08-125344.
- Tong Q, et al. Interferon-gamma inhibits T84 epithelial cell migration by redirecting transcytosis of beta1 integrin from the migrating leading edge. J Immunol. 2005;175(6):4030–4038. doi:10.4049/jimmunol.175.6.4030.
- Garcia-Hernandez V, Quiros M, Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann N Y Acad Sci. 2017;1397(1):66–79. doi:10.1111/nyas.13360.
- Nighot PK, et al. ClC-2 is required for rapid restoration of epithelial tight junctions in ischemic-injured murine jejunum. Exp Cell Res. 2009;315(1):110–118. doi:10.1016/j.yexcr.2008.10.001.
- Nighot PK, Leung L, Ma TY. Chloride channel ClC- 2 enhances intestinal epithelial tight junction barrier function via regulation of caveolin-1 and caveolar trafficking of occludin. Exp Cell Res. 2017;352(1):113–122. doi:10.1016/j.yexcr.2017.01.024.
- Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. 2011;17(17):2161–2171. doi:10.3748/wjg.v17.i17.2161.
- Blais M, et al. A gene expression programme induced by bovine colostrum whey promotes growth and wound-healing processes in intestinal epithelial cells. J Nutr Sci. 2014;3:e57. doi:10.1017/jns.2014.56.
- Bu H-F, et al. Milk fat globule–EGF factor 8/lactadherin plays a crucial role in maintenance and repair of murine intestinal epithelium. J Clin Invest. 2007;117(12):3673–3683. doi:10.1172/JCI31841.
- Cario, et al., Effects of exogenous zinc supplementation on intestinal epithelial repair in vitro. Eur J Clin Invest, 2000. 30(5): p. 419–428. 10.1046/j.1365-2362.2000.00618.x
- Ciacci C, Lind SE, Podolsky DK. Transforming growth factor beta regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology. 1993;105(1):93–101. doi:10.1016/0016-5085(93)90014-4.
- Liu L, et al. HuR enhances early restitution of the intestinal epithelium by increasing Cdc42 translation. Mol Cell Biol. 2017;37(7):e00574–16. doi:10.1128/MCB.00574-16.
- Moyer RA, et al. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. 2007;87(8):807–817. doi:10.1038/labinvest.3700595.
- Rhoads JM, et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut. 2004;53(4):514–522. doi:10.1136/gut.2003.027540.
- Abey SK, et al. Data supporting the effects of lysozyme on mRNA and protein expression in a colonic epithelial scratch wound model. Data in Brief. 2017;11:15–18. doi:10.1016/j.dib.2016.12.043.
- Davudian S, et al. BACH1 silencing by siRNA inhibits migration of HT-29 colon cancer cells through reduction of metastasis-related genes. Biomed Pharmacother. 2016;84:191–198. doi:10.1016/j.biopha.2016.09.021.
- Kelm M, et al. Targeting epithelium-expressed sialyl Lewis glycans improves colonic mucosal wound healing and protects against colitis. JCI Insight. 2020;5(12). doi:10.1172/jci.insight.135843.
- Yue PY, et al. A simplified method for quantifying cell migration/wound healing in 96-well plates. J Biomol Screen. 2010;15(4):427–433. doi:10.1177/1087057110361772.
- Yarrow JC, et al. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004;4(1):21. doi:10.1186/1472-6750-4-21.
- Jonkman JEN, et al. An introduction to the wound healing assay using live-cell microscopy. Cell Adh Migr. 2014;8(5):440–451. doi:10.4161/cam.36224.
- Pirkmajer S, Chibalin AV. Serum starvation: caveat emptor. American J Physiol-Cell Physiol. 2011;301:C272–C279.
- Cormier N, et al. Optimization of the wound scratch assay to detect changes in murine mesenchymal stromal cell migration after damage by soluble cigarette smoke extract. J Vis Exp. 2015;(106):e53414. doi:10.3791/53414.
- Flomerfelt FA, Gress RE. Analysis of cell proliferation and homeostasis using EdU labeling. Methods Mol Biol. 2016;1323:211–220.
- Polk DB. Epidermal growth factor receptor–stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;114(3):493–502. doi:10.1016/S0016-5085(98)70532-3.
- Rhoads JM, et al. Arginine Stimulates cdx2-transformed intestinal epithelial cell migration via a mechanism requiring both nitric oxide and phosphorylation of p70 S6 kinase. J Nutr. 2008;138(9):1652–1657. doi:10.1093/jn/138.9.1652.
- Dise RS, et al. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G276–85. doi:10.1152/ajpgi.00340.2007.
- Kam Y, et al. A novel circular invasion assay mimics in vivo invasive behavior of cancer cell lines and distinguishes single-cell motility in vitro. BMC Cancer. 2008;8(1):198. doi:10.1186/1471-2407-8-198.
- Poujade M, et al. Collective migration of an epithelial monolayer in response to a model wound. Proc Natl Acad Sci. 2007;104(41):15988. doi:10.1073/pnas.0705062104.
- Pratt BM, et al. Mechanisms of cytoskeletal regulation. modulation of aortic endothelial cell spectrin by the extracellular matrix. Am J Pathol. 1984;117(3):349–354.
- Glenn HL, Messner J, Meldrum DR. A simple non-perturbing cell migration assay insensitive to proliferation effects. Sci Rep. 2016;6(1):31694. doi:10.1038/srep31694.
- Omelchenko T, Hall A. Myosin-IXA regulates collective epithelial cell migration by targeting RhoGAP activity to cell-cell junctions. Curr Biol: CB. 2012;22(4):278–288. doi:10.1016/j.cub.2012.01.014.
- Nyegaard S, Christensen B, Rasmussen JT. An optimized method for accurate quantification of cell migration using human small intestine cells. Metab Eng Commun. 2016;3:76–83. doi:10.1016/j.meteno.2016.03.002.
- Luissint AC, Parkos CA, Nusrat A. Inflammation and the intestinal barrier: leukocyte-epithelial cell interactions, cell junction remodeling, and mucosal repair. Gastroenterology. 2016;151(4):616–632. doi:10.1053/j.gastro.2016.07.008.
- Phillipson M, Kubes P. The healing power of neutrophils. Trends Immunol. 2019;40(7):635–647. doi:10.1016/j.it.2019.05.001.
- Hall CHT, Campbell EL, Colgan SP. Neutrophils as components of mucosal homeostasis. Cell Mol Gastroenterol Hepatol. 2017;4(3):329–337. doi:10.1016/j.jcmgh.2017.07.001.
- Brazil JC, et al. Neutrophil migration across intestinal epithelium: evidence for a role of CD44 in regulating detachment of migrating cells from the luminal surface. J Immunol. 2010;185(11):7026–7036. doi:10.4049/jimmunol.1001293.
- Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177(2):512–524. doi:10.2353/ajpath.2010.100168.
- Gu AY, et al. In vitro wounding models using the electric cell-substrate impedance sensing (ECIS)-ztheta technology. Biosensors (Basel). 2018;8(4).
- Keese CR, et al. Electrical wound-healing assay for cells in vitro. Proc Natl Acad Sci U S A. 2004;101(6):1554–1559. doi:10.1073/pnas.0307588100.
- Kucharzik T, et al. Activation of epithelial CD98 glycoprotein perpetuates colonic inflammation. Lab Invest. 2005;85(7):932–941. doi:10.1038/labinvest.3700289.
- Charrier L, et al. ADAM-15 inhibits wound healing in human intestinal epithelial cell monolayers. Am J Physiol Gastrointest Liver Physiol. 2005;288(2):G346–53. doi:10.1152/ajpgi.00262.2004.
- Ootani A, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–706. doi:10.1038/nm.1951.
- Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi:10.1038/nature09637.
- Spence JR, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470(7332):105–109. doi:10.1038/nature09691.
- Montenegro-Miranda PS, et al. A novel organoid model of damage and repair identifies HNF4alpha as a critical regulator of intestinal epithelial regeneration. Cell Mol Gastroenterol Hepatol. 2020;10(2):209–223. doi:10.1016/j.jcmgh.2020.02.007.
- Qu M, et al. Establishment of intestinal organoid cultures modeling injury-associated epithelial regeneration. Cell Res. 2021;31(3):259–271. doi:10.1038/s41422-020-00453-x.
- Dutton JS, et al. Primary cell-derived intestinal models: recapitulating physiology. Trends Biotechnol. 2019;37(7):744–760. doi:10.1016/j.tibtech.2018.12.001.
- Quaroni A, Wands J, Trelstad RL, Isselbacher KJ. Epithelioid cell cultures from rat small intestine. Characterization by morphologic and immunologic criteria. J Cell Biol. 1979;80(2):248–265. doi:10.1083/jcb.80.2.248.
- Zhang L,Zhang M, Chen X, He Y, Chen R, Zhang J, Huang J, Ouyang C, Shi G. Identification of the tubulointerstitial infiltrating immune cell landscape and immune marker related molecular patterns in lupus nephritis using bioinformatics analysis. Ann Transl Med. 2020;8(23):1596. doi:10.21037/atm-20-7507.
- Puthia MK,Sio SW, Lu J, Tan KS. Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect Immun. 2006;74(7):4114–4123. doi:10.1128/IAI.00328-06.
- Thomas C, Oates PS. IEC-6 cells are an appropriate model of intestinal iron absorption in rats. J Nutr. 2002;132(4):680–687. doi:10.1093/jn/132.4.680.
- Bhattacharya S, Ray RM, Johnson LR. Prevention of TNF-alpha-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am J Physiol Gastrointest Liver Physiol. 2004;286(3):G479–90. doi:10.1152/ajpgi.00342.2003.
- Liao Y, Zhang M, Lönnerdal B. Growth factor TGF-β induces intestinal epithelial cell (IEC-6) differentiation: miR-146b as a regulatory component in the negative feedback loop. Genes Nutr. 2013;8(1):69–78. doi:10.1007/s12263-012-0297-3.
- Kolinska J, et al. Constitutive expression of IL-18 and IL-18R in differentiated IEC-6 cells: effect of TNF-alpha and IFN-gamma treatment. J Interferon Cytokine Res. 2008;28(5):287–296. doi:10.1089/jir.2006.0130.
- Wang JY. Cellular signaling in rapid intestinal epithelial restitution: implication of polyamines and K+ channels. Sheng Li Xue Bao. 2003;55:365–372.
- Rao JN, et al. Differentiated intestinal epithelial cells exhibit increased migration through polyamines and myosin II. Am J Physiol. 1999;277(6):G1149–58. doi:10.1152/ajpgi.1999.277.6.G1149.
- McCormack SA, et al. Polyamine depletion alters the relationship of F-actin, G-actin, and thymosin beta4 in migrating IEC-6 cells. Am J Physiol. 1999;276(2):C459–68. doi:10.1152/ajpcell.1999.276.2.C459.
- Zhang CL, et al. Modulation of intestinal epithelial cell proliferation, migration, and differentiation in vitro by Astragalus polysaccharides. PLoS One. 2014;9(8):e106674. doi:10.1371/journal.pone.0106674.
- Xu DZ, et al. The effect of hypoxia/reoxygenation on the cellular function of intestinal epithelial cells. J Trauma. 1999;46(2):280–285. doi:10.1097/00005373-199902000-00014.
- Jia Z, et al. Ischemic postconditioning protects against intestinal ischemia/reperfusion injury via the HIF-1alpha/miR-21 axis. Sci Rep. 2017;7(1):16190. doi:10.1038/s41598-017-16366-6.
- Simon-Assmann P, et al. In vitro models of intestinal epithelial cell differentiation. Cell Biol Toxicol. 2007;23(4):241–256. doi:10.1007/s10565-006-0175-0.
- Pinto M, et al. Enterocytic differentiation of cultured human colon cancer cells by 482 replacement of glucose by galactose in the medium. 481 Zweibaum, A. Btol Celt. 1982;44:193–196.
- Gayet J, et al. Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene. 2001;20(36):5025–5032. doi:10.1038/sj.onc.1204611.
- DeMarco VG, et al. Glutamine and barrier function in cultured Caco-2 epithelial cell monolayers. J Nutr. 2003;133(7):2176–2179. doi:10.1093/jn/133.7.2176.
- Khan N, Binder L, Pantakani DVK, Asif AR. MPA modulates tight junctions‘ permeability via midkine/PI3K pathway in Caco-2 cells: a possible mechanism of leak-flux diarrhea in organ transplanted patients. Front Physiol. 2017;8(438). doi:10.3389/fphys.2017.00438.
- Peng L, et al. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr Res. 2007;61(1):37–41. doi:10.1203/01.pdr.0000250014.92242.f3.
- Fernando EH, et al. Inhibition of intestinal epithelial wound healing through protease-activated receptor-2 activation in Caco2 Cells. J Pharmacol Exp Ther. 2018;367(2):382–392. doi:10.1124/jpet.118.249524.
- Czulkies BA, et al. Loss of LSR affects epithelial barrier integrity and tumor xenograft growth of CaCo-2 cells. Oncotarget. 2017;8(23):37009–37022. doi:10.18632/oncotarget.10425.
- Lian P, et al. Hypoxia and heat stress affect epithelial integrity in a Caco-2/HT-29 co-culture. Sci Rep. 2021;11(1):13186. doi:10.1038/s41598-021-92574-5.
- Tazuke Y, et al. The effect of hypoxia on permeability and bacterial translocation in Caco-2 adult and I-407 fetal enterocyte cell culture models. Pediatr Surg Int. 2003;19(5):316–320. doi:10.1007/s00383-003-1002-9.
- Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102(3):581–600. doi:10.1242/jcs.102.3.581.
- Rufino AT, et al. Differential effects of the essential oils of Lavandula luisieri and Eryngium duriaei subsp. juresianum in cell models of two chronic inflammatory diseases. Pharm Biol. 2015;53(8):1220–1230. doi:10.3109/13880209.2014.970701.
- Behrens I, et al. Transport of lipophilic drug molecules in a new mucus-secreting cell culture model based on HT29-MTX cells. Pharm Res. 2001;18(8):1138–1145. doi:10.1023/A:1010974909998.
- Giromini C, et al. In vitro-digested milk proteins: evaluation of angiotensin-1-converting enzyme inhibitory and antioxidant activities, peptidomic profile, and mucin gene expression in HT29-MTX cells. J Dairy Sci. 2019;102(12):10760–10771. doi:10.3168/jds.2019-16833.
- Lozoya-Agullo I, et al. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture. Mol Pharm. 2017;14(4):1264–1270. doi:10.1021/acs.molpharmaceut.6b01165.
- Gillois K, Stoffels C, Leveque M, Fourquaux I, Blesson J, Mils V, Cambier S, Vignard J, Terrisse H, Mirey G, et al. Repeated exposure of Caco-2 versus Caco-2/HT29-MTX intestinal cell models to (nano)silver in vitro: comparison of two commercially available colloidal silver products. Sci Total Environ. 2021;754:142324. doi:10.1016/j.scitotenv.2020.142324.
- Tawiah A, Moreau F, Kumar M, Tiwari S, Falguera J, Chadee K. High MUC2 mucin biosynthesis in goblet cells impedes restitution and wound healing by elevating endoplasmic reticulum stress and altered production of growth factors. Am J Pathol. 2018;188(9):2025–2041. doi:10.1016/j.ajpath.2018.05.013.
- Iseri OD, Sahin FI, Terzi YK, Yurtcu E, Erdem SR, Sarialioglu F. beta-Adrenoreceptor antagonists reduce cancer cell proliferation, invasion, and migration. Pharm Biol. 2014;52(11):1374–1381. doi:10.3109/13880209.2014.892513.
- He L, et al. Administration of alpha-ketoglutarate improves epithelial restitution under stress injury in early-weaning piglets. Oncotarget. 2017;8(54):91965–91978.
- Ali AA, et al. Salutary effect of calcium channel blockade following hypoxic and septic insult. J Trauma Acute Care Surg. 2014;77(1):40–46. discussion 45-6. doi:10.1097/TA.0000000000000260.
- Devriese S, Van den Bossche L, Van Welden S, Holvoet T, Pinheiro I, Hindryckx P, De Vos M, Laukens D. T84 monolayers are superior to Caco-2 as a model system of colonocytes. Histochem Cell Biol. 2017;148(1):85–93. doi:10.1007/s00418-017-1539-7.
- Ao M, Venkatasubramanian J, Boonkaewwan C, Ganesan N, Syed A, Benya RV, Rao MC. Lubiprostone activates Cl- secretion via cAMP signaling and increases membrane CFTR in the human colon carcinoma cell line, T84. Dig Dis Sci. 2011;56(2):339–351. doi:10.1007/s10620-010-1495-8.
- Sumagin R, et al. Neutrophil interactions with epithelial-expressed ICAM-1 enhances intestinal mucosal wound healing. Mucosal Immunol. 2016;9(5):1151–1162. doi:10.1038/mi.2015.135.
- Nusrat A, et al. Neutrophil migration across model intestinal epithelia: monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113(5):1489–1500. doi:10.1053/gast.1997.v113.pm9352851.
- Vergauwen H. The IPEC-J2 cell line, in the impact of food bioactives on health: in vitro and ex vivo models Verhoeckx K, editor 2015:Cham (CH) 125–134
- Brosnahan AJ, Brown DR. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet Microbiol. 2012;156(3–4):229–237. doi:10.1016/j.vetmic.2011.10.017.
- Schierack P,Nordhoff M, Pollmann M, Weyrauch KD, Amasheh S, Lodemann U, Jores J, Tachu B, Kleta S, Blikslager A, Tedin K. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol. 2006;125(3):293–305. doi:10.1007/s00418-005-0067-z.
- Zakrzewski S, J.r SK, Lee I, Lee I, Schulzke J, Schulzke J, Schulzke J, Schulzke J, Gunzel D, Gunzel D. Improved cell line IPEC J2, characterized as a model for porcine jejunal epithelium. PLoS One. 2013;8(11):e79643. doi:10.1371/journal.pone.0079643.
- Madara JL, Stafford J, Dharmsathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92(5 Pt 1):1133–1145. doi:10.1016/S0016-5085(87)91069-9.
- Domingue JC,Ao M, Sarathy J, Rao MC. Chenodeoxycholic acid requires activation of EGFR, EPAC, and Ca2+ to stimulate CFTR-dependent Cl- secretion in human colonic T84 cells. Am J Physiol Cell Physiol. 2016;311(5):C777–C792. doi:10.1152/ajpcell.00168.2016.
- Shapiro M, Matthews J, Hecht G, Delp C, Madara JL. Stabilization of F-actin prevents cAMP-elicited Cl- secretion in T84 cells. J Clin Invest. 1991;87(6):1903–1909. doi:10.1172/JCI115215.
- Tabcharani J,Low W, Elie D, Hanrahan JW. Low‐conductance chloride channel activated by cAMP in the epithelial cell line T84. FEBS Lett. 1990;270(1–2):157–164. doi:10.1016/0014-5793(90)81257-O.
- Vajanaphanich M,Kachintorn UD, Barrett KE, Cohn JA, Dharmsathaphorn KI, Traynor-Kaplan AL. Phosphatidic acid modulates Cl- secretion in T84 cells: varying effects depending on mode of stimulation. Am J Physiol. 1993;264(5 Pt 1):C1210–8. doi:10.1152/ajpcell.1993.264.5.C1210.
- Panjwani N. Role of galectins in re-epithelialization of wounds. Ann Transl Med. 2014;2(9):89. doi:10.3978/j.2305-5839.2014.09.09.
- Wang R,Zhao H, Zhang Y, Zhu H, Su Q, Qi H, Deng J, Xiao C. Identification of microRNA-92a-3p as an essential regulator of tubular epithelial cell pyroptosis by targeting Nrf1 via HO-1. Front Genet. 2020;11:616947. doi:10.3389/fgene.2020.616947.
- Boger KB, Blikslager, Anthony CP, Madan C, Laumas S, Krishnan B, Jin Y. Establishment and characterization of a leaky porcine jejunal cell line grown as a 2-dimensional monolayer using crypt culture media and their response to the tight junction agent larazotide acetate. Gastroenterology. 2019;156(6):S–486. Su1017 doi: 10.1016/S0016-5085(19)38076-X
- Bosch-Camos L,López E, Navas MJ, Pina-Pedrero S, Accensi F, Correa-Fiz F, Park C, Carrascal M, Domínguez J, Salas ML, Nikolin V. Identification of promiscuous African swine fever virus T-cell determinants using a multiple technical approach. Vaccines (Basel). 2021;9(1).
- Ma X, X. Fan P, Li LS, Qiao SY, Zhang GL, Li DF. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J Anim Sci. 2012;90(Suppl suppl_4):266–268. doi:10.2527/jas.50965.
