ABSTRACT
This paper shows how SARS-CoV-2 alters tight junctions (TJs) in human organs. The effect of SARS-CoV-2 on the ACE/Ang II/AT1R pathway and immune cells culminates in the release of numerous pro-inflammatory mediators, leading to the presence of certain symptoms in COVID-19, such as acute lung injury (ALI), pulmonary hypertension, and pulmonary fibrosis. Furthermore, the cytokines released alter different TJs components. The study shows how the irregular release of pro-inflammatory cytokines leads to claudin disruption in various tissues of the body, resulting in different symptoms, such as alveolar fibrosis, pulmonary edema, conjunctivitis, altered fertility in males, gastrointestinal symptoms, Covid toes, and others. SARS-CoV-2 also alters occludin expression in the endothelial and blood-testis barriers (BTB) resulting in edema and altered fertility. Viral disruption of JAM-A leads to activation of the RhoA GTPase, which leads to ALI. Taken together, these results define ACE/Ang II/AT1R pathway receptors and tight junctional components as potential therapeutic targets in COVID-19.
1. Background
By the end of 2019, the first cases of COVID-19 were reported from Wuhan city in China, before the disease spread and caused the first coronavirus pandemic that extended globally within a short period.Citation1,Citation2 Since that time, many vaccines have been developed for COVID-19, such as the mRNA Moderna vaccineCitation3 and other types of COVID-19 vaccines.Citation4 Various countries have tried to prevent the spread of COVID-19 through vaccination, and this has led to a notable reduction in the spread of the disease internationally, despite the presence of people’s reluctance to take vaccinations at the beginning of the vaccination process.Citation5 New antiviral agents were developed in response to COVID-19, such as Nirmatrelvir-Ritonavir, which inhibits the main protease, 3CL protease, of SARS-CoV-2,Citation6 and Molnupiravir, which is a nucleoside analogue.Citation7 However, these agents were not proposed for use in all SARS-CoV-2 infected people (molnupiravir (>18 years) and nirmatrelvir-ritonavir (≥12 years; >40 kg) for the outpatient treatment of mild-to-moderate COVID-19 patients who are at risk for progression).[Citation7] Thus, researchers are still looking for a drug that could be given to infected people of different ages and in different situations,Citation8 especially with the appearance of different strains. The World Health Organization (WHO) designated the Omicron variant as the variant of concern (VOC) on November 26, 2021.Citation9
SARS-CoV-2, the pathogen that causes COVID-19, is a positive sense, single-stranded RNA virus with envelope and surface-spike S proteins that infects the alveolar epithelium.Citation10
Knowledge regarding the mechanism of pathogenesis of COVID-19 may be very useful in finding therapies to prevent SARS-CoV-2 infection of alveolar cells. The use of specific drugs that block specific receptors such as ACE receptors, Ang II receptors, and other receptors has been suggested to be useful for relieving symptoms of COVID-19.Citation11 Furthermore, TJs have been introduced as potential therapeutic targets in COVID-19.Citation12 Thus, it is important to explain why many of the proposed drugs have been suggested to be useful in treating COVID-19. This article will focus on the receptors that SARS-CoV-2 affects during the processes of pathogenesis and cell entry. It also explains the reasons why certain medications may be beneficial for patients with COVID-19, such as ACE inhibitors and Angiotensin Receptor Blockers (ARBs). The paper also reviews current knowledge about the effects of SARS-CoV-2 on different components of TJs, and clarifies their roles in pathogenesis.
2. SARS-CoV-2 entry mechanism: A brief overview
The main entry mediator for SARS-CoV-2 is suggested to be ACE2. However, the virus entry mechanism is affected by other cellular receptors. A study by Wang et al. showed that viral entry points were more than normal (twice) under high cholesterol.Citation13 This was explained by the cholesterol concomitantly trafficking of ACE2 to the viral binding sites where SARS-CoV-2 docks to complete the entry process. They further demonstrated that the apoE protein carries cholesterol to the alveolar cell and binds to the cell’s LDL receptor to load cholesterol into it. This loading process is essential for the number and size of monosialotetrahexosylganglioside1 (GM1) rafts on the cell surface. The study found that ACE2 receptor sites were bound to GM1 lipid rafts more than 3-fold in the cell-loaded condition. Cholesterol-enriched lipid rafts on cell surfaces represent a platform for viruses to enter the host cell.Citation14 Thus, in the case of SARS-CoV-2, apoE-mediated cholesterol loading after binding to LDLr leads to the presence of GM1 rafts where ACE2 localization occurs at viral binding sites. As this depends on LDLr, binding of virus in the unloaded cell state is not associated with proper localization of ACE2, thus binding of SARS-CoV-2 to ACE2 would not be effective in the absence of LDLr. Another study demonstrated a different and additional potential role for LDLr in COVID-19. That is, SARS-CoV-2 was found to interact with LDLr while cholesterol is used for RNA replication.Citation15
Regarding the binding of SARS-CoV-2 to ACE2, it was found that SARS-CoV-2 binds to ACE2 via the interaction of the viral spike protein with the receptor-binding domain on ACE2,Citation16 in which the spike protein is activated by the human transmembrane serine protease 2 (TMPRSS2).Citation17 However, the binding is not entirely dependent on the proteases on the host cell surface. Furin proprotein convertase was found to play a role in the pre-activation of SARS-CoV-2.Citation16 Studies have detected increased expression of ACE2 receptors in the lungs of COVID-19 patients with comorbidities that have a detrimental effect on immunity and are associated with increased mortality among COVID-19 patients,Citation18,Citation19 such as diabetes and hypertension.Citation20–22 These studies demonstrate that ACE2 is an effective therapeutic target, and targeting ACE2 with a newly synthesized monoclonal antibody, called h11B11, shows efficacy against the virus.Citation23
Regarding viral endocytosis, recent research has confirmed that clathrin-mediated cellular endocytosis of SARS-CoV-2 is a major aspect of virus infection.Citation24 Then, viral endocytosis is followed by endosome acidification, the step by which SARS-CoV-2 enters the cytoplasm and begins replication.Citation25 It has been found that inhibition of endosome acidification in COVID-19 by various agents – including chloroquine – impaired viral replication and improved the prognosis of viral pneumonia in vivo.Citation25,Citation26 However, chloroquine is not currently used in COVID-19 due to poor therapeutic effects and adverse side effects.Citation27 These results present clathrin as a mediator of cellular endocytosis for SARS-CoV-2, which opens the door to the discovery of more potentially potent drugs that act by interfering with endosome acidification that follows clathrin-mediated endocytosis of SARS-CoV-2, such as Salibinin, which was expected to be a good treatment for the management of COVID-19 from a multi-target perspective.Citation28
3. Role of the ACE/Ang II/AT1R Pathway and cytokines release in the Pathogenesis of Inflammatory COVID-19 Symptoms
3.1. The molecular aspect
ACE/Ang II/AT1R pathway is considered a strong pro-inflammatory pathway. Once the pathway is activated, pro-inflammatory and anti-inflammatory modulators are activated. Here, the focus will be only on the pro-inflammatory ones, as those are the ones by which the disruption of TJs in COVID-19 occurs.
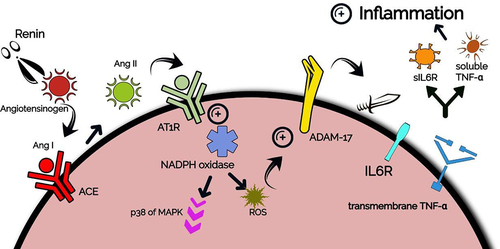
Angiotensinogen, the only precursor of all angiotensin peptides in humans, is an important protein produced by hepatocytes. It has 10 N-terminal amino acids that are cleaved by renin (an aspartyl-protease produced by juxtaglomerular cells in kidney) to provide Angiotensin I, which is the source for an array of active angiotensin peptides.Citation29,Citation30 Renin and Angiotensinogen were found to be expressed in multiple tissues, and not only in kidneys where they play important role in Renin-Angiotensin-Aldosterone System (RAS). That is, regulating blood pressure.Citation31 After Angiotensin I synthesis, it binds to a special receptor localized in different cells and tissues, like the lungs, macrophages, pancreas, liver, blood vessels, and others, namely Angiotensin-Converting Enzyme (ACE).Citation32 ACE then converts Angiotensin I to Angiotensin II (Ang II), an important immune regulator produced in different infections. Ang II binds to Angiotensin 2 Receptor Type 1 (AT1R) which is found to be localized on neutrophils, monocytes, B-lymphocytes, T-lymphocytes, and dendritic cells.Citation33,Citation34 AT1R is a transmembrane G protein that encourages different intracellular pathways via the activation of subunits of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, several protein kinases, Janus Activated Kinases (JAKs), and others. Furthermore, it turns on several downstream signals, like mitogen-activated protein kinase/extracellular signal-regulated kinases (MAPK/ERK) (a pro-inflammatory pathway).Citation35 Ang II-mediated activation of AT1R triggers a signaling cascade via the intracellular NADPH oxidase, which induces reactive oxygen species (ROS) formation and p38 family of MAPK activation, which leads to A Disintegrin And Metalloproteinase-17 (ADAM-17) phosphorylation.Citation36,Citation37 Phosphorylation of ADAM-17 protease enhances its catalytic activity, characterized by the cleavage of the ectodomains of various transmembrane proteins located in the membrane of immune cells, such as TNFα and its receptors,Citation38 through the catalytic or metalloenzyme domain of ADAM-17.Citation38 This produces the active form of TNFα, which has important inflammatory roles in COVID-19 and other infections,Citation39 as mentioned next. ADAM-17 also cleaves the membrane-bound IL-6 receptor (IL6R), generating the soluble form which – after complexing with IL-6- induces important pro-inflammatory roles.Citation40 See . Let us shed the light on how IL-6 and other cytokines are produced in COVID-19, leading to inflammatory symptoms with involvement from the ACE/Ang II/AT1R pathway.
It is noteworthy that after SARS-CoV-2 is captured by first-line immune cells (like dendritic cells, macrophages, and neutrophils), it binds to certain Toll-like receptors (TLRs) (transmembrane proteins that recognize bacterial lipopolysaccharides and viruses on immune cells membranes), such as TLR 7/8. Then, an inflammatory signaling pathway is activated.Citation41 After SARS-CoV-2 binding to TLRs, a cytoplasmic protein that binds cytoplasmic tails of TLRs, namely Myeloid Differentiation factor 88 (MyD88), phosphorylates a protein that connects to it at its N-terminal, IRAK1 (which is one of the IL-1 Receptor-Associated Kinases (IRAKs)).Citation42 IRAK1 then phosphorylates TNF Receptor-Associated Factor 6 (TRAF6), which is an E3 ubiquitin ligase and one of the important downstream molecules. TRAF6 then forms poly-ubiquitin chains making a complex with other molecules including transforming growth factor β-activated kinase (TAK1).Citation43 TAK1 is then activated, leading to the activation of the I kappa B (IkB) kinase (IKK) complex, which consists of three subunits; alpha, beta, and NF-κB Essential Modulator (NEMO).Citation44 IKK in turn phosphorylates IkB protein, degrading it and liberating NFκB dimer toward the nucleus. IKK also activates the pro-inflammatory p38 family of MAPK.Citation42 NF-κB then binds its promoters on DNA and induces pro-inflammatory cytokines and chemokines expression, and leads to the activation and differentiation of innate immune cells and inflammatory T cells, as reported by Liu et al.Citation45 Liu et al. reported that in macrophages, NF-κB induces the expression of a large number of inflammatory genes, including those encoding TNFα, IL-1β (an IL-1 subtype with pro-inflammatory activity), IL-6, IL-12p40 (a component of the cytokines IL-12. It acts as a chemoattractant for macrophagesCitation46) and cyclooxygenase-2. Furthermore, they found that NF-κB also regulates T-cell differentiation and effector function, as it induces CD4 + T cells differentiation into different subsets of T cells, including T-helper 1, T-helper 2, T-helper 17, and follicular T-helper (Tfh) cells, which secrete distinct cytokines and mediate different aspects of immune responses. NF-κB was found to play a potentially important role in Tfh development, which is important for the maturation of B cells and subsequent production of antibodies. Furthermore, it is essential in T-helper 1 cells’ production of IFNγ.Citation44 The cytokines produced by mentioned cells were found to have important roles in alveolar fibrosis. She and colleaguesCitation47 found that, after its secretion from cells and moving toward the extracellular matrix where fibroblasts locate, IL-6 promotes the proliferation of fibroblasts, the synthesis of collagen, and the transition of special anti-inflammatory macrophages, M2, to hyper-profibrotic phenotype. They also reported that IL-1β promotes collagen synthesis and recruitment of more lymphocytes and leukocytes, with other cytokines playing similar roles. Certain cytokines produced by the aforementioned cells were also found to induce thrombus formation. Najem et al.Citation48 reported that IFN-γ enhances thrombus formation and delays thrombus resolution through the downregulation of Matrix Metalloproteinase-9 (MMP-9). They also found that IL-1β has a compelling role in thrombus formation characterized by its contribution to platelet aggregation. Moreover, they mentioned that IL-6 induces thrombus formation and increases the possibility of post-thrombotic syndrome by the inflammatory cascades it induces – mentioned next –. Post-thrombotic syndrome is an important chronic consequence of deep venous thrombosis, clinically characterized by leg pain, sensations of leg heaviness, fatigue, and limb swelling.Citation49
One of the important cytokines regulated through the NF-κB pathway is IL-6.Citation50 IL-6 has two forms of receptors; the membrane-bound receptor expressed on hepatocytes and some leukocytes (IL6R), and the soluble receptor (sIL6R).Citation51 After IL-6 secretion, it binds to sIL6R, forming IL-6/sIL6R complex, a complex that is known to induce a pro-inflammatory signaling pathway, namely the trans-signaling pathway.Citation52 This complex binds to the Glycoprotein 160 (gp160) signal-transducing subunit that is ubiquitously expressed in different body tissues. This leads to the homodimerization of gp160, which is followed by the activation of gp130-associated JAKs.Citation53 Consequently, receptor-associated JAKs become activated and phosphorylate each other and the intracellular tail of gp130 receptors, thereby creating docking sites for latent, cytoplasmic transcription factors termed Signal Transducers and Activators of Transcription (STATs), which in turn dimerize, move to nucleus, and bind to STAT Binding Element (SBE) on DNA to regulate gene expression.Citation54 In the case of the IL-6/sIL6R complex, the activation of JAK was found to be followed by the activation of STAT1, which then binds to MMP promoters, expressing both MMP-1 (Collagenase 1) and MMP-3 (Strommelysin).Citation55 These two MMPs were found to release soluble TNFα from its transmembrane precursor. TNFα in turn activates cyclooxygenase-2.Citation56 Cyclooxygenase-2 is an integral membrane protein that expresses the inflammatory mediator, Prostaglandin E2 (PGE2), which binds Prostaglandin E receptor 4 (EP4) leading to the activation of other MMPs (like MMP-9) aiding in the recruitment of more immune cells, like dendritic cells.Citation57 TNFα also participates in vasodilatation, edema formation, and leukocyte adhesion to epithelium through the expression of different adhesion molecules. Furthermore, it regulates blood coagulation and contributes to oxidative stress in sites of inflammation.Citation58
Another pro-inflammatory role that the IL-6/sIL6R complex plays in inflammation is protecting T cells from apoptosis through the activation of the apoptosis suppressor protein, B cell lymphoma-2 (Bcl-2),Citation59 thereby sustaining the inflammatory cycle.
In COVID-19, the virus induces IL-6 production. Furthermore, the initial activation of ADAM-17 by the action ACE/Ang II/AT1R pathway leads to the activation of the IL-6/sIL6R complex and its aforementioned consequences (i.e., activating different immune cells and inducing the secretion of different cytokines). ADAM-17 also liberates the active form of TNFα, which has been shown to activate different immune cells. Thus, the ACE/Ang II/AT1R pathway is considered one of the main inducers of COVID-19 inflammatory symptoms.
3.2. The clinical aspect
It has been found that increased frequency of Ang II binding to ACE receptors and AT1R has been associated with pulmonary inflammation, fibrosis, and consequently progressive tissue damage in pathological conditions.Citation60 Another concomitant manifestation of this binding is pulmonary hypertension associated with AT1R-induced vascular smooth muscle remodeling.Citation61 These manifestations have been reported in patients with COVID-19 infection due to activation of the ACE/Ang II/AT1R pathway leading to proinflammatory, profibrotic, prothrombotic, and vasoconstrictor effects.Citation62
Since ACE is a major contributor to this pathway, its inhibition will result in decreased pathway activation. A study found that ALI was shown to be less severe in ACE complete knocked out (ACE -/-) mice and even lesser in partially knocked out (ACE ±) mice, indicating that the increased level of expression of ACE has been associated with higher severity of lung injury.Citation63 The same research reported that injury was less severe in AT1R knocked out mice than in wild-type mice. Another different study showed that injection of recombinant SARS protein leads to an increased level of Ang II expression in mice. It also showed that treating these mice with an AT1R antagonist alleviated acute respiratory distress in them.Citation64 These studies show that ACE/Ang II/AT1R pathway receptors mediate the pathogenesis of ALI in COVID-19.Citation65 Thus, SARS-CoV-2 infection to the alveoli is followed by inflammatory roles of the ACE/AT1R receptors. In more detail, these inflammatory roles occur in response to the presence of virus in lung cells, where activation of the ACE/AngII/AT1R pathway leads to increased activation of the AT1R receptor by Ang II. Ang II has been shown to reduce the number of ACE2 receptors by activating their internalization through ADAM-17 activation, which promotes the shedding of the ACE2 receptor from the membrane to the cytosol, resulting in soluble ACE2 (sACE2), consequently leading to its degradation by lysosomes.Citation37,Citation66 As the level of ACE2 is elevated by the virus stimulating its expression,Citation18,Citation19 activation of AT1R will limit SARS-CoV-2 entry into cells by decreasing ACE2 by stimulating ACE2 internalization and degradation by lysosomes. However, further activation of this pathway would result in the aforementioned inflammatory damage, making its receptors potential therapeutic targets. A study showed that the use of the AT1R blocker Valsartan in COVID-19 was associated with a beneficial therapeutic effect, but this effect was not mediated by altered ACE2 expression.Citation67 This demonstrates the efficacy of attenuating the inflammatory damage caused by activation of the ACE/Ang II/AT1R pathway in COVID-19. Other studies have also shown that ACE inhibitors/ARB’s therapy is associated with a lower risk of severe COVID-19 infection and reduced mortality by decreasing inflammation caused by activation of these receptors.Citation12,Citation68 Some studies have reported other drugs that are good at relieving symptoms used in the treatment of patients with COVID-19 and Hepatitis C, such as FavipiravirCitation69,Citation70 and Sofosbuvir/daclatasvir.Citation71 The mechanism of action of these drugs does not depend on the inactivation of the ACE2 enzyme. However, it is likely to reduce serum ACE activity, as one study showed a decrease in serum ACE activity in patients with chronic hepatitis C after treatment with antiviral therapies.Citation72 Thus, these drugs had a potential therapeutic effect on COVID-19 due to reduced ACE activity.
4. The pathological effect of SARS-CoV-2 on tight junction components
Tight junctions (also known as zonula occludens) are intercellular structures between the apical membranes of epithelial cells, crucial for building the epithelial barriers and maintaining epithelial polarity by providing a suitable electrochemical gradient. They connect cells in the endothelial and epithelial tissues together, forming extracellular channels between the lateral membranes of cells, thus regulating tissue permeability for ions, cations, and water.Citation73 They consist of multiple proteins, but the most common ones in different TJs are classified as claudin, occludin, and JAMs.Citation74 Proteins are not the only ones found to be important in TJs’ functionality. Membrane lipids (cholesterol) and mechanical force also play roles in maintaining TJs integrity.Citation75
In pathological conditions, the inflammatory response has an important role in altering TJs, where the alteration is not caused by the pathogen itself, but by the immune response it causes. It was found that inflammatory cytokines play different roles in the alteration of TJs. Furthermore, certain cytokines were found to have pleiotropic effects. In their review paper, Capaldo et al. have reported many important roles for cytokines in TJs alteration.Citation76 For example, they mentioned that IFN- γ increase the permeability of endothelial and epithelial monolayers through the induction of actin-myosin fiber’s restructuring and contraction (actin-myosin fibers are important intracellular regulators of cytoskeleton and TJs), which changes the localization of the TJs components that are in contact with these fibers, leading to their internalization by macropinocytosis. However, in the airway epithelial cells, IFN- γ promotes epithelial barrier function. Another cytokine, TNF-α, was also found by them to increase the permeability of endothelial barriers by the remodeling of TJs proteins, but in intestinal epithelial cells, it increases the permeability by another additional mechanism. He et al.Citation77 found that TNF-α activation of NF-κB and the subsequent activation of myosin light chain kinase (MLCK) – which in turn hyperphosphorylates MLCs – attenuates actin-myosin fibers’ connections with TJs, thus increasing the permeability. In a different research, TNF-α, IL-4, and IFN-γ were found to induce TJs disassembly and potentially alter epithelial permeability in the airways by the activation of the pro-inflammatory, Epidermal Growth Factor Receptor (EGFR)-dependent MAPK/ERK1/2 signaling pathway.Citation78
As the mentioned cytokines are secreted in COVID-19, the mentioned alterations in TJs following their secretion is expected to be present in COVID-19, manifesting in different symptoms. COVID-19-associated alterations in TJs will be reviewed next.
4.1. Claudins
Claudins are transmembrane proteins that play a critical role in the TJs between epithelial and endothelial cells. That is, they regulate paracellular permeability and maintain cell polarity in cell sheets.Citation79,Citation80 To date, 27 members of the claudins family were discovered. Claudins are ubiquitously present in the body, and their roles differ according to the organ they localize in. Certain claudins work as “sealing” claudins, with no permeability to ions or water, such as claudins 1, 3, 5, 6, 9, 11, and 18.Citation81 Of note, all the mentioned sealing claudins are present in the airways, working as a fence that prevents liquid movement to the airway in physiological conditions.Citation81 However, other claudins allow liquid and ions movements through TJs, representing “pore-forming” claudins. These include claudin 2,10, 15, 16, and other ones.Citation81 Of note, all mentioned pore-forming claudins are present in kidneys’ nephorns.Citation81 It is noteworthy that claudin-2, and recently claudin-15, were found to be permeable to water.Citation82 Other claudins were found to do both things. That is, being permeable in certain conditions -or tissues- and working as fences in other ones. For example, claudin-19 is impermeable in peripheral neurons, but when became in contact with claudin-16 in the thick ascending limb of kidneys’ nephrons, it forms a permeable pore for ions.Citation81 Other similar claudins include claudins 4, 7, 8, and 12.Citation81
Besides their involvement in TJs, recent studies reported claudins’ involvement in intracellular signaling that influences cellular behavior, including proliferation, differentiation, and migration.Citation83
In COVID-19, the alterations in claudins were found to be associated with many manifestations in the patients. Detailing the roles of different claudins in pathogenesis would be beneficial for a better understanding of the disease. Thus, the claudins affected in COVID-19, and how they are affected, will be discussed here.
It was found that high cholesterol levels are not uncommon in the elderly, and that cholesterol levels in different tissues increase with age as a result of diseases such as acute and chronic infections.Citation84 High cholesterol levels have been found to increase the severity and likelihood of symptomatic COVID-19,Citation14 which may be explained in part by the detrimental effect of increased cholesterol on claudins permeability in alveolar and other tissues, which is heightened by the pathological effect of COVID-19. Studies have estimated that the lipid environment is an important factor in regulating claudin function.Citation85,Citation86 Also, Fuladi et al. hypothesized that the lipid environment influences claudin oligomerization.Citation87 Furthermore, some simulations by Fuladi et al. aforementioned study indicate that the presence of cholesterol reduces the frequency of dimerization of claudins and, consequently, increases the possibility of strand formation in the lipid bilayer. Thus, as claudin dimerization is reduced, its binding specificity to other transmembrane structures and cytoskeleton will decrease,Citation88 thus it is more likely to be affected pathologically in COVID-19. The release of TNFα – which is associated with COVID-19 – has been discovered to alter lipid composition in membrane microdomains of the TJs, which constitutes a mechanism by which TNFα contributes to the aberrant TJs in COVID-19.Citation85
In the lungs, alveolar squamous cells were found to have apical intercellular junctions containing claudin 1, 2, 3, 4, and 7 under physiological conditions. These claudins have been estimated to have a stronger claudin expression in usual interstitial pneumonia (UIP) seen in interstitial lung diseases (ILD).Citation89 This change in claudin expression contributes to certain clinical manifestations. It mainly involves regenerating alveolar cells or cells that have been replaced by metaplastic epithelial tissue.Citation90 It has been reported that the claudin expression pattern in regenerating metaplastic cells compared to alveolar cells would lead to focal changes in the permeability of alveolar walls, which are potentially detrimental to lung function.Citation90 It is noteworthy that these metaplastic manifestations were detected in patients with COVID-19,Citation91 and that several groups admitted to hospitals after COVID-19-associated pneumonia had persistent parenchymal abnormalities similar to those mentioned, which are described as post-COVID ILD.Citation92 Moreover, other manifestations similar to those seen in UIP-associated ILD have also been detected in COVID-19, such as chronic airway inflammation, pulmonary interstitial fibrosis, and non-cardiogenic pulmonary edema associated with elevated extravascular pulmonary water index.Citation93,Citation94 These different manifestations occur due to activation of the immune response in UIP and COVID-19.Irregular release of cytokines in COVID-19 will cause a change in the expression of alveolar TJs proteins, leading to altered expression of claudin 1, 2, 3, 4, and 7 thus altering the permeability of TJs of the alveolar walls in COVID-19.
In detail, Fujita et al.Citation95 reported that in asthmatic patients, IL-1β and TNF-α were found to upregulate claudin-1 expression in the airway smooth muscle (ASM) cells. They also caused claudin-1 localization to the nucleus and cytoplasm, away from their usual location (lateral membranes). Synchronously, a proliferation in ASM cells was detected, leading to airway remodeling, one of the aforementioned manifestations present in COVID-19. Regarding claudin-5, the increase in TNFα and subsequent activation of NF-κB downregulates claudin-5 promoter activity, decreasing claudin-5 expression in the lungs after different viral infections.Citation96 As claudin-5 is an important regulator of the lung endothelial barrier, its downregulation potentially culminates in edema.Citation97 TNFα and IFN-γ were also found to decrease the assembly of claudin-4 (a TJ-sealing claudin whose expression correlates with better tightness), leading to epithelial barrier dysfunction.Citation98
At the blood–brain barrier (BBB), claudin-5 is the most enriched TJ protein and its dysfunction may cause various neurodegenerative disorders.Citation99 Although SARS-CoV-2 was found to infect the brain, it was discovered that the TJs were not affected in the mechanism of virus entry or pathogenesis, implying that neither claudin-5 nor the TJs play roles in the pathogenesis of the neuronal injury associated with COVID-19.Citation100,Citation101 It has been found that viral binding and overexpression of the ACE2 enzyme is the main factor disrupting the BBB in COVID-19.Citation102
Away from the brain, claudin-5 expression has been found to be decreased in the decidua and choroid villus of women with severe COVID-19, leading to abnormalities and thus leakage of the endothelium, increasing disease severity.Citation103 This is attributed to the irregular immune response after COVID-19 during pregnancy, which leads to increased activation of natural killer cells and T cells. This is followed by the release of various inflammatory cytokines, including TNFα and various interleukins.Citation104 TNFα was found to change the localization of claudin-5 in human umbilical vascular endothelial cells.Citation76 In these cells, IL-6 alters TJs by actin restructuring.Citation76 These cytokines thus play important roles in altering the placenta. Consequently, participating in the pathogenesis of several symptoms, like signs of maternal and fetal malperfusion such as infarcts, decidual vasculopathy, thrombi in the fetal circulation, and possibly vertical transmission.Citation105
At the BTB, SARS-CoV-2 was found to increase claudin-11 expression associated with increased release of TNFα, IL1β, and IL6, which disrupts junctional proteins and leads to a decrease in the number of Sertoli cells,Citation106 and thus testicular discomfort and altered hormone levels in malesCitation107–109
In an in vitro experiment on the T84 cell line (cells derived from metastatic colon cancer cells, widely used in studying epithelial barriers), IFN-γ was found to induce the internalization of claudin-1 and claudin-4, which alters the permeability.Citation76 Furthermore, the simultaneous exposure to IFN-γ and TNF-α was found to stimulate claudin-4 and 5 internalizations.Citation76 In the same cell line, exposure to IL-4 was found to increase the expression of claudin-2 (pore-forming claudin), increasing the permeability.Citation76 Thus, the increase in the mentioned cytokines in COVID-19 explains different gastrointestinal symptoms, such as diarrhea, abdominal discomfort, loss of appetite, vomiting, and others.Citation110–112
Overexpression of claudin-1 has been observed in conjunctival epithelial cell inflammation occurring in atopic dermatitis due to numerous cytokines secretion.Citation113 A recent study did not find evidence of significant expression of ACE2 in conjunctival samples in COVID-19, making conjunctival infection with SARS-CoV-2 via these mediators unlikely,Citation114 highlighting the potential for altered claudin-1 to underline COVID-19-associated conjunctivitis.Citation115
Claudin-1, 4, and 7 are expressed in renal tubular cells in response to TNFα, which leads to a change in cell permeability due to the change in claudin-1 expression, but not changes in claudin 4 or 7, as these two expression is potentially associated with better tightness.Citation76,Citation116 Collectively, this has the potential to manifest in COVID-19 as an acute kidney injury mostly associated with immune changes and direct virus cytopathic lesions.Citation117
In the microvascular endothelial cells, IFN-γ was found to induce actin restructuring, and TNF-α was also found to play the same role.Citation76 However, the simultaneous release for both of them was found to cause mislocalization of claudin-5,Citation76 which in turn alters the permeability of endothelial cells. One of the unusual symptoms seen in COVID-19 that is plausibly attributed to what was mentioned is COVID toes, characterized by pernio-like lesions on the toes that are predominantly caused by endothelial cell inflammation.Citation118
The reported findings suggest potential efficacy in targeting such intercellular structures in the treatment of COVID-19. This is supported by the results of a study by Adil et al. that found an essential role in intercellular junctions in COVID-19, indicating its therapeutic potential.Citation119 Among the methods that can be used to target claudins are claudin-binders anti-claudin monoclonal antibodies, which have been suggested for use in other claudin-dependent viral infections.Citation120,Citation121 These factors can be modified to overcome the effects of altered claudin expressed in response to the release of inflammatory cytokines in COVID-19. This can occur by targeting different domains of the newly expressed claudin with appropriate antibodies. Such types of therapies would be beneficial for those who can’t tolerate immune modulators (i.e., patients having immunodeficiency).
4.2. Occludin
Occludin is a transmembrane protein that plays a vital role in the regulation of TJs. That is, it is involved in the stability of the TJs and barrier function in different epithelial tissues.Citation122 Occludin has been found to play an important role in the pathogenesis of COVID-19. Expression of the SARS-CoV-2 entry mediator correlates with expression of this TJ protein, through which ACE2 is localized at the apical cell–cell junctions of epithelial cells.Citation123 Moreover, the inflammatory response and consequent release of TNF-α, IL-4, or IFN-γ in COVID-19 are detrimental to occludin, as it has been found to cause a remarkable reduction in the expression of this protein at alveolar TJs in severe cases of COVID-19, altering the permeability of cells to cations, and – to a lesser extent – altering claudin permeability to ions.Citation78,Citation124,Citation125 Occludin has also been found to have low expression in the BTB in COVID-19 due to the erratic release of inflammatory cytokines, resulting in altered fertility.Citation106 It was found that TNFα reduces occludin expression and alters occludin phosphorylation in endothelial cells, causing altered permeability.Citation76,Citation126 It is likely that this inflammatory mechanism is present in the endothelial damage associated with COVID-19, but not in the BBB damage, since intact TJs were found in COVID-19 brain infection.Citation100,Citation101
In T84 cell lines, IFN-γ induces the internalization of occludin.Citation76 In an intestinal epithelial line model (Caco-2), IL-1 was found to decrease the levels of occludin via the reduction of occludin mRNA levels.Citation76 Taken together, these findings suggest that occludin potentially participates in the pathogenesis of the aforementioned gastrointestinal symptoms seen in COVID-19
From another point of view, Na-K ATPase, a ubiquitous transporter in the apical cell junctions that regulates TJs permeability through regulation and complexing with phosphatase 2A, was found to regulate TJs permeability via occludin phosphorylation.Citation127,Citation128 SARS-CoV-2 has been found to alter the expression of Na-K ATPase subunits in epithelial lines.Citation129 This disrupts the Na-K ATPase regulation of occludin phosphorylation, leading to occludin hyperphosphorylation and thus altered permeability at epithelial tight junctions.Citation128
4.3. Junctional adhesion molecules (JAMs)
JAMs are cell–cell adhesion molecules of the immunoglobulin superfamily. They include JAM-A, -B, -C, and other JAMs. JAMs are expressed by a variety of tissues during development and in adulthood.Citation130 They play important roles in various vital processes, such as inflammation, angiogenesis, hemostasis, and epithelial barrier formation. These processes depend on the important role that JAMs play. That is, making contacts with other junctional proteins, such as integrin, zona occludin (ZO), and others, in order to provide a stable cellular barrier.Citation131,Citation132 ZOs are TJs scaffolding proteins that link and regulate integral transmembrane proteins, such as claudins, occludins, and JAMs.Citation133
JAM-A, the most studied member of the family to date, plays important role in regulating the epithelia of the lung, liver, and other epithelial organs. It does this by binding to other tight junctional components, such as ZO-1 and ZO-2.Citation134 The contact between JAM-A and ZO-1 and ZO-2 has been shown to be direct and intermediated via the partner-specific PDZ domain and the PDZ domain-binding motif of JAM-A.Citation134 PDZ domain (postsynaptic density protein [PSD95]/Drosophila disc large tumor suppressor (DlgA), and Zonula occludens-1 protein [zo-1]) is a component of scaffolding proteins that joins proteins into the appropriate cellular complex. It acts by binding to C-terminal peptide stretch, other PDZ domains, phospholipids, or other structures.Citation135 The connection between JAM-A and ZO-2 – as part of the JAM-A, ZO-2, afadin, and RAPGEF2 junction complex – has been found to control the contraction of the junction-associated apical cytoskeleton by controlling RhoA activity, in order to maintain a functional and selective epithelial barrier.Citation132 RhoA is a GTPase of the Rho GTPases family. It exists in two forms, the RhoA-RhoGDI complex present in the cytosol, and the active, GTP-binding form of RhoA located on the membrane. It plays a variety of functions in the regulation of cytoskeletal proteins, cellular morphology, and migration along with reproduction and transcriptional activity in cells.Citation136 JAM-A has been found to suppress RhoA activity, which is critical in inhibiting peri-junctional actomyosin contractility that is associated with impaired barrier function in epithelial cells,Citation134 a mechanism by which JAM-A stabilizes epithelial permeability.
Regarding JAM-A and COVID-19, SARS-CoV-2 was found to reduce the expression of JAM-A.Citation137 Loss of JAM-A has been shown to cause decreased epithelial layer resistance, reduced expression of TJ’s ZO-1, and disruption of junctional localization of claudin-18 at the lung epithelial lines.Citation138 Furthermore, the SARS-CoV-2 C-terminus protein E was found to contain a PDZ-binding motif that binds to the PDZ domain-2 of ZO-1 at host TJs, resulting in junction damage and epithelium altered permeability.Citation139,Citation140 These deleterious effects – the direct or indirect ones – of the virus on JAM-A and ZOs alter their interactions, which are critical for inhibiting RhoA activation. This leads to the activation of RhoA GTPase, which contributes to a burst in inflammatory features, immune cell migration, apoptosis, coagulation, and alters cellular TJs in pulmonary endothelial cells, leading to endothelium barrier dysfunction and edema. In other words, it leads to ALI.Citation141 Highlighting the pathogenic mechanism of SARS-CoV-2 on JAM-A explains the efficacy of Rho-kinase inhibitors in COVID-19.Citation141
Regarding cytokines roles, TNF-α was found to mislocalize JAM-A in endothelial cells, and IFN- γ was found to do the same in the T84 cell line.Citation76 Thus, the cytokines-induced mislocalization of JAM-A may alter permeability in epithelial cells of the gut.
In an in vitro experiment, IL-4 and 13 were found to decrease the expression of JAM-A in sinonasal epithelial layers, providing a likely mechanism for the epithelial permeability changes.Citation142 Thus, the temporary loss of smell and taste was seen in a significant number of COVID-19 patients even after vaccination may be due to altered JAM-A.Citation143–146
Away from JAM-A, other JAMs have been found to be involved in the endothelial and epithelial TJs, such as JAM-B, ESAM, and CAR. These JAMs were found to be co-localized with ZO-1 at TJs.Citation134 Since SARS-CoV-2 disrupts ZO-1ʹs communication with JAM-A, it may disrupt junctions permeability by interfering with ZO-1ʹs interactions with JAM-B, ESAM, and CAR. However, this case remains a hypothesis until it is confirmed or rejected by future studies.
As the irregular release of cytokines in COVID-19 is a major cause of TJs alteration, targeting cytokines in the treatment of the disease was found to correlate with a better prognosis.Citation147–149
The following table () summarizes the types of receptors targeted and affected by the virus and how they are affected.
Table 1. Receptors and mediators that play roles in the pathogenesis of COVID-19.
5. Conclusion
SARS-CoV-2 showed the potential for other pathogenic mediators besides ACE2, namely LDLr, AT1R, and ACE. These receptors mediate the pathogenic inflammatory mechanism of SARS-CoV-2 to alveolar cells. These receptors have been demonstrated to be potential therapeutic targets for the treatment of COVID-19. Various claudins are also affected by the pathogenesis of SARS-CoV-2 which contributes to the increase of cytokines levels. In COVID-19, claudin 1, 2, 3, 4, and 7 were found to be overexpressed in alveolar cells, while claudin-5 was found to have decreased expression in the endothelia of lungs, decidua, and chorionic villus. Claudin-5 also had mislocalization in umbilical vascular and microvascular endothelia in the placentas of severely affected pregnant women. Claudin-11 was found to be overexpressed in the BTB, while claudin-18 is mislocalized in the lungs. Claudin-1 is overexpressed in the conjunctiva and many claudins – as well as occludin and JAM-A – had the potential for mislocalization in the gastrointestinal tract. All these effects alter the permeability of the TJs and lead to atypical symptoms, such as alveolar fibrosis, altered male fertility, conjunctivitis, acute kidney injury, and others. Occludin has also been found to be altered due to the immune response caused by COVID-19, increasing the permeability of endothelial cells – far from the brain – and altering the BTB. JAM-A has been found to be reduced in alveoli of COVID-19 patients. Also, it has been found to be altered in COVID-19 due to viral binding to PDZ domain-2 of ZO-1 and consequent inactivation of the complexes made by ZOs. This leads to a change in permeability. It also causes activation of the RhoA GTPase, which contributes to ALI in COVID-19. Many drugs used in the treatment of COVID-19 have proven effective due to the disruption of the mentioned receptors that mediate the pathogenesis of COVID-19, such as some drugs used in the treatment of hepatitis C along with other drugs such as ACE inhibitors and ARBs. TNFα inhibitors and immune modulators are also considered important therapeutic agents. Targeting TJs potentially results in good therapeutic outcomes.
Further studies are needed to test claudins and JAMs from different tissues’ TJs that may be potentially affected by COVID-19. Also, further studies are needed to test more therapeutic agents that act by inactivating said mediators of the COVID-19 pathogenesis.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Liu YC, Kuo RL, Shih SR. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43(4):192–209. doi:10.1016/j.bj.2020.04.007.
- Al-Kassim Hassan M, Adam Bala A, Jatau AI. Low rate of COVID-19 vaccination in Africa: a cause for concern. Therapeutic Advances in Vaccines and Immunotherapy. 2022 January;10:251513552210881. doi:10.1177/25151355221088159.
- Mayfield J, Bandi S, Ganti L, Rubero J. Anaphylaxis after Moderna COVID-19 vaccine. Therapeutic Advances in Vaccines and Immunotherapy. 2021 January;9:251513552110484. doi:10.1177/25151355211048418.
- Yap C, Ali A, Prabhakar A, Prabhakar A, Pal A, Lim YY, Kakodkar P. Comprehensive literature review on COVID-19 vaccines and role of SARS-CoV-2 variants in the pandemic. Therapeutic Advances in Vaccines and Immunotherapy. 2021 January;9:251513552110597. doi:10.1177/25151355211059791.
- Ashour L, Funjan K. Assessment of medical students’ knowledge and access to scientific journal articles in Jordan: insufficient knowledge has potentially negative effects on the social response to COVID-19. Internet Ref Serv Q. 2022:1–14. doi:10.1080/10875301.2022.2075071.
- Hung YP, Lee JC, Chiu CW, Lee -C-C, Tsai P-J, Hsu I-L, Ko W-C. Oral nirmatrelvir/ritonavir therapy for COVID-19: the dawn in the dark? Antibiotics (Basel). 2022 [Published 2022 Feb 9];11(2):220. doi:10.3390/antibiotics11020220.
- Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral COVID antiviral drugs [published online ahead of print, 2022 Mar 4]. Clin Infect Dis. 2022:ciac180. doi:10.1093/cid/ciac180.
- Izda V, Jeffries MA, Sawalha AH. COVID-19: a review of therapeutic strategies and vaccine candidates. Clin Immunol. 2021 Jan;222:108634. doi:10.1016/j.clim.2020.108634.
- World Health Organization. Classification of Omicron (B.1.1.529): SARS‐CoV‐2 variant of concern. November 26, 2021. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern.
- Wang MY, Zhao R, Gao LJ, Gao X-F, Wang D-P, Cao J-M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol. 2020;10:587269. doi:10.3389/fcimb.2020.587269.
- Hippisley-Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020 Oct;106(19):1503–1511. doi:10.1136/heartjnl-2020-317393.
- Linfield DT, Raduka A, Aghapour M, Rezaee F. Airway tight junctions as targets of viral infections. Tissue Barriers. 2021;9(2):1883965. doi:10.1080/21688370.2021.1883965.
- Kočar E, Režen T, Rozman D. Cholesterol, lipoproteins, and COVID-19: basic concepts and clinical applications. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866(2):158849. doi:10.1016/j.bbalip.2020.158849.
- Li X, Zhu W, Fan M, et al. Dependence of SARS-CoV-2 infection on cholesterol-rich lipid raft and endosomal acidification. Comput Struct Biotechnol J. 2021;19:1933–1943. doi: 10.1016/j.csbj.2021.04.001.
- Cure E, Cumhur Cure M. Strong relationship between cholesterol, low-density lipoprotein receptor, Na+/H+ exchanger, and SARS-COV-2: this association may be the cause of death in the patient with COVID-19. Lipids Health Dis. 2021;20(1):179. doi:10.1186/s12944-021-01607-5.
- Shang J, Wan Y, Luo C, Ye G, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020 May 26;117(21):11727–11734. doi:10.1073/pnas.2003138117
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N-H, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 Apr 16;1812:271–280.e8. 10.1016/j.cell.2020.02.052
- Pinto BGG, Oliveira AER, Singh Y, et al. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J Infect Dis. 2020;222(4):556–563. doi:10.1093/infdis/jiaa332.
- Gheware A, Ray A, Rana D, et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci Rep. 2022;12(1):4058. doi:10.1002/lary.29964.
- Parveen R, Sehar N, Bajpai R, et al. Association of diabetes and hypertension with disease severity in covid-19 patients: a systematic literature review and exploratory meta-analysis. Diabetes Res Clin Pract. 2020 Aug;166:108295. doi:10.1016/j.diabres.2020.108295.
- Mahamat-Saleh Y, Fiolet T, Rebeaud ME, Mulot M, Guihur A, El Fatouhi D, Laouali N, Peiffer-Smadja N, Aune D, Severi G, et al. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open. 2021;11(10):e052777. doi:10.1136/bmjopen-2021-052777.
- Sun Y, Guan X, Jia L, et al. Independent and combined effects of hypertension and diabetes on clinical outcomes in patients with COVID-19: a retrospective cohort study of huoshen mountain hospital and guanggu fangcang shelter hospital. J Clin Hypertens (Greenwich). 2021;23(2):218–231. doi:10.1111/jch.14146.
- Du Y, Shi R, Zhang Y, Duan X, Li L, Zhang J, Wang F, Zhang R, Shen H, Wang Y, et al. A broadly neutralizing humanized ACE2-targeting antibody against SARS-CoV-2 variants. Nat Commun. 2021 Aug 17;121:5000. 10.1038/s41467-021-25331-x
- Bayati A, Kumar R, Francis V, McPherson PS. SARS-CoV-2 infects cells after viral entry via clathrin-mediated endocytosis. J Biol Chem. 2021 Jan-Jun;296:100306. doi:10.1016/j.jbc.2021.100306.
- Shang C, Zhuang X, Zhang H, Li Y, Zhu Y, Lu J, Ge C, Cong J, Li T, Tian M, et al. Inhibitors of endosomal acidification suppress SARS-CoV-2 replication and relieve viral pneumonia in hACE2 transgenic mice. Virol J. 2021;18(1):46. doi:10.1186/s12985-021-01515-1.
- Mao B, Le-Trilling VTK, Wang K, Mennerich D, Hu J, Zhao Z, Zheng J, Deng Y, Katschinski B, Xu S, et al. Obatoclax inhibits SARS-CoV-2 entry by altered endosomal acidification and impaired cathepsin and furin activity in vitro. Emerg Microbes Infect. 2022;11(1):483–497. doi:10.1080/22221751.2022.2026739.
- Ho TC, Wang YH, Chen YL, Tsai W-C, Lee C-H, Chuang K-P, Chen YMA, Yuan C-H, Ho S-Y, Yang M-H, et al. Chloroquine and hydroxychloroquine: efficacy in the treatment of the COVID-19. Pathogens. 2021;10(2):217. doi:10.3390/pathogens10020217.
- Speciale A, Muscarà C, Molonia MS, Cimino F, Saija A, Giofrè SV. Silibinin as potential tool against SARS-Cov −2: in silico spike receptor-binding domain and main protease molecular docking analysis, and in vitro endothelial protective effects. Phytother Res. 2021 Aug;35(8):4616–4625. doi:10.1002/ptr.7107.
- Lu H, Cassis LA, Kooi CW, Daugherty A. Structure and functions of angiotensinogen [published correction appears in hypertens res. Hypertens Res. 2016;39(7):492–500. doi:10.1038/hr.2016.17. 2016 Nov;39(11):827]
- Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology (Bethesda). 2007;22:310–319. doi:10.1152/physiol.00024.2007.
- Satou R, Kobori H. Regulation of a novel angiotensin II precursor, proangiotensin-12, in the tissues by blockade of the renin-angiotensin system. Hypertens Res. 2012;35(2):153–154. doi:10.1038/hr.2011.172.
- Khurana V, Goswami B. Angiotensin converting enzyme (ACE). Clin Chim Acta. 2022;524:113–122. doi:10.1016/j.cca.2021.10.029.
- Rasini E, Cosentino M, Marino F, et al. Angiotensin II type 1 receptor expression on human leukocyte subsets: a flow cytometric and RT-PCR study. Regul Pept. 2006;134(2–3):69–74. doi:10.1016/j.regpep.2006.01.007.
- Lu X, Zhang J, Wen Y, Ren J, Griffiths R, Rudemiller NP, Ide S, Souma T, Crowley SD. Type 1 angiotensin receptors on CD11c-expressing cells protect against hypertension by regulating dendritic cell-mediated T cell activation. Hypertension. 2022;79(6):1227–1236. doi:10.1161/HYPERTENSIONAHA.121.18734.
- Verma K, Pant M, Paliwal S, Dwivedi J, Sharma S. An insight on multicentric signaling of angiotensin II in cardiovascular system: a recent update. Front Pharmacol. 2021 Published 2021 Aug 20;12:734917. doi:10.3389/fphar.2021.734917.
- Zhang Y, Wang Y, Zhou D, Zhang L-S, Deng F-X, Shu S, Wang L-J, Wu Y, Guo N, Zhou J, et al. Angiotensin II deteriorates advanced atherosclerosis by promoting MerTK cleavage and impairing efferocytosis through the AT 1 R/ROS/p38 MAPK/ADAM17 pathway. Am J Physiol Cell Physiol. 2019;317(4):C776–C787. doi:10.1152/ajpcell.00145.2019.
- Stepanova G. Biologia futura: is ADAM 17 the reason for COVID-19 susceptibility in hyperglycemic and diabetic patients? Biol Futur. 2021;72(3):291–297. doi:10.1007/s42977-021-00092-2.
- Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45(2):146–169. doi:10.3109/10409231003628015.
- Iwasaki M, Saito J, Zhao H, Sakamoto A, Hirota K, Ma D. Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: molecular mechanisms and implications. Inflammation. 2021;44(1):13–34. doi:10.1007/s10753-020-01337-3.
- Chalaris A, Gewiese J, Paliga K, Fleig L, Schneede A, Krieger K, Rose-John S, Scheller J. ADAM17-mediated shedding of the IL6R induces cleavage of the membrane stub by gamma-secretase. Biochim Biophys Acta. 2010;1803(2):234–245. doi:10.1016/j.bbamcr.2009.12.001.
- Khanmohammadi S, Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93(5):2735–2739. doi:10.1002/jmv.26826.
- Vayalanellore Giridharan V, Collodel A, Doifode T, Barichello T. Brain infections, encephalitis. And Meningitis: Bacteria, Encyclopedia of Infection and Immunity, Elsevier. 2022:287–301. doi:10.1016/B978-0-12-818731-9.00161-0.
- Sadeghalvad M, Mohammadi-Motlagh H-R. Nima Rezaei, Toll-Like Receptors. Encyclopedia of Infection and Immunity, Elsevier. 2022:130–143. doi:10.1016/B978-0-12-818731-9.00044-6.
- Oh H, Ghosh S. NF-κB: roles and regulation in different CD4(+) T-cell subsets. Immunol Rev. 2013;252(1):41–51. doi:10.1111/imr.12033.
- Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):17023. doi:10.1038/sigtrans.2017.23.
- Cooper AM, Khader SA. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2007;28(1):33–38. doi:10.1016/j.it.2006.11.002.
- She YX, Yu QY, Tang XX, Tang XX. Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov. 2021 [Published 2021 Mar 15];7(1):52. doi:10.1038/s41420-021-00437-9.
- Najem MY, Couturaud F, Lemarié CA. Cytokine and chemokine regulation of venous thromboembolism. J Thromb Haemost. 2020;18(5):1009–1019. doi:10.1111/jth.14759.
- Kahn SR. The post-thrombotic syndrome. Hematology Am Soc Hematol Educ Program. 2016;2016(1):413–418. doi:10.1182/asheducation-2016.1.413.
- Magro G. SARS-CoV-2 and COVID-19: is interleukin-6 (IL-6) the ‘culprit lesion’ of ARDS onset? What is there besides tocilizumab? SGP130Fc. Cytokine X. 2020;2(2):100029. doi:10.1016/j.cytox.2020.100029.
- Chalaris A, Garbers C, Rabe B, Rose-John S, Scheller J. The soluble interleukin 6 receptor: generation and role in inflammation and cancer. Eur J Cell Biol. 2011;90(6–7):484–494. doi:10.1016/j.ejcb.2010.10.007.
- Wolf J, Rose-John S, Garbers C. Interleukin-6 and its receptors: a highly regulated and dynamic system. Cytokine. 2014;70(1):11–20. doi:10.1016/j.cyto.2014.05.024.
- Fiebelkow J, Guendel A, Guendel B, Mehwald N, Jetka T, Komorowski M, Waldherr S, Schaper F, Dittrich A. The tyrosine phosphatase SHP2 increases robustness and information transfer within IL-6-induced JAK/STAT signalling. Cell Commun Signal. 2021 [Published 2021 Sep 16];19(1):94. doi:10.1186/s12964-021-00770-7.
- O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66(1):311–328. doi:10.1146/annurev-med-051113-024537.
- Cutler SJ, Doecke JD, Ghazawi I, Yang J, Griffiths LR, Spring KJ, Ralph SJ, Mellick AS. Novel STAT binding elements mediate IL-6 regulation of MMP-1 and MMP-3. Sci Rep. 2017 [Published 2017 Aug 17];7(1):8526. doi:10.1038/s41598-017-08581-y.
- Steenport M, Khan KM, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: evidence for the role of TNF-alpha and cyclooxygenase-2. J Immunol. 2009;183(12):8119–8127. doi:10.4049/jimmunol.0901925.
- Yen JH, Kocieda VP, Jing H, Ganea D. Prostaglandin E2 induces matrix metalloproteinase 9 expression in dendritic cells through two independent signaling pathways leading to activator protein 1 (AP-1) activation. J Biol Chem. 2011;286(45):38913–38923. doi:10.1074/jbc.M111.252932.
- Zelová H, Hošek J. TNF-α signalling and inflammation: interactions between old acquaintances. Inflamm Res. 2013;62(7):641–651. doi:10.1007/s00011-013-0633-0.
- Nowell MA, Richards PJ, Horiuchi S, Yamamoto N, Rose-John S, Topley N, Williams AS, Jones SA. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J Immunol. 2003;171(6):3202–3209. doi:10.4049/jimmunol.171.6.3202.
- Kuba K, Imai Y, Penninger JM. Angiotensin-converting enzyme 2 in lung diseases. Curr Opin Pharmacol. 2006;6(3):271–276. doi:10.1016/j.coph.2006.03.001.
- Becker MO, Kill A, Kutsche M, Guenther J, Rose A, Tabeling C, Witzenrath M, Kühl AA, Heidecke H, Ghofrani HA, et al. Vascular receptor autoantibodies in pulmonary arterial hypertension associated with systemic sclerosis. Am J Respir Crit Care Med. 2014 Oct 1;190(7):808–817. 10.1164/rccm.201403-0442OC
- Aksoy H, Karadag AS, Wollina U. Angiotensin II receptors: impact for COVID-19 severity. Dermatol Ther. 2020 Nov;33(6):e13989. doi:10.1111/dth.13989.
- Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436(7047):112–116. doi:10.1038/nature03712.
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus –induced lung injury. Nat Med. 2005;11(8):875–879. doi:10.1038/nm1267.
- Liu MY, Zheng B, Zhang Y, et al. Role and mechanism of angiotensin-converting enzyme 2 in acute lung injury in coronavirus disease 2019. Chron Dis and Transl Med. 2020;98–105. doi:10.1016/j.cdtm.2020.05.003.
- Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014 Dec;64(6):1368–1375. doi:10.1161/HYPERTENSIONAHA.114.03743.
- de Ligt M, Hesselink MKC, Jorgensen J, Jocken JWE, Blaak EE, Goossens GH. The angiotensin II type 1 receptor blocker valsartan in the battle against COVID-19. Obesity (Silver Spring). 2021 Sep;29(9):1423–1426. doi:10.1002/oby.23221.
- Liu X, Long C, Xiong Q, Chen C, Ma J, Su Y, Hong K. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID −19, inflammation level, severity, and death in patients with COVID −19: a rapid systematic review and meta-analysis. Clin Cardiol. 2020 Aug 5. doi:10.1002/clc.23421.
- Hassanipour S, Arab-Zozani M, Amani B, Heidarzad F, Fathalipour M, Martinez-de-hoyo R. The efficacy and safety of Favipiravir in treatment of COVID-19: a systematic review and meta-analysis of clinical trials. Sci Rep. 2021 May 26;11(1):11022. doi:10.1038/s41598-021-90551-6
- Avila A, Gallego I, Soria ME, Gregori J, Quer J, Esteban JI, Rice CM, Domingo E, Perales C. Lethal mutagenesis of hepatitis C virus induced by favipiravir. PLoS One. 2016;11(10). doi:10.1371/journal.pone.0164691.
- Roozbeh F, Saeedi M, Alizadeh-Navaei R, Hedayatizadeh-Omran A, Merat S, Wentzel H, Levi J, Hill A, Shamshirian A. Sofosbuvir and daclatasvir for the treatment of COVID-19 outpatients: a double-blind, randomized controlled trial. J Antimicrob Chemother. 2021 Feb 11;76(3):753–757. doi:10.1093/jac/dkaa501
- Husic-Selimovic A, Sofic A, Huskic J, Bulja A. Effect of antiviral therapy on serum activity of angiotensin converting enzyme in patients with chronic hepatitis C. Med Arch. 2016 Apr;70(2):92–96. doi:10.5455/medarh.2016.70.92-96.
- Krug SM, Schulzke JD, Fromm M. Tight junction, selective permeability, and related diseases. Semin Cell Dev Biol. 2014;36:166–176. doi:10.1016/j.semcdb.2014.09.002.
- Vermette D, Hu P, Canarie MF, Funaro M, Glover J, Pierce RW. Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp. 2018 [Published 2018 Sep 26];6(1):37. doi:10.1186/s40635-018-0203-4.
- Otani T, Furuse M. Tight junction structure and function revisited [published correction appears in trends cell biol. Trends Cell Biol. 2020;30(10):805–817. doi:10.1016/j.tcb.2020.08.004. Dec;30(12):1014]
- Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta. 2009;1788(4):864–871. doi:10.1016/j.bbamem.2008.08.027.
- He F, Peng J, Deng XL, Yang L-F, Camara AD, Omran A, Wang G-L, Wu L-W, Zhang C-L, Yin F, et al. Mechanisms of tumor necrosis factor-alpha-induced leaks in intestine epithelial barrier. Cytokine. 2012;59(2):264–272. doi:10.1016/j.cyto.2012.04.008.
- Petecchia L, Sabatini F, Usai C, Caci E, Varesio L, Rossi GA. Cytokines induce tight junction disassembly in airway cells via an EGFR-dependent MAPK/ERK1/2-pathway. Lab Invest. 2012;92(8):1140–1148. doi:10.1038/labinvest.2012.67.
- al-Nag M, Morin PJ. The claudins. Genome Biol. 2009;10(8):235. doi:10.1186/gb-2009-10-8-235.
- Hu YJ, Wang YD, Tan FQ, Yang W-X. Regulation of paracellular permeability: factors and mechanisms. Mol Biol Rep. 2013 Nov;40(11):6123–6142. doi:10.1007/s11033-013-2724-y.
- Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93(2):525–569. doi:10.1152/physrev.00019.2012.
- Rosenthal R, Günzel D, Piontek J, Krug SM, Ayala‐Torres C, Hempel C, Theune D, Fromm M. Claudin-15 forms a water channel through the tight junction with distinct function compared to claudin-2. Acta Physiol (Oxf). 2020;228(1):e13334. doi:10.1111/apha.13334.
- Furuse M, Takai Y. Recent advances in understanding tight junctions. Fac Rev. 2021 Published 2021 Feb 23;10:18. doi:10.12703/r/10-18.
- Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015 Feb;15(2):104–116. doi:10.1038/nri3793.
- Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, Li N, Li Y, Xu G, Li J, et al. Interferon-γ and tumor necrosis factor-α disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol. 2008;126(1):67–80. doi:10.1016/j.clim.2007.08.017.
- Chen-Quay SC, Eiting KT, AWA L, Lamharzi N, Quay SC. Identification of tight junction modulating lipids. J Pharm Sci. 2009;98(2):606–619. doi:10.1002/jps.21462.
- Fuladi S, Jannat R, Shen L, et al. Computational modeling of claudin structure and function. Int J of Mol Sci. 2020;1-2:4–9. doi:10.3390/ijms21030742.
- Marianayagam NJ, Sunde M, Matthews JM. The power of two: protein dimerization in biology. Trends in Biochemical Sciences. 2004;29(11):618–625. doi:10.1016/j.tibs.2004.09.006.
- Kaarteenaho-Wiik R, Soini Y. Claudin-1, −2, −3, −4, −5, and −7 in usual interstitial pneumonia and sarcoidosis. J Hisochem Cytochem. 2009;57(3):187–195. doi:10.1369/jhc.2008.951566.
- Soini Y. Claudins in lung diseases. Respir Res. 2011;12(1). doi:10.1186/1465-9921-12-70.
- Vishwajeet V, Purohit A, Kumar D, Parag V, Tripathi S, Kanchan T, Kothari N, Dutt N, Elhence PA, Bhatia PK, et al. Evaluation of Pathological findings of COVID-19 by minimally invasive autopsies: a single tertiary care center experience from India. J Lab Physicians. 2021 Jun;13(2):97–106. doi:10.1055/s-0041-1730750.
- Myall KJ, Martinovic JL, West A. How COVID-19 interacts with interstitial lung disease. Breathe (Sheff). 2022;18(1):210158. doi:10.1183/20734735.0158-2021.
- Rasch S, Schmidle P, Sancak S, et al. Increased extravascular lung water index (EVLWI) reflects rapid non-cardiogenic oedema and mortality in COVID-19 associated ARDS. Sci Rep. 2021;11(1):11524. doi:10.1038/s41598-021-91043-3.
- Cui X, Chen W, Zhou H, Gong Y, Zhu B, Lv X, Guo H, Duan J, Zhou J, Marcon E, et al. Pulmonary Edema in COVID-19 patients: mechanisms and treatment potential. Front Pharmacol. 2021 Published 2021 Jun 7;12:664349. doi:10.3389/fphar.2021.664349.
- Fujita H, Chalubinski M, Rhyner C, Indermitte P, Meyer N, Ferstl R, Treis A, Gomez E, Akkaya A, O’Mahony L, et al. Claudin-1 expression in airway smooth muscle exacerbates airway remodeling in asthmatic subjects. J Allergy Clin Immunol. 2011;127(6):1612–21.e8. doi:10.1016/j.jaci.2011.03.039.
- Wittekindt OH. Tight junctions in pulmonary epithelia during lung inflammation. Pflugers Arch. 2017;469(1):135–147. doi:10.1007/s00424-016-1917-3.
- Armstrong SM, Wang C, Tigdi J, Si X, Dumpit C, Charles S, Gamage A, Moraes TJ, Lee WL, et al. Influenza infects lung microvascular endothelium leading to microvascular leak: role of apoptosis and claudin-5. PLoS One. 2012;7(10):e47323. doi:10.1371/journal.pone.0047323.
- Capaldo CT, Farkas AE, Hilgarth RS, Krug SM, Wolf MF, Benedik JK, Fromm M, Koval M, Parkos C, Nusrat A, et al. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell. 2014;25(18):2710–2719. doi:10.1091/mbc.E14-02-0773.
- Greene C, Hanley N, Campbell M. Claudin-5: gatekeeper of neurological function. Fluids Barriers CNS. 2019 [Published 2019 Jan 29];16(1):3. doi:10.1186/s12987-019-0123-z.
- Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M, Appelt-Menzel A, Cubukova A, Barenberg J, Leu J, et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Reports. 2022;17(2):307–320. doi:10.1016/j.stemcr.2021.12.011.
- Zhang L, Zhou L, Bao L, Liu J, Zhu H, Lv Q, Liu R, Chen W, Tong W, Wei Q, et al. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther. 2021;6(1):337. doi:10.1038/s41392-021-00719-9.
- DeOre BJ, Tran KA, Andrews AM, Ramirez SH, Galie PA. SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation. J Neuroimmune Pharmacol. 2021;16(4):722–728. doi:10.1007/s11481-021-10029-0.
- Flores-Pliego A, Miranda J, Vega-Torreblanca S, Valdespino-Vázquez Y, Helguera-Repetto C, Espejel-Nuñez A, Borboa-Olivares H, Espino Y Sosa S, Mateu-Rogell P, León-Juárez M, et al. Molecular insights into the thrombotic and microvascular injury in placental endothelium of women with mild or severe COVID-19. Cells. 2021;10(2):364. doi:10.3390/cells10020364.
- Juttukonda LJ, Wachman EM, Boateng J, Jain M, Benarroch Y, Taglauer ES. Decidual immune response following COVID-19 during pregnancy varies by timing of maternal SARS-CoV-2 infection [published online ahead of print, 2022 Feb 24]. J Reprod Immunol. 2022;151:103501. doi:10.1016/j.jri.2022.103501.
- Menter T, Tzankov A, Bruder E. SARS-CoV-2/COVID-19-Auswirkungen auf die plazenta [Impact of SARS-CoV-2/COVID-19 on the placenta]. Pathologe. 2021;42(6):591–597. doi:10.1007/s00292-021-00952-7.
- Peirouvi T, Aliaghaei A, Eslami Farsani B, et al. COVID-19 disrupts the blood-testis barrier through the induction of inflammatory cytokines and disruption of junctional proteins. Inflamm Res. 2021;70(10–12):1165–1175. doi:10.1007/s00011-021-01497-4.
- Edenfield RC, Easley CA. 4th. Implications of testicular ACE2 and the renin-angiotensin system for SARS-CoV-2 on testis function. Nat Rev Urol. 2022 Feb;19(2):116–127. doi:10.1038/s41585-021-00542-5.
- Tur-Kaspa I, Tur-Kaspa T, Hildebrand G, Cohen D. COVID-19 may affect male fertility but is not sexually transmitted: a systematic review. F S Rev. 2021 Apr;2(2):140–149. doi:10.1016/j.xfnr.2021.01.002.
- Lee WY, Mok A, Chung JPW. Potential effects of COVID-19 on reproductive systems and fertility; assisted reproductive technology guidelines and considerations: a review. Hong Kong Med J. 2021 Apr;27(2):118–126. doi:10.12809/hkmj209078.
- Rokkas T. Gastrointestinal involvement in COVID-19: a systematic review and meta-analysis. Ann Gastroenterol. 2020;33(4):355–365. doi:10.20524/aog.2020.0506.
- Groff A, Kavanaugh M, Ramgobin D, McClafferty B, Aggarwal CS, Golamari R, Jain R. Gastrointestinal manifestations of COVID-19: a review of what we know. Ochsner J. 2021;21(2):177–180. doi:10.31486/toj.20.0086.
- Marasco G, Cremon C, Barbaro MR, Salvi D, Cacciari G, Kagramanova A, Bordin D, Drug V, Miftode E, Fusaroli P, et al. Prevalence of gastrointestinal symptoms in severe acute respiratory syndrome coronavirus 2 infection: results of the prospective controlled multinational GI-COVID-19 study. Am J Gastroenterol. 2022 Jan 1;1171:147–157. 10.14309/ajg.0000000000001541
- Allou TMP, Orfali RL, Sotto MN, et al. Increased expression of filaggrin and claudin-1 in the ocular surface of patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2021 Oct 27; 10.1111/jdv.17768.
- Lange C, Wolf J, Auw-Haedrich C, Schlecht A, Boneva S, Lapp T, Horres R, Agostini H, Martin G, Reinhard T, et al. Expression of the COVID-19 receptor ACE2 in the human conjunctiva. J Med Virol. 2020 Oct;92(10):2081–2086. doi:10.1002/jmv.25981.
- Aggarwal K, Agarwal A, Jaiswal N, Dahiya N, Ahuja A, Mahajan S, Tong L, Duggal M, Singh M, Agrawal R, et al. Ocular surface manifestations of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One. 2020 Nov 5;1511:e0241661. 10.1371/journal.pone.0241661
- Amoozadeh Y, Dan Q, Anwer S, et al. Tumor necrosis factor-α increases claudin-1, 4, and 7 expression in tubular cells: role in permeability changes. J Cell Physiol. 2017 Aug;232(8):2210–2220. doi:10.1002/jcp.25736.
- Bitencourt L, Pedrosa AL, de Brito SBCS, Fróes ACF, de Carvalho ST, Fonseca GG, Ferreira GC, Fradico PF, Simões E Silva AC. COVID-19 and renal diseases: an update. Curr Drug Targets. 2021;22(1):52–67. doi:10.2174/1389450121999201013151300.
- Burke KT, McGinnis KS, Petronic-Rosic V. COVID toes. Clin Dermatol. 2020. doi:10.1016/j.clindermatol.2020.12.002.
- Adil MS, Khulood D, Narayanan SP, et al. Bioinformatics analyses reveal cell-barrier junction modulations in lung epithelial cells on SARS-CoV-2 infection. Tissue Barriers. Nov 2021;5(2000300). doi:10.1080/21688370.2021.2000300.
- Hashimoto Y, Okada Y, Shirakura K, et al. Anti-claudin antibodies as a concept for development of claudin-directed drugs. J Pharmacol Exp Ther. 2019 Feb;368(2):179–186. doi:10.1124/jpet.118.252361.
- Hashimoto Y, Fukasawa M, Kuniyasu H, et al. Claudin-targeted drug development using anti-claudin monoclonal antibodies to treat hepatitis and cancer. Ann N Y Acad Sci. 2017 Jun;1397(1):5–16. doi:10.1111/nyas.13337.
- Cummins PM. Occludin: one protein, many forms. Mol Cell Biol. 2012;32(2):242–250. doi:10.1128/MCB.06029-11.
- Rouaud F, Méan I, Citi S. The ACE2 receptor for coronavirus entry is localized at apical cell-cell junctions of epithelial cells. Cells. 2022 [Published 2022 Feb 11];11(4):627. doi:10.3390/cells11040627.
- Michalick L, Weidenfeld S, Grimmer B, Fatykhova D, Solymosi PD, Behrens F, Dohmen M, Brack MC, Schulz S, Thomasch E, et al. Plasma mediators in patients with severe COVID-19 cause lung endothelial barrier failure. Eur Respir J. 2021;57(3):2002384. doi:10.1183/13993003.02384-2020.
- Yu AS, McCarthy KM, Francis SA, et al. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288(6):C1231–C1241. doi:10.1152/ajpcell.00581.2004.
- Ni Y, Teng T, Li R, Simonyi A, Sun GY, Lee JC, Zhu D. TNFα alters occludin and cerebral endothelial permeability: role of p38MAPK. PLoS One. 2017 [Published 2017 Feb 7];12(2):e0170346. doi:10.1371/journal.pone.0170346.
- Fedosova NU, Habeck M, Nissen P. Structure and function of Na,K-ATPase-the sodium-potassium pump. Compr Physiol. 2021 [Published 2021 Dec 29];12(1):2659–2679. doi:10.1002/cphy.c200018.
- Rajasekaran SA, Barwe SP, Gopal J, Ryazantsev S, Schneeberger EE, Rajasekaran AK. Na-K-ATPase regulates tight junction permeability through occludin phosphorylation in pancreatic epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G124–G133. doi:10.1152/ajpgi.00297.2006.
- Kryvenko V, Vadász I. Molecular mechanisms of Na,K-ATPase dysregulation driving alveolar epithelial barrier failure in severe COVID-19. Am J Physiol Lung Cell Mol Physiol. 2021;320(6):L1186–L1193. doi:10.1152/ajplung.00056.2021.
- Ebnet K. Junctional adhesion molecules (JAMs): cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol Rev. 2017;97(4):1529–1554. doi:10.1152/physrev.00004.2017.
- Kummer D, Ebnet K. Junctional adhesion molecules (JAMs): the JAM-integrin connection. Cells. 2018 [Published 2018 Mar 26];7(4):25. doi:10.3390/cells7040025.
- Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, et al. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;24(18):2849–2860. doi:10.1091/mbc.E13-06-0298.
- Tsukita S, Katsuno T, Yamazaki Y, Umeda K, Tamura A, Tsukita S. Roles of ZO-1 and ZO-2 in establishment of the belt-like adherens and tight junctions with paracellular permselective barrier function. Ann N Y Acad Sci. 2009;1165(1):44–52. doi:10.1111/j.1749-6632.2009.04056.x.
- Hartmann C, Schwietzer YA, Otani T, Furuse M, Ebnet K. Physiological functions of junctional adhesion molecules (JAMs) in tight junctions. Biochim Biophys Acta Biomembr. 2020;1862(9):183299. doi:10.1016/j.bbamem.2020.183299.
- Ernst A, Appleton BA, Ivarsson Y, Zhang Y, Gfeller D, Wiesmann C, Sidhu SS. A structural portrait of the PDZ domain family. J Mol Biol. 2014;426(21):3509–3519. doi:10.1016/j.jmb.2014.08.012.
- Kim JG, Islam R, Cho JY, Jeong H, Cap K-C, Park Y, Hossain AJ, Park J-B. Regulation of RhoA GTPase and various transcription factors in the RhoA pathway. J Cell Physiol. 2018;233(9):6381–6392. doi:10.1002/jcp.26487.
- Raghavan S, Kenchappa DB, Leo MD. SARS-CoV-2 spike protein induces degradation of junctional proteins that maintain endothelial barrier integrity. Front Cardiovasc Med. 2021 Published 2021 Jun 11;8:687783. doi:10.3389/fcvm.2021.687783.
- Mitchell LA, Ward C, Kwon M, et al. Junctional adhesion molecule A promotes epithelial tight junction assembly to augment lung barrier function. Am J Pathol. 2015;185(2):372–386. doi:10.1016/j.ajpath.2014.10.010.
- Shepley-McTaggart A, Sagum CA, Oliva I, Rybakovsky E, DiGuilio K, Liang J, Bedford MT, Cassel J, Sudol M, Mullin JM, et al. SARS-CoV-2 envelope (E) protein interacts with PDZ-domain-2 of host tight junction protein ZO1. PLoS One. 2021;16(6):e0251955. doi:10.1371/journal.pone.0251955.
- Zhu Y, Alvarez F, Wolff N, Mechaly A, Brûlé S, Neitthoffer B, Etienne-Manneville S, Haouz A, Boëda B, Caillet-Saguy C, et al. Interactions of severe acute respiratory syndrome coronavirus 2 protein e with cell junctions and polarity PSD-95/Dlg/ZO-1-containing proteins. Front Microbiol. 2022 Published 2022 Feb 23;13:829094. doi:10.3389/fmicb.2022.829094.
- Abedi F, Rezaee R, Karimi G. Plausibility of therapeutic effects of Rho kinase inhibitors against severe acute respiratory syndrome coronavirus 2 (COVID-19). Pharmacol Res. 2020;156:104808. doi:10.1016/j.phrs.2020.104808.
- Wise SK, Laury AM, Katz EH, Den Beste KA, Parkos CA, Nusrat A. Interleukin-4 and interleukin-13 compromise the sinonasal epithelial barrier and perturb intercellular junction protein expression. Int Forum Allergy Rhinol. 2014;4(5):361–370. doi:10.1002/alr.21298.
- Vaira LA, De Vito A, Lechien JR, Chiesa‐Estomba CM, Mayo‐Yàñez M, Calvo‐Henrìquez C, Saussez S, Madeddu G, Babudieri S, Boscolo‐Rizzo P, et al. New onset of smell and taste loss are common findings also in patients with symptomatic COVID −19 after complete vaccination. Laryngoscope. 2022 Feb;132(2):419–421. doi:10.1002/lary.29964.
- Mastrangelo A, Bonato M, Cinque P. Smell and taste disorders in COVID-19: from pathogenesis to clinical features and outcomes. Neurosci Lett. 2021 Mar 23;748:135694. doi:10.1016/j.neulet.2021.135694.
- Salcan İ, Karakeçili F, Salcan S, Ünver E, Akyüz S, Seçkin E, Cingi C. Is taste and smell impairment irreversible in COVID-19 patients? Eur Arch Otorhinolaryngol. 2021;278(2):411–415. doi:10.1007/s00405-020-06560-0.
- Kumar L, Kahlon N, Jain A, Kaur J, Singh M, Pandey AK. Loss of smell and taste in COVID-19 infection in adolescents. Int J Pediatr Otorhinolaryngol. 2021 Mar;142:110626. doi:10.1016/j.ijporl.2021.110626.
- Tharmarajah E, Buazon A, Patel V, Hannah JR, Adas M, Allen VB, Bechman K, Clarke BD, Nagra D, Norton S, et al. IL-6 inhibition in the treatment of COVID-19: a meta-analysis and meta-regression. J Infect. 2021;82(5):178–185. doi:10.1016/j.jinf.2021.03.008.
- Guo Y, Hu K, Li Y, Lu C, Ling K, Cai C, Wang W, Ye D. Targeting TNF-α for COVID-19: recent advanced and controversies. Front Public Health. 2022 Published 2022 Feb 11;10:833967. doi:10.3389/fpubh.2022.833967.
- Salesi M, Shojaie B, Farajzadegan Z, Salesi N, Mohammadi E. TNF-α blockers showed prophylactic effects in preventing COVID-19 in patients with rheumatoid arthritis and seronegative spondyloarthropathies: a case-control study. Rheumatol Ther. published online ahead of print, 2021 Jul 23;2021:1–16. doi:10.1007/s40744-021-00342-8.