Abstract
The present study described biosynthesis of silver nanoparticles (AgNPs) using a bacterial strain Novosphingobium sp. THG-C3, isolated from soil, and their application in antibacterial activity. The maximum absorbance values of the synthesized AgNPs was measured at 406 nm in ultraviolet-visible spectrophotometry and were mostly spherical in shape with particle size in range of 8–25 nm by field emission transmission electron microscopy analysis. X-ray diffraction pattern corresponding to planes (111), (200), (220), and (311) demonstrated the crystalline nature of the AgNPs. The synthesized AgNPs exhibited antimicrobial activity against various pathogens inculding Staphylococcus aureus, Candida tropicalis, Pseudomonas aeruginosa, Escherichia coli, Vibrio parahaemolyticus, Candida albicans, Salmonella enterica, Bacillus subtilis, and Bacillus cereus. In addition, the AgNPs in combination with commercial antibiotics enhanced antimicrobial activity against P. aeruginosa, S. enterica, E. coli, and V. parahaemolyticus. The AgNPs synthesized by strain Novosphingobium sp. THG-C3 are comparatively simple, green, cost-effective, and may serve as a potential antimicrobial agent.
Introduction
Nanotechnology is one of the most attractive areas of research in modern material science due to their potential applications in numerous fields, including mechanics, biomedical, energy science, catalysis, optoelectronics, magnetics sciences, and sensors (Araujo et al. Citation2012, Sulaiman et al. Citation2013). Metal nanoparticles (MNPs) pave significance attraction of researchers because of their unique properties such as increased catalytic activity due to morphologies with highly active facets as compared to bulk material (Ahmed et al. Citation2015). Among MNPs, silver nanoparticles (AgNPs) play a significant role in the field of biology and medicine. A variety of chemical and physical approaches are available for the synthesis of AgNPs such as chemical reduction, pyrolysis, laser ablation, electrochemical techniques, sol gel, and photochemical reduction (Kouvaris et al. Citation2012, Loo et al. Citation2015). However, the eco-friendly process of nanoparticle synthesis is a growing need. Interdisciplinary research has extended the horizons of material research, various comparatively safer and cheaper strategies using microorganisms, enzymes, fungi, plants, or plant extracts have been made to develop for nanoparticle biosynthesis (Mata et al. Citation2015). Furthermore, these eco-friendly approaches efficiency produce a wide variety of shapes with sizes ranging from 1 to 100 nm (Basavegowda and Lee Citation2013).
Human beings are often infected by pathogenic microorganisms such as bacteria, yeasts, and molds in the living environment. There has been intensive research in bactericidal material containing multifarious natural and inorganic substances (Ahamed et al. Citation2010). However, the past two decades have seen a significant decline in the discovery and development of novel antibiotics and marked increase in resistance of pathogen to those currently bactericides and antibiotics (Bush Citation2010, Li et al. Citation2006). This emergency makes bactericidal nanomaterials emerged up as novel antimicrobial agents owing to their remarkable properties different from both the ion and the bulk material (Cao and Liu Citation2010, Morones et al. Citation2005). AgNPs have been considered to be effective as it has good antimicrobial efficacy and is shown to be potential to against multidrug-resistant microorganisms (Gong et al. Citation2007). Considering this, the present study highlighted the synthesis of AgNPs by strain Novosphingobium sp. THG-C3 and their antimicrobial activity against clinical microorganisms. The further extend to use the synthesized AgNPs of control antimicrobial resistance in clinical microorganisms was also studied.
Materials and methods
Materials and bacterial strains
Chemicals used were purchased from Sigma Chemicals (St. Louis, MO). All the media and standard antibiotics discs were procured from Oxoid Ltd. (Basingstoke, England). The antibiotics used were erythromycin 15 μg/disc (E 15), lincomycin 15 μg/disc (MY 15), novobiocin 30 μg/disc (NV 30), penicillin G 10 units/disc (P 10), vancomycin 30 μg/disc (VA 30), and oleandomycin 15 μg/disc (OL 15). Pathogenic bacterial cultures Staphylococcus aureus (ATCC 6538), Candida tropicalis (ATCC 18807), Pseudomonas aeruginosa (ATCC 10145), Escherichia coli (ATCC 10798), Vibrio parahaemolyticus (ATCC 33844), Candida albicans (ATCC 18804), Salmonella enterica (ATCC 13076), Bacillus subtilis (ATCC 6633), and Bacillus cereus (ATCC 14579) were employed from American Type Culture Collection (ATCC) and used for antimicrobial activity assay.
Isolation and molecular identification of bacteria
Soil samples were selected from Suwon, South Korea. For the screening of bacteria which could synthesis AgNPs, soil samples were serially diluted in sterile 0.85% NaCl and then spread onto nutrient agar (NA) contains filter-sterilized AgNO3 solution at 1 mM final concentration. The plates were incubated at 28 °C and growth was observed. The isolated colony was purified and routinely cultured on NA and maintained with 25% (v/v) glycerol at −80 °C.
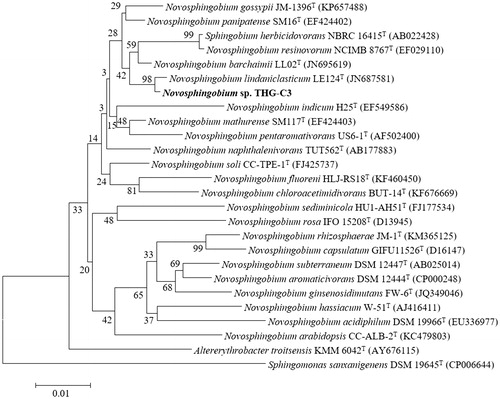
Molecular identification of the isolated strain was performed by using a 16S rRNA sequencing-based method. The 16S rRNA gene of the isolated strain was amplified and sequenced using the universal bacterial primer pairs 27F (5′-AGAGTTTGATCCTGGCTCAG–3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′), 518F (5′-CCAGCAGCCGCGGTAATACG-3′), and 805R (5′-TACCAGGGTATCTAATCC-3′) (Weisburg et al. Citation1991). The 16S rRNA gene sequences of related taxa were obtained from the EzTaxon-e server and GenBank database (Kim et al. Citation2012). Phylogenetic tree was constructed with neighbor-joining method (Saitou and Nei Citation1987) by using the MEGA 6 program package (Tamura et al. Citation2013) to demonstrate the phylogenetic position of the isolated strain.
Biosynthesis of AgNPs
For the synthesis of AgNPs, the selected strain was cultured in 100 ml of nutrient broth (NB) and incubated in shaking incubator at 28 °C and 80 rpm. After 48-h incubation, the cells were completely removed by centrifugation at 9000 rpm for 10 min. 100 ml of the supernatant was added with 0.1 ml filter-sterilized AgNO3 solution (1 M/ml) and kept for incubation of 2 d in an orbital shaker at 120 rpm and 25 °C. The extracellular synthesis of AgNPs was confirmed due to the color change in the culture medium. Then, the mixture was first centrifuged at 2000 rpm for 5 min to remove the undesirable contaminants, and the AgNPs were collected by high speed centrifugation at 24,000 rpm for 10 min. The pellets of AgNPs were washed thoroughly and used for characterization and antimicrobial studies.
Characterization of AgNPs
The reduction of silver ions to AgNPs was confirmed by UV-vis spectrophotometer (OPTIZEN POP-BIO, Mecasys, Daejeon, Korea) in the range of 300–800 nm. The synthesized AgNPs were further analyzed by using field emission transmission electron microscopy (FE-TEM) (JEM-2100F, JEOL, Japan), 10 μl of the re-dispersed AgNPs solution was placed on the carbon coated copper grid. The FE-TEM grid was dried at room temperature and operated at a voltage of 200 kV. The dried AgNPs were coated on X-ray diffraction (XRD) grid and detected on X-ray diffractometer (D8 Advance, Bruker, Germany), at 40 kV with CuKα radiation. In addition, the elemental mapping, selected-area electron diffraction (SAED) and energy dispersive X-ray spectroscopy (EDX) of the AgNPs were performed with JEM-2100F (JEOL).
Antimicrobial activity assay
The antimicrobial activity of synthesized AgNPs was determined using disk diffusion method. Briefly, 100 μl fresh overnight inoculums of each culture were spread evenly on Mueller–Hinton agar (MHA) plates to form a bacterial lawn. The sterile paper discs (8 mm diameter) impregnated with 30 μl of AgNPs (500 ppm in water) were placed over the bacterial lawn. The zone of inhibition (in mm) was measured after 24-h incubation. The standard antibiotic discs (6 mm diameter) such as E 15, My 15, NV 30, P 10, VA 30, and OL 15 μg and each antibiotic discs containing 30 μl of synthesized AgNPs were tested in same manner. The study was done in triplets and the average value was calculated.
Results and discussion
Screening and identification of bacteria
During the screening study, a bacterial strain THG-C3 showed growth on NA supplemented with 1 mM of AgNO3 solution, which indicated that strain THG-C3 was capable of tolerating silver salts. The almost complete 16S rRNA gene sequence of strain THG-C3 (1459 bp) was analyzed and submitted to NCBI (accession number: KU523694). On the basis of the sequence, strain THG-C3 showed 98.68% similarity with Novosphingobium lindaniclasticum. Members of the genus Novosphingobium were found to be gram-negative, aerobic, rod-shaped organisms, which played an important role in biodegradation of organic pollutants such as polycyclic aromatic hydrocarbons, aromatic hydrocarbons, dibenzofuran, and 2,4-dichlorophenoxyacetic (Dai et al. Citation2015, Fujii et al. Citation2003, Sohn et al. Citation2004, Suzuki and Hiraishi Citation2007, Yuan et al. Citation2009). The phylogenetic trees also indicated that strain THG-C3 was affiliated with the genus Novosphingobium and formed a phyletic line distinct from the clades of related species ().
Synthesis and characterization of nanoparticles
The formation of AgNPs was monitored by a visual color change of the reaction mixture, as a result of the surface plasmon resonance phenomenon (Eustis and el-Sayed Citation2006). The color of THG-C3 culture supernatant was changed from light yellow to deep brown within 48 h. The use of microorganisms such as bacteria, fungi, yeast, and actinomycetes has been reported for the biosynthesis of nanoparticles and their applications (Mohanpuria et al. Citation2008). The present work first synthesized AgNPs by species of genus Novosphingobium, the methodology was extracellular, simple, green, and cost-effective.
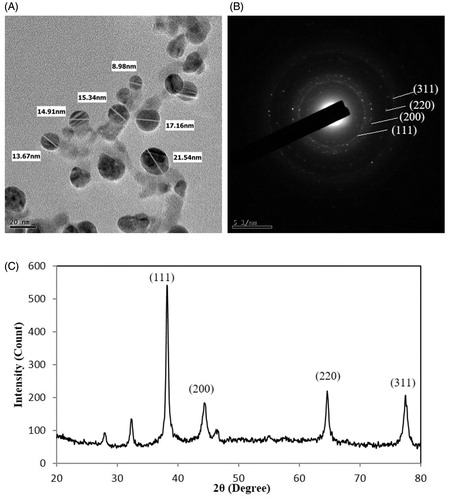
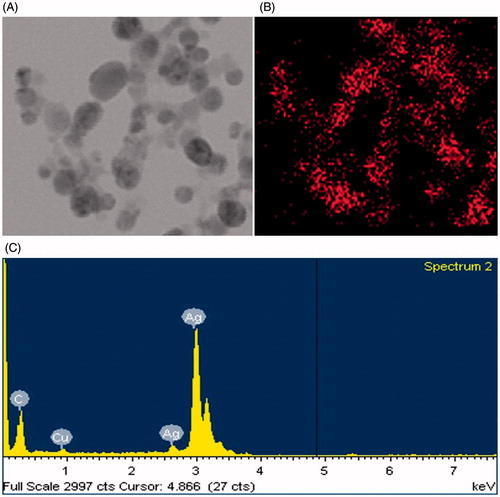
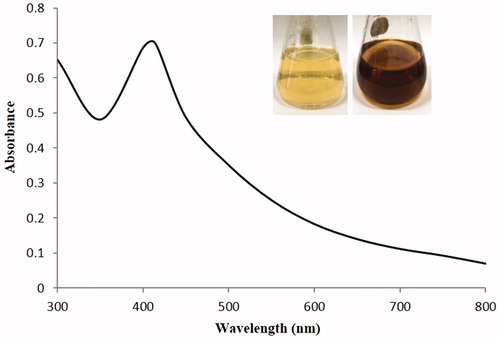
The formation of AgNPs was confirmed by UV-vis spectral scanning at 300–800 nm and a strong peak at around 406 nm was observed (), which is attributed to the surface plasmon resonance band of AgNPs. According to the FE-TEM micrographs shown in , the majorities of AgNPs formed were spherical in shape and measured 8–25 nm. The SAED pattern () showed four bright rings which can be indexed to (111), (200), (220), and (311) lattice planes of the crystalline silver face-centered cubic structure, respectively. showed the XRD pattern of the synthesized AgNPs, the diffraction peaks at 2θ values of 38.20°, 44.37°, 64.43°, and 77.47° match the respective (111), (200), (220), and (311) lattice planes of AgNPs, corresponding to the result of SAED, confirmed the crystalline character of the AgNPs. The elemental mapping results showed the maximum distribution of silver elements, indicated that silver were the predominant elements in the AgNPs (). The purity and composition of the synthesized AgNPs was analyzed by EDX, showed the characteristic signals of silver atoms in AgNPs approximately at 3 keV (), and the presence of signals of copper and carbon was because of the use of FE-TEM grid (Magudapathy et al. Citation2001).
Figure 2. UV-vis spectra of reaction mixture. The figure inset shows NB with AgNO3 solution as control and synthesized AgNPs.
Antibacterial efficacies of the AgNPs
Until now, most antibiotics were produced by soil isolates, and synthetic approaches were unable to replace this platform. Antibiotic resistance was an increasing problem and caused the requirement for a constant introduction of new compounds (Ling et al. Citation2015). However, antimicrobial drug discovery was uniquely difficult (Payne et al. Citation2007). In order to overcoming this limited resource, AgNPs had been studied and reported to be an effective antimicrobial agent (Naqvi et al. Citation2013). In this study, the synthesized AgNPs exhibited significant antimicrobial activity against a range of pathogenic microorganisms including S. aureus, C. tropicalis, P. aeruginosa, E. coli, V. parahaemolyticus, C. albicans, S. enterica, B. subtilis, and B. cereus (). The main mechanisms of AgNPs against microorganisms were attach to the surface of the cell membrane or cell wall and drastically disturb its proper function, cause further damage of DNA and possibly other sulfur- and phosphorus-containing compounds (Morones et al. Citation2005). The antibacterial activity against the pathogenic microorganisms was measured in terms of zones of inhibition (mm) (). In addition, the synthesized AgNPs in combination with commercial antibiotics enhanced antimicrobial activity against P. aeruginosa, S. enterica, E. coli, and V. parahaemolyticus (). The increase in fold area was calculated for antibiotics with AgNPs as compared with standard antibiotics control (). The pathogenic strains P. aeruginosa, S. enterica, and E. coli exhibited resistant to commercial antibiotics, but AgNPs in combination with commercial antibiotics were inhibited the pathogenic strains and formed the zone of inhibition. Strain V. parahaemolyticus was sensitive to the commercial discs, and the results showed increase in sensitivity after the addition of AgNPs. The results demonstrated that the AgNPs restrict the microorganisms to develop resistance. In case of microorganisms develops resistance to one of them, the other bactericidal agent would kill the microorganisms. However, more the detailed mechanism needs to be studied to develop a novel antibacterial agent against multidrug-resistant bacteria.
Figure 5. Antimicrobial activities of synthesized AgNPs (30 μl) at 500 ppm concentrations in water against S. aureus, V. parahaemolyticus, B. cereus, P. aeruginosa, C. tropicalis, C. albicans, E. coli, B. subtilis, and S. enterica.
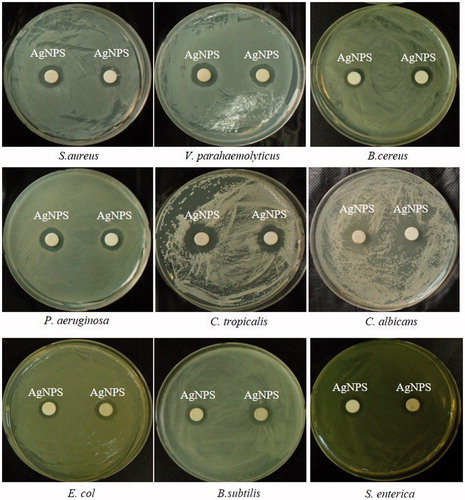
Figure 6. Antimicrobial activities against P. aeruginosa of standard antibiotics (A1) and antibiotics loaded with synthesized AgNPs 30 μl (500 ppm) (A2); against S. enterica (B1, B2); against V. parahaemolyticus (C1, C2); against E. coli (D1, D2), respectively. Abbreviation: E 15 (erythromycin, 15 μg/disc), NV 30 (novobiocin, 30 μg/disk), OL 15 (oleandomycin, 15 μg/disk), MY 15 (lincomycin, 15 μg/disc), P 10 (penicillin G, 10 Units/disc), VA 30 (vancomycin, 30 μg/disk).
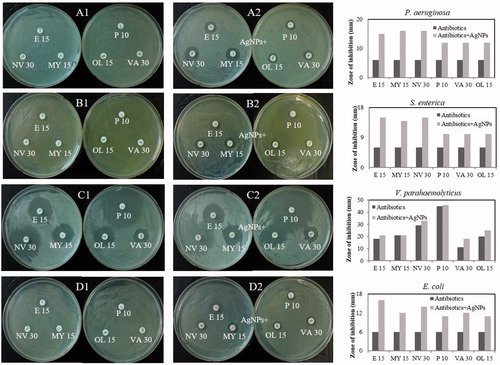
Table 1. Antimicrobial activity of silver nanoparticles against selected pathogenic strains.
Table 2. Individual and combined efficacy of synthesized AgNPs and antibiotics against selected pathogenic strains.
Conclusion
This study reported a biosynthesis of AgNPs using the cultural filtrate of strain Novosphingobium sp. THG-C3 in an eco-friendly manner. The characterization study supported the formation of AgNPs. The synthesized AgNPs proved excellent antimicrobial activity against varies of gram-negative and gram-positive pathogenic microorganisms. In addition, the AgNPs showed the potential application of antibacterial agent against multidrug-resistant bacteria.
Acknowledgements
This work was conducted under the industrial infrastructure program (No. N0000888) for fundamental technologies which is funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahamed M, Alsalhi MS, Siddiqui MK. 2010. Silver nanoparticle applications and human health. Clin Chim Acta. 411:1841–1848.
- Ahmed MJ, Murtaza G, Mehmood A, Bhatti TM. 2015. Green synthesis of silver nanoparticles using leaves extract of Skimmia laureola: characterization and antibacterial activity. Mater Lett. 153:10–13.
- Araujo EA, Andrade NJ, da Silva LH, Bernardes PC, de CTAV de Sa JP, et al. 2012. Antimicrobial effects of silver nanoparticles against bacterial cells adhered to stainless steel surfaces. J Food Prot. 75:701–705.
- Basavegowda N, Lee YR. 2013. Synthesis of silver nanoparticles using Satsuma mandarin (Citrus unshiu) peel extract: a novel approach towards waste utilization. Mater Lett. 109:31–33.
- Bush K. 2010. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr Opin Microbiol. 13:558–564.
- Cao HL, Liu XY. 2010. Silver nanoparticles-modified films versus biomedical device-associated infections. Wires Nanomed Nanobi. 2:670–684.
- Dai Y, Li NN, Zhao Q, Xie SG. 2015. Bioremediation using Novosphingobium strain DY4 for 2,4-dichlorophenoxyacetic acid-contaminated soil and impact on microbial community structure. Biodegradation. 26:161–170.
- Eustis S, el-Sayed MA. 2006. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 35:209–217.
- Fujii K, Satomi M, Morita N, Motomura T, Tanaka T, Kikuchi S. 2003. Novosphingobium tardaugens sp. nov., an oestradiol-degrading bacterium isolated from activated sludge of a sewage treatment plant in Tokyo. Int J Syst Evol Microbiol. 53:47–52.
- Gong P, Li HM, He XX, Wang KM, Hu JB, Tan WH, et al. 2007. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology. 18:285604.
- Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, et al. 2012. Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 62:716–721.
- Kouvaris P, Delimitis A, Zaspalis V, Papadopoulos D, Tsipas SA, Michailidis N. 2012. Green synthesis and characterization of silver nanoparticles produced using Arbutus unedo leaf extract. Mater Lett. 76:18–20.
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis. 6:589–601.
- Ling LL, Schneider T, Peoples AJ, Spoering AL, Engels I, Conlon BP, et al. 2015. A new antibiotic kills pathogens without detectable resistance. Nature. 517:455–459.
- Loo SL, Krantz WB, Fane AG, Hu X, Lim TT. 2015. Effect of synthesis routes on the properties and bactericidal activity of cryogels incorporated with silver nanoparticles. RSC Adv. 5:44626–44635.
- Magudapathy P, Gangopadhyay P, Panigrahi BK, Nair KGM, Dhara S. 2001. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Physica B. 299:142–146.
- Mata R, Reddy Nakkala J, Rani Sadras S. 2015. Catalytic and biological activities of green silver nanoparticles synthesized from Plumeria alba (frangipani) flower extract. Mater Sci Eng C Mater Biol Appl. 51:216–225.
- Mohanpuria P, Rana NK, Yadav SK. 2008. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 10:507–517.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, et al. 2005. The bactericidal effect of silver nanoparticles. Nanotechnology. 16:2346–2353.
- Naqvi SZH, Kiran U, Ali MI, Jamal A, Hameed A, Ahmed S, et al. 2013. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomed. 8:3187–3195.
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. 2007. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 6:29–40.
- Saitou N, Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4:406–425.
- Sohn JH, Kwon KK, Kang JH, Jung HB, Kim SJ. 2004. Novosphingobium pentaromativorans sp. nov., a high-molecular-mass polycyclic aromatic hydrocarbon-degrading bacterium isolated from estuarine sediment. Int J Syst Evol Microbiol. 54:1483–1487.
- Sulaiman GM, Mohammed WH, Marzoog TR, Al-Amiery AA, Kadhum AA, Mohamad AB. 2013. Green synthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Eucalyptus chapmaniana leaves extract. Asian Pac J Trop Biomed. 3:58–63.
- Suzuki S, Hiraishi A. 2007. Novosphingobium naphthalenivorans sp. nov., a naphthalene-degrading bacterium isolated from polychlorinated-dioxin-contaminated environments. J Gen Appl Microbiol. 53:221–228.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 30:2725–2729.
- Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 1991. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 173:697–703.
- Yuan J, Lai Q, Zheng T, Shao Z. 2009. Novosphingobium indicum sp. nov., a polycyclic aromatic hydrocarbon-degrading bacterium isolated from a deep-sea environment. Int J Syst Evol Microbiol. 59:2084–2088.