Abstract
Snail-1 known as one of the important transcription factor is a mediator of survival and cell migration, and expression is raised in numerous cancer types. Snail-1 gene may show a role in recurrence of several cancers including bladder cancer by down-regulating E-cadherin, inducing an epithelial to mesenchymal transition (EMT) and its related microRNAs (miRNAs). The aim of this study was to investigate the effect of a specific Snail-1 siRNA on apoptosis and alter EMT related miRNAs of EJ-138 (bladder cancer) cells. The cells were transfected with siRNAs using transfection reagent. The cytotoxic effects of Snail-1 siRNA, on bladder cancer cells were determined using MTT assay. Relative Snail-1 mRNA levels were measured by QRT- PCR, respectively. Apoptosis was measured by TUNEL test based on labeling of DNA strand breaks. We also evaluated miR-29b, miR-21, and miR-203 expression by QRT-PCR to determine alteration in miRNAs expression involved in EMT. Snail-1 siRNA significantly reduced mRNA expression levels in 48 h after transfection at the concentration of 60 pmol in bladder cancer cells. We also showed that the silencing of Snail-1 led to the induction of apoptosis. miR-21 and miR-29b depression have been shown in Snail-1 suppressed group in EJ-138 cells in vitro. These results propose that Snail-1 might play an important role in the progression of bladder cancer, and be a potential therapeutic target for trigger apoptosis and suppression of EMT-related miRNAs in bladder cancer.
Introduction
Bladder cancer is the most regularly detected urologic cancer (Sartini et al. Citation2013). The incidence of bladder cancer ranks fourth among the cancer types in men, and bladder cancer is one of the main causes of cancer-related deaths in United States (Jemal et al. Citation2009). The number of bladder cancer patients in China has been progressively increasing over current years (Shao et al. Citation2007). These results suggest for more attention on bladder cancer studies.
Snail factor is a copy of Zinc finger family that plays a role in creation of invasive phenotype in cancer, differentiation of neural cells, cell division, and tumor cells apoptosis. The footprint of this factor has been observed in metastatic breast cancer to the bone (Yun et al. Citation2011). Snail causes inhibition in protein expression corresponsive to epithelial cells as E-cadherin’s (Baritaki et al. Citation2007, Baritaki et al. Citation2009, Jordà et al. Citation2007).
RNA interference (RNAi) is a mechanism for gene silencing. Such mechanism possesses uncanny ability in targeting cancer-related genes (Kachalaki et al. Citation2015, Mansoori et al. Citation2014a, Mansoori et al. Citation2016, Montazami et al. Citation2015). A majority of gene products involved in tumor genesis have recently been utilized as targets in RNAi-based therapy. The evidence from these studies indicates that RNAi application for targeting functional carcinogenic molecules, tumor resistance to chemotherapy and radiotherapy is required in today’s cancer treatment (Mansoori et al. Citation2014b).
According to the previous studies about Snail-1, Snail-1 can regulate the expression of several genes including E-cadherin and vimentin and miRNAs, miR-21 and miR-29b which interfere in epithelial mesenchymal transition (EMT) process. These results indicate that Snail-1 may be an important regulator during the invasion and metastasis of tumor (Li et al. Citation2015, Luwor et al. Citation2013, Olmeda et al. Citation2007a, Sugimachi et al. Citation2003, Sun et al. Citation2012, Zhang et al. Citation2008). So Snail can play a role in epithelial cancers metastasis.
In this study, we determined Snail-1 mRNA expression in bladder cancer cell line (EJ-138) and analyzed the association of Snail-1 with apoptosis and invasive potential in breast cancer cell in vitro.
Materials and methods
Reagents
Cell culture products and Thiazolyl Blue Tetrazolium Bromide (MTT) powder were purchased from Sigma-Aldrich (St. Louis, MO). Snail-1 specific small interfering RNA (siRNA), siRNA transfection reagent, and transfection media were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). SYBR Premix Ex Taq was purchased from Takara BIO (Otsu, Shiga, Japan). In Situ Cell Death Detection Kit and Taq DNA polymerase were purchased from Roche Diagnostics (Risch-Rotkreuz, Switzerland). MMLV reverse transcriptase was purchased from Thermo scientific (Waltham, MA). RNX-PLUS, primer, and DEPC were purchased from CinnaGen (Tehran, Iran). dNTP and buffer PCR were purchased from Fermentas (Helsinki, Finland). miRCURY LNA™ Universal RT microRNA PCR kit and miRCURY™ RNA Isolation kits were purchased from Exiqon (Vedbæk, Denmark).
Cell lines and culture conditions
Ej-138 bladder cancer cell line was purchased from the Pasteur institute, Tehran, Iran, and was maintained in RPMI-1640 medium supplemented with 10% FBS and antibiotics (100 unit/ml penicillin and 100 μg/ml streptomycin). Cell lines were cultured at 37 °C in a humidified atmosphere containing 5% CO2. The culture medium was changed every other day and the cells were passaged when they reached 80–90% confluency.
Transfection of siRNA
Snail-1-specific siRNA includes of pooled three different siRNA duplexes sequences (). Cells were transfected with siRNA and transfection reagent according to the manufacturer’s instructions. Briefly, cells were seeded in a 6-well-plate at a density of 1 × 106 cells/well with antibiotics-free medium 40 min before the transfection. Then 6 μl of the siRNA concentrations was mixed with 6 μl transfection reagent in 200 μl optimal medium and were incubated at room temperature for 30 min to form a complex. After washing cells with PBS, the 212 μl transfection mixtures were added to each well with 800 μl optimal medium at a different concentration of siRNA (40–80 pmol). Six hours after the transfection, the medium was replaced with the fresh 1 ml RPMI-1640 medium containing 20% FBS. At 24, 48, and 72 h after the transfection, cells were collected for RNA isolation.
Table 1. Snail-1 siRNA sequences.
Real-time RT-PCR
Total RNA was isolated from cells using RNX -PLUS reagent according to the manufacturer’s protocol. One microliter total RNA (5 ng) was converted to cDNA using 1 μl random hexamer primer, 1 μl MMLV (Moloney Murine Leukemia Virus 200 units), 4 μl 5X reaction buffer and 2 μl of 10 mM dNTP. Then, 500 ng of cDNA were amplified by real-time PCR using SYBR Green-1 dye universal master mix on a Rotor – Gene TIM 6000 (Corbett Life Science, Mortlake, NSW, Australia) Sequence Detection System. To confirm the PCR specificity, PCR products were subjected to a melting-curve analysis. The comparative threshold method was used to calculate the relative amount of mRNA of treated sample in comparison with control samples ().
Table 2. The primers sequences.
miRNA isolation was performed by using Exiqon chromatography columns according to the manufacturer’s protocol. Eight microliters of Trizol extracted RNA, in 20 μl of total volume, was subjected to reverse transcription with miRCURY LNA™ Universal cDNA synthesis kit (Exiqon, Vedbæk, Denmark), and incubated for 60 min at 42 °C followed by enzyme heat-inactivation for 5 min at 95 °C. Quantitative RT-PCR was carried out for miR-21 and miR-29b in total volume of 20 μl reaction mixture using miRCURY LNA™ Universal RT microRNA PCR, SYBR Green master mix (Exiqon, Vedbæk, Denmark) according to the manufacturer’s protocol. Amplification was performed as follows: 95 °C for 10 min, 40 cycles of 95 °C for 10 s and 60 °C for 10 s, ramp rate 100% under standard condition. U6 snRNA was used as references.
Cytotoxicity assay
In this study, cytotoxicity of the treatments was assessed using MTT assay kit. Briefly, the cells were treated with the agents as described above and incubated in humidified CO2 incubator. Following on 100 μl of MTT reagent (0.5 mg/ml in PBS) was added to each well and then the plates were returned in the incubator for 4 h. The water insoluble formazan crystals were formed during incubation period that solubilized by adding 100 μl of the solubilization (DMSO + Sorensen’s buffer) to each well. After 30 min incubation in above-mentioned conditions, the absorbance of the solubilized formazan dyes was measured using an ELISA plate reader.
Detection of DNA strand breaks by the terminal deoxynucleotidyl transferase nick end labeling (TUNEL) assay
To determine the induction of apoptosis after Snail-1 suppression on the EJ-138 cells, TUNEL assay was used. Cells were cultured at a density of 15 × 10³ cell/well in 96-well cell culture plates and then transfected with 60 pmol siRNAs. After 48 h, the cells were fixed with a freshly prepared fixation solution (in 4% paraformaldehyde in PBS, in pH: 7.4), for 1 h at 15–25 °C, then the slides were rinsed with PBS. After that, slides were incubated with blocking solution (3% H2O2 in methanol) for 10 min at 15–25 °C, then rinsed slides with PBS and incubated in permeabilization solution (0.1% Triton X-100 in 0.1% sodium citrate, freshly prepared) for 2 min on ice (2–8 °C). Then, slides were rinsed twice with PBS and dried area around sample. After that, 50 μl TUNEL reaction mixture was added on sample, then added lid and incubated for 1 h at 37 °C in a humidified atmosphere in the dark. Then the slides were rinsed three times with PBS. Samples were analyzed in drop of PBS under light microscope.
Statistical analysis
Data were presented as mean ± standard deviation (SD). t Test and analysis of variance (ANOVA) followed by Dennett’s test were used to determine the significant differences between groups. Values of P less than 0.05 were considered significant. All statistical analyses performed using GraphPad prism 6.01 software (GraphPad Software Inc., San Diego, CA).
Results
Specific siRNAs down regulated Snail-1 mRNA expression in bladder cancer cells
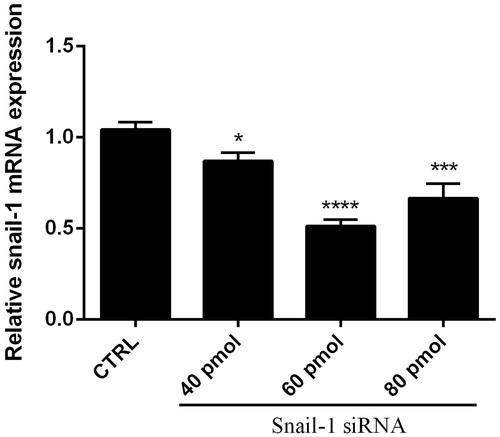
We examined the effect of siRNA on mRNA expression in tumor cells. The cells were transfected with siRNA and relative gene expressions were determined using qRT-PCR. The quantitative data from each sample was normalized against β-actin and relative gene expression calculated in relation to control (untreated cells), which was considered as 100%. Results showed that, treatment with specific siRNA markedly reduced Snail-1 mRNA levels (P < 0.05; and ) compared with the control. At 24, 48, and 72 h post transfection, the relative expression of Snail-1 was 83.33, 56, and 72.67%, respectively, while relative expression of different concentration of Snail-1 siRNA on Snail-1 mRNA was 87, 51, and 66%, respectively (P < 0.05).
Figure 1. Knockdown of Snail-1 by siRNA in EJ-138 cells. Cells were transfected with 60 pmol of siRNA as described in methods. At 24, 48, and 72 h post transfection, total RNA was extracted and mRNA levels were examined by qRT-PCR. Relative mRNA expression levels were quantified by the qRT-PCR method, using β-actin as an internal control. The data represent mean ± SD (n = 3); *P < 0.05, ***P < 0.001, ****P < 0.0001 versus control (CTRL).
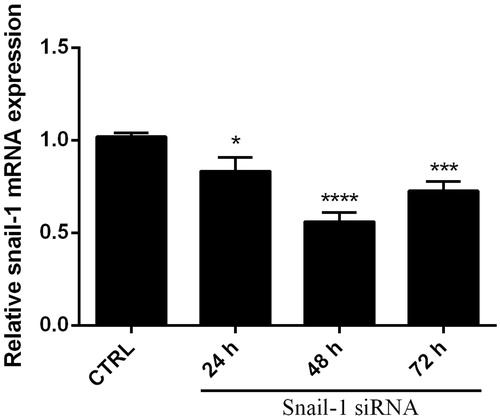
Figure 2. Knockdown of Snail-1 by siRNA in Ej-138 cells. Cells were transfected with 40, 60, and 80 pmol of siRNA as described in methods. At 48 h post transfection, total RNA was extracted and mRNA levels were examined by qRT-PCR. Relative mRNA expression levels were quantified by the qRT-PCR method, using β-actin as an internal control. The data represent mean ± SD (n = 3); *P < 0.05, ***P < 0.001, ****P < 0.0001 versus control (CTRL).
The expression levels of β-actin mRNA (served as an internal control for the qRT-PCR) were also similar in all groups (P > 0.05). Notably, treatment with NC siRNA (negative control) has minimal effect on mRNA levels compared with the control (P > 0.05). These data indicated that Snail-1 specific siRNAs could effectively suppress Snail-1 mRNA expression in bladder cancer cells, without nonspecific effects on the β-actin mRNA expression. PCR products were performed at the end of PCR reaction to confirm a single product is amplified and that no dimers interfere with the reaction.
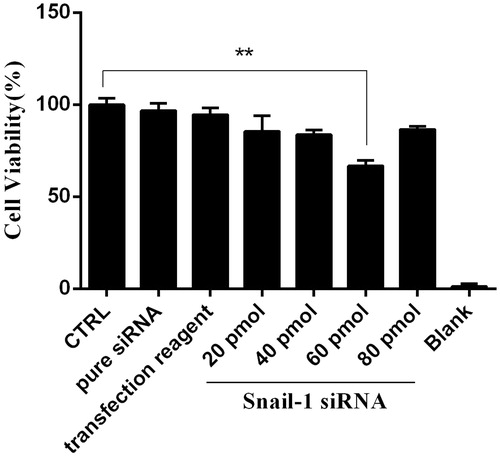
Snail-1 suppression caused cytotoxic effect on Ej-138 cells by dose dependent manner
The effect of Snail-1 down regulation on Ej-138 cells was also investigated. As shown in , monotreatment with Snail-1 specific siRNA induced cytotoxicity. The results of MTT assay showed that 60 pmol Snail-1 siRNA group significantly decreased the cell survival rate, compared with control group (P < 0.01).
Knockdown of Snail-1 induced apoptosis in bladder cancer cells
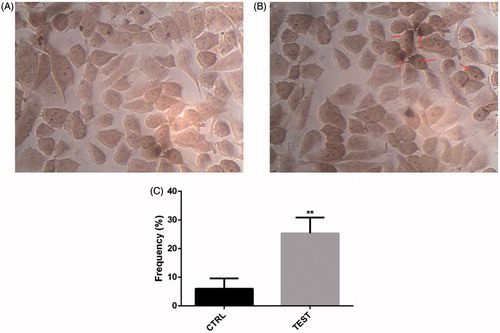
To analyze observed sensitizing effect of Snail-1 siRNA was linked to the enhancement of apoptosis. When the Snail-1 siRNA was transfected into Ej-138 cells, the number of surviving cells was decreased at 48 h after transfection and at the dose of 60 pmol Snail-1 siRNA compared with the group control (). The amount of this decrease was impressive ().
Figure 4. siRNA-mediated targeting of Snail-1 strongly sensitized Ej-138 cells by stimulation of apoptosis. (A) Untreated siRNA (control) with the In Situ Cell Death Detection Kit, POD. (B) Treated 60 pmol Snail-1 siRNA (test) with the In Situ Cell Death Detection Kit, POD. (C) Percentage of TUNEL-positive cells in control and test group. The results are expressed as mean ± SD (n = 3); **P < 0.001 versus control.
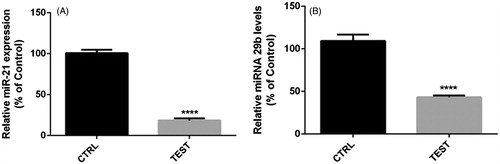
Down-regulation of Snail-1 by RNAi, reduced miR-21, and miR-29b expression
To investigate the expression of metastatic related microRNA (miRNA) with Snail-1, we performed the qRT-PCR test (). The miRNA expression fold of miR-21 and miR-29b prominently were decreased following siRNA knockdown of Snail-1 gene as shown in . At 48 h post transfection, the relative expression of miR-21 and miR-29b was 18.46 and 43%, respectively, () (P < 0.05).
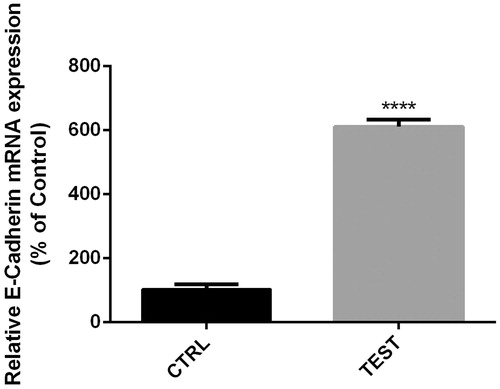
Snail-1 suppression caused E-cadherin up regulation in bladder cancer cells
E-cadherin was linked to invasion and metastasis by reducing EMT. We prompted to find its expression level in Ej-138 cell line and also their relation with Snail-1 knockdown. The mRNA level expression was attenuated after siRNA knockdown as tested by RT-PCR. At 48 h post transfection, the relative expression of E-cadherin was 611.15% () (P < 0.05).
Discussion
One appealing target for the development of pharmaceutical agents is Snail. Blocking Snail function potentially can prevent tumor cell metastasis by interfering with processes such as EMT, invasion and metastasis. Besides, suggests that Snail inhibitors could prevent tumor recurrence. The expression of Snail which is a critical regulator of multiple signaling pathways leading to EMT is closely related to the cancer metastasis. The requirement for Snail for lymph node metastasis of human breast carcinoma has been demonstrated (Olmeda et al. Citation2007b). Also, the elevation of level of Snail expression in metastatic lesions in ovarian cancer has been observed (Elloul et al. Citation2005). According to a recent study, metastasis is accelerated through induction of immunosuppression by snail-induced EMT. Tumor growth and metastasis is significantly inhibited through knock downing of Snail (Elloul et al. Citation2005). So, Snail is an effective target for preventing metastasis.
Accumulated evidence demonstrates that Snail presents a broad spectrum of biological functions. Therefore, Snail advances the bladder cancer cells metastasis and overexpression of Snail is a biomarker of poor clinical outcome for patients with bladder cancer.
The effects of expression and silencing of the Snail-1 transcription factor on certain functional properties of EJ-138 cells was demonstrated by the results of this study. The data indicated that the silencing of Snail-1 in EJ-138 cell line reduced the Snail-1; miR-21 and miR-29b expression levels in contrast the expression of E-cadherin. It reduced survival significantly, and significant increase of apoptosis.
Some studies have been conducted on several types of cancers, but there are not many studies about Snail-1 in bladder cancer and its knockdown. Most studies were about Snail-1 gene suppression but there were not enough studies about silencing effects on apoptosis and micro RNA and gene regulation. The study indicates that, Snail-1 knockdown-increased apoptosis in cancer cells has a significant importance because apoptosis is one of the ways to fight against cancer cells. However, our results are similar to those of others (Borrás et al. Citation2008, Sahranavard et al. Citation2010, Valdivieso et al. Citation2012).
It has been shown that Snail-1 knockdown causes a significant decrease in metastasis related miRNA including miR-21 and miR-29b expression. As we know, metastasis in cancer, especially in bladder cancer has a significant importance because it causes a lot of deaths, so metastasis significant decline is an important factor. Silencing of Snail-1 in LNCaP and PC3 cells led to a MET-like process, increasing epithelial characteristics and decreasing tumor cell migration and invasion (Borrás et al. Citation2008, Ma and Jemal Citation2013, Winer et al. Citation2009).
Bastian Keck et al. showed that Snail overexpression also was increased in bladder cancer cells compared to non-transduced cells (Vincent et al. Citation2009). Lundgren et al. indicated that knocking down of Snail resulted in significant reduction of the migratory capacity of metastatic cells including MDA-MB-468 and MCF-7 (Baritaki et al. Citation2009). Also, the MTT assay has showed the cytotoxicity caused through gene silencing and the results showed Snail-1 may play critical role in cancerous cells survival.
Expression of the Snail-1 gene was observed in EJ-138 bladder cancer cells and this expression was suppressed by Snail-1 siRNA. The knockdown of the Snail-1 gene resulted in a significant decreased in EJ-138 cell survival. Furthermore, apoptosis was induced through suppression of the Snail-1 gene in EJ-138 cells. Collectively, these results show that the Snail-1 gene has a positive effect on bladder cancer cell survival, metastasis, and apoptosis by regulating miR-21, miR-29b, and E-cadherin. Therefore, siRNA mediated knockdown of the Snail-1 gene could be considered as a novel therapeutic tool for bladder cancer treatment.
Disclosure statement
The authors declare no conflict of interest.
References
- Baritaki S, Chapman A, Yeung K, Spandidos D, Palladino M, Bonavida B. 2009. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene. 28:3573–3585.
- Baritaki S, Huerta-Yepez S, Sakai T, Spandidos DA, Bonavida B. 2007. Chemotherapeutic drugs sensitize cancer cells to TRAIL-mediated apoptosis: up-regulation of DR5 and inhibition of Yin Yang 1. Mol Cancer Therap. 6:1387–1399.
- Borrás JM, Pareja L, Peris M, Espinàs JA. 2008. Análisis de la incidencia, la supervivencia y la mortalidad según las principales localizaciones tumorales, 1985-2019: cáncer colorrectal. Med Clín. 131:58–62.
- Elloul S, Bukholt Elstrand M, Nesland JM, Tropé CG, Kvalheim G, Goldberg I, Reich R, Davidson B. 2005. Snail, Slug, and Smad‐interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 103:1631–1643.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. 2009. Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
- Jordà M, Vinyals A, Marazuela A, Cubillo E, Olmeda D, Valero E, Cano A, Fabra À. 2007. Id-1 is induced in MDCK epithelial cells by activated Erk/MAPK pathway in response to expression of the Snail and E47 transcription factors. Exp Cell Res. 313:2389–2403.
- Kachalaki S, Baradaran B, Majidi J, Yousefi M, Shanehbandi D, Mohammadinejad S, Mansoori B. 2015. Reversal of chemoresistance with small interference RNA (siRNA) in etoposide resistant acute myeloid leukemia cells (HL-60). Biomed Pharmacother. 75:100–104.
- Li H, Chen X, Gao Y, Wu J, Zeng F, Song F. 2015. XBP1 induces snail expression to promote epithelial- to-mesenchymal transition and invasion of breast cancer cells. Cell Signal. 27:82–89.
- Luwor R, Baradaran B, Taylor L, Iaria J, Nheu T, Amiry N, et al. 2013. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene. 32:2433–2441.
- Ma J, Jemal A. 2013. Breast cancer statistics. Breast Cancer Metastasis and Drug Resistance. New York: Springer.
- Mansoori B, Mohammadi A, Goldar S, Shanehbandi D, Mohammadnejad L, Baghbani E, et al. 2016. Silencing of high mobility group isoform IC (HMGI-C) enhances paclitaxel chemosensitivity in breast adenocarcinoma cells (MDA-MB-468). Adv Pharm Bull.
- Mansoori B, Mohammadi A, Shirjang S, Baradaran B. 2014a. Micro-RNAs: the new potential biomarkers in cancer diagnosis, prognosis and cancer therapy. Cell Mol Biol (Noisy-le-Grand). 61:1–10.
- Mansoori B, Shotorbani SS, Baradaran B. 2014b. RNA interference and its role in cancer therapy. Adv Pharm Bull. 4:313–321.
- Montazami N, Kheirandish M, Majidi J, Yousefi M, Yousefi B, Mohamadnejad L, et al. 2015. siRNA-mediated silencing of MDR1 reverses the resistance to oxaliplatin in SW480/OxR colon cancer cells. Cell Mol Biol (Noisy-le-Grand). 61:98.
- Olmeda D, Jorda M, Peinado H, Fabra A, Cano A. 2007a. Snail silencing effectively suppresses tumour growth and invasiveness. Oncogene. 26:1862–1874.
- Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, PortillO F, Cano A. 2007b. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 67:11721–11731.
- Sahranavard S, Naghibi F, Mosaddegh M, Esmaeili S, Sarkhail P, Taghvaei M, Ghafari S. 2010. Cytotoxic activities of selected medicinal plants from Iran and phytochemical evaluation of the most potent extract. Res Pharm Sci. 4:133–137.
- Sartini D, Muzzonigro G, Milanese G, Pozzi V, Vici A, Morganti S, et al. 2013. Upregulation of tissue and urinary nicotinamide N-methyltransferase in bladder cancer: potential for the development of a urine-based diagnostic test. Cell Biochem Biophys. 65:473–483.
- Shao J, Gu M, Xu Z, Hu Q, Qian L. 2007. Polymorphisms of the DNA gene XPD and risk of bladder cancer in a Southeastern Chinese population. Cancer Genet Cytogenet. 177:30–36.
- Sugimachi K, Tanaka S, Kameyama T, Taguchi KI, Aishima SI, Shimada M, Sugimachi K, Tsuneyoshi M. 2003. Transcriptional repressor snail and progression of human hepatocellular carcinoma. Clin Cancer Res. 9:2657–2664.
- Sun M, Guo X, Qian X, Wang H, Yang C, Brinkman KL, et al. 2012. Activation of the ATM-Snail pathway promotes breast cancer metastasis. J Mol Cell Biol. 4:304–315.
- Valdivieso M, Kujawa AM, Jones T, Baker LH. 2012. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci. 9:163–173.
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, et al. 2009. A SNAIL1–SMAD3/4 transcriptional repressor complex promotes TGF-β mediated epithelial–mesenchymal transition. Nat Cell Biol. 11:943–950.
- Winer E, Gralow J, Diller L, Karlan B, Loehrer P, Pierce L, et al. 2009. Clinical cancer advances 2008: major research advances in cancer treatment, prevention, and screening—a report from the American Society of Clinical Oncology. J Clin Oncol. 27:812–826.
- Yun J, Frankenberger CA, Kuo WL, Boelens MC, Eves EM, Cheng N, et al. 2011. Signalling pathway for RKIP and Let-7 regulates and predicts metastatic breast cancer. EMBO J. 30:4500–4514.
- Zhang A, Chen G, Meng L, Wang Q, Hu W, Xi L, et al. 2008. Antisense-Snail transfer inhibits tumor metastasis by inducing E-cadherin expression. Anticancer Res. 28:621–628.