?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The separation and purification methods are extremely important for the hemoglobin (Hb) which is a crucial biomolecule. The adsorption technique is popular among these methods and the cryogels have been used quite much due to their macropores and interconnected flow channels. In this study, the Hb adsorption onto the Cu(II) immobilized poly(2-hydroxyethyl methacrylate-glycidyl methacrylate), poly(HEMA-GMA)-Cu(II), cryogels was investigated under different conditions (pH, interaction time, initial Hb concentration, temperature and ionic strength) to optimize adsorption conditions. The swelling test, Fourier transform infrared (FT-IR) spectroscopy, scanning electron microscope (SEM), surface area (BET), elemental and ICP-OES analysis were performed for the characterization of cryogels. Polyethyleneimine (PEI) molecule was used as a Cu(II)-chelating ligand. The Hb adsorption capacity of cryogels was determined as 193.8 mg Hb/g cryogel. The isolation of Hb from human blood was also studied under optimum adsorption conditions determined and the Hb (124.5 mg/g cryogel) was isolated. The adsorption model was investigated in the light of Langmuir and Freundlich adsorption isotherm models and it was determined to be more appropriate to the Langmuir adsorption isotherm model.
Introduction
The lack of biomolecules’ purity during selective separation from mixtures is one of the important problems encountered in biotechnological and biomedical fields. The various methods such as ultra-filtration, electrophoretic separation, liquid chromatography, membrane chromatography and adsorption have been developed to remove the proteins today (El-Safty et al. Citation2011). The adsorption method is considered to be the most effective technique for the selective separation of proteins among these methods (Anirudhan et al. Citation2011, Köse and Uzun Citation2016).
The hemoglobin (Hb) located in red blood cells is one of the most abundant proteins for the human and animals. Hb is an iron-containing biomolecule carrying oxygen through 2α and 2β globin chains, exactly the same with each other, in the internal structure. The hereditary diseases such as sickle cell, hemoglobinopathies, thalassemia rupture occur with hemolysis of Hb found in high concentrations in the blood vessels (Lezcano et al. Citation2006, Pourreza and Golmohammadi Citation2015, Sun et al. Citation2016).
In the literature, the techniques such as molecular recognition, ion exchange chromatography, immobilized metal affinity chromatography (IMAC) have been used for the selective isolation of the Hb, abundant in the blood, with the help of nanoparticles, membranes, polymeric spheres and gels utilized in these methods (Altıntaş et al. Citation2011, Bhattacharya et al. Citation2007, Derazshamshir et al. Citation2010, Dimino and Palmer Citation2007, Liu et al. Citation2016, Ma et al. Citation2005a, Ma et al. Citation2005b, Ma et al. Citation2006, Ringrose et al. Citation2008, Williams et al. Citation2010, Zhan et al. Citation2016, Zhang et al. Citation2010, Zhang et al. Citation2011).
IMAC is a technique developed first in 1975 by Porath et al. (Citation1975). In this technique, the metal ions are immobilized on the solid support through chelating ligand and the target molecule can bind to metal ions. Ligands play a critical role in this technique. The iminodiacetic acid (IDA) and nitrilotriacetic acid (NTA) are the chelating compounds used most widely (Sun et al. Citation2015). The divalent ions such as Zn(II), Cu(II), Co(II) and Ni(II) are used as transition metals. The performance of the IMAC technique is directly related with the type of chelating compound (Karakuş et al. Citation2015).
The polyethyleneimine (PEI) is a polyamine comprising a variety of secondary amine groups in the macromolecular backbone (Jin and Yuan Citation2005, Matsukizono et al. Citation2009). Despite the insolubility in an aqueous medium at room temperature, it can dissolve in hot water (60 °C and above) and crystallized in cold environments (Yao et al. Citation2016).
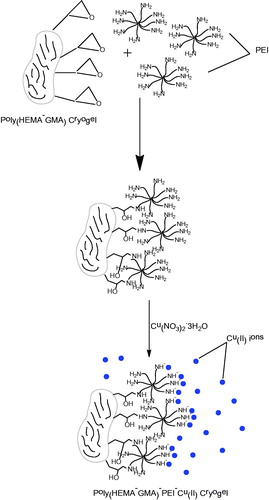
PEI is very suitable for IMAC technique as a chelating agent due to a variety of amine groups it contains, and it has been immobilized as a ligand on the poly(2-hydroxyethyl methacrylate-glycidyl methacrylate), poly(HEMA-GMA), cryogels synthesized within the scope of this study. The metal ion to be immobilized onto the structure was chosen as Cu(II). First, the optimum adsorption conditions (optimum pH, interaction time, initial concentration, etc.) to be applied for the Hb adsorption from blood were determined in aqueous solution containing Hb standard and the Hb adsorption was applied under those conditions for blood sample obtained from a volunteer.
Materials and methods
Materials
2-Hydroxyethyl methacrylate (HEMA), glycidyl methacrylate (GMA), ethylene glycol dimethacrylate (EGDMA), copper (II) nitrate trihydrate, ammonium persulfate (APS), sodium dodecyl sulphate (SDS), N,N,N′,N′-Tetramethylethylenediamine (TEMED), polyethyleneimine (average Mw ∼25.000 by LS, average Mn ∼10.000 by GPC, branched) and Hb were obtained from the company Sigma (St. Louis, MO). All other chemicals were of an analytical purity and ultra-pure water (18 mω.cm) was used in all the studies.
Methods
The synthesis of poly(HEMA-GMA) cryogels
The GMA (500 μL), HEMA (5000 μL) and distilled water (6500 μL) were mixed to obtain monomer phase. The disperse phase was prepared by mixing sodium dodecyl sulphate (SDS) (1 g), distilled water (25.60 mL) and EGDMA (2.4 mL). After mixing these two phases, the resultant solution was cooled in an ice bath for 10–15 min. The polymerization reaction was started with the addition of APS (20 mg) and TEMED (100 μL) and continued at −20 °C for 24 h.
The resulting cryogels were cut in the shape of the membrane (disc). To get rid of sodium dodecyl sulphate and other components, all cryogels were stirred in distilled water via rotator (Multi Bio RS-24 Biosen, Riga, Latvia) at 10 rpm until the all unwanted particles were removed.
PEI and cu(II) binding on poly(HEMA-GMA) cryogels
The cryogels (20 items) were stirred by mixing NaOH (1M, 10 mL) for 2 h and washed with distilled water several times. The resulting cryogels were interacted with polyethyleneimine solution of 50 mg/mL (10 mL) for 24 h before mixing with Cu(NO3)2.3H2O of 5 mg/mL (10 mL) for 6 h. The blue cryogels were obtained at the end of Cu(II) immobilization and washed several times with distilled water and ethanol ().
Characterization studies
Swelling test
The water retention capacity of poly(HEMA-GMA)-PEI-Cu(II) cryogels was determined using distilled water. To do this, all cryogels were weighed carefully and then placed in a beaker filled with distilled water in the isothermal water bath and waited for 30 min at 25 °C. As the next stage, membranes were taken and placed over a filter paper and swept to get rid of water clinging to the surface and weighed again to calculate the water retention capacity. The following equation was used to determine the capacity of water retention.
(1)
(1)
where, Wo and Ws are the weights (g) of dry and swelled membrane, respectively.
SEM analysis.
The surface morphology of cryogel membranes was examined using scanning electron microscopy (SEM; FEI/Quanta 450 FEG, Hillsboro, OR). The membrane dried by lyophilization was tailored for SEM analysis and attached with double-sided carbon tape on the SEM holder. The sample was then coated with a thin gold layer under vacuum. At the end, the sample was placed in the device and imaged.
FT-IR analysis.
The Fourier transform infrared (FT-IR, Thermo Scientific Nicolet 6700 FT-IR spectrometer, Waltham, MA) spectrophotometer was used for the determination of the characteristic functional groups of poly(HEMA-GMA) cryogels. Cryogels (about 2 mg) were dried and pulverized primarily and mixed homogeneously with anhydrous potassium bromide powder (KBr) (98 mg, IR grade, Merck, Darmstadt, Germany) to make into pellets and the FT-IR spectrum was obtained in the wave number range of 400–4000 cm−1.
Elemental analysis.
The totally dried sample was weighed carefully in a special elemental analysis (Elementar Vario PYRO cube, Hanau, Germany) container. Approximately, 1–2 mg sample was weighed and then put in sample holder compartment. Then the analysis was started. The N% value was obtained under the condition of the combustion tube at 1120 °C and the reduction tube at 850 °C.
The determination of cu(II) amount immobilized onto the cryogel.
To determine the amount of Cu(II) immobilized onto the poly(HEMA-GMA)–PEI cryogels, the ICP-OES (Spectro Arcos, Kleve, Germany) device was used. In this technique, the sample is excited by argon plasma at 10,000 K by electromagnetic induction and the number of elements are determined according to the specific wavelength emitted by excited elements.
Surface area analysis.
The specific surface area of the membranes was determined using the device Brunauer-Emmett-Teller (BET; Quantachrome Autosorb® iQ-Chemi, Boynton Beach, FL). The cryogel samples dried with lyophilization were remained under vacuum (10 mbar) at 35 °C for 6 h to eliminate oxygen and moisture in the pores. The cryogel samples, then, were treated with nitrogen gas at room temperature.
Adsorption-desorption studies.
The adsorption of Hb onto the poly(HEMA-GMA)-PEI-Cu(II) cryogels have been studied batch wise. The buffer solution of 4 mL (acetate, phosphate or carbonate) and the Hb solution of 1 mL (1000 mg/L) were mixed in a test tube and stirred via rotator for 15 min. Then, prior to adsorption, the sample of 200 μL was taken in order to determine the initial protein concentration and a cryogel disk was placed into the remaining solution and stirred via rotator at 20 rpm for 30 min. The membrane was then removed from the adsorption medium and 2 mL sample was taken from the adsorption medium to determine the concentration of protein remained. The amount of Hb was determined via UV-VIS spectrophotometer (Double Beam PC 8 Auto Cell Scanning UVD-3200 Labomed, INC., Los Angeles, CA) at 280 nm. The Hb adsorption capacity of a cryogel disk was determined according to the following formula.
(2)
(2)
wherein, q is the amount of adsorption (mg/g), Ci and Cf are the concentrations of Hb solution before and after adsorption (mg/L), respectively, V is the volume of the adsorption medium (L) and m is the amount of dry adsorbent (g).
The desorption studies were performed using NaCl solution (1M, 10 mL). In the adsorption-desorption-cycle studies were made to determine the reusability of cryogels, NaOH solution (50 mM, 10 mL) was used for the regeneration of the cryogel used in the desorption process.
The Hb adsorption performance of cryogels from human blood.
The batch system was conducted to determine the Hb adsorption performance of cryogels from a real natural medium; the blood, obtained voluntarily from a blood donor. There was no additive used for the blood preparation before centrifugation (14.000 rpm) process performed at laboratory conditions for 3 min. The normal saline solution was used to get rid of buffy coat from the packed red blood cells (RBCs). In the next step, prior to sedimentation at high speed (12.000 rpm, 40 min), red blood suspension was hemolyzed in ice-cold distilled water. The incubation of the hemolysate solution (10 mL) was achieved with the poly(HEMA-GMA)-PEI-Cu(II) cryogels at 25 °C for 2 h. The photometric method was used to determine the amount of Hb binding. Equation 2 was used to calculate the Hb amount bounded in hemolysate per unit mass of dry cryogel.
Results and discussion
Characterization studies
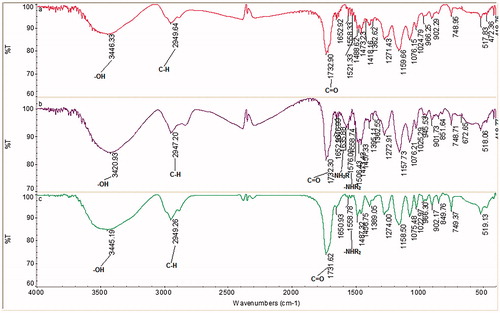
The swelling ratio of cryogels was determined as 683%. Accordingly, 1 g of dry cryogel has a retention capacity of about 6.83 g water. In the case of SEM image analysis of cryogels, the macropores and the interconnected flow channels are seen clearly (). According to the FT-IR spectra of poly(HEMA-GMA) cryogels; the peaks at 3446 cm−1 (alcohol, -OH), 2949 cm−1 (alkane, C–H) and 1732 cm−1 (carboxylic acid, C=O) are observed (). The peaks for poly(HEMA-GMA)–PEI at 3420 cm−1 (alcohol, –OH), 2947 cm−1 (alkane, CH) and 1732 cm−1 (carboxylic acid C=O) and also at 1635 cm−1 primary amine (–NH2R) and secondary amine 1576 cm−1 (–NHR2) peaks are clearly seen (). These results confirm the incorporation of polyethyleneimine into the polymeric structure. Almost same peaks are seen for poly(HEMA-GMA)-PEI-Cu(II) cryogels, by the way, the primary amine peak was not observed (). This result shows the incorporation of Cu(II) ions into the structure and binding to the primary amine groups. According to the elemental analysis results, the nitrogen amount incorporated in the poly(HEMA-GMA)-PEI-Cu(II) cryogels was obtained as 39.5 mg/g polymer. The amount of Cu(II) immobilized onto the poly(HEMA-GMA)-PEI-Cu(II) cryogels with the surface area of 8.372 m2/g is 265.5 μmol/g.
Adsorption-desorption studies
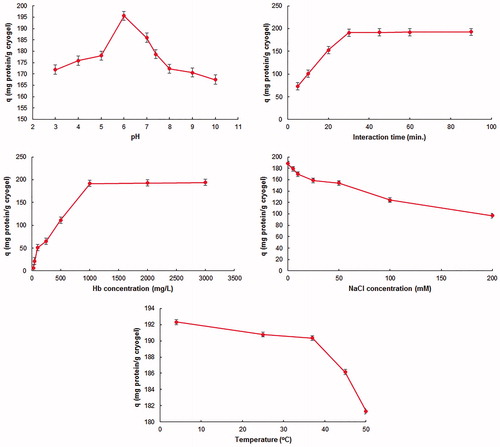
To determine the optimum conditions within the scope of the adsorption studies, the parameters; pH, interaction time, Hb initial concentration, ionic strength, and temperature were studied. In the light of the experiments conducted, the maximum Hb adsorption was observed at pH 6.0 (). The isoelectric point of the Hb is pH: 6.8. The surface of Hb is positively charged at pH values below that value but negatively charged at higher pH values (Anirudhan and Rejeena Citation2013). Therefore, the positively charged groups on the surface of Hb interacts with the negatively charged groups on the adsorbent electrostatically at the pH value of 6.0, and thus the maximum adsorption capacity was achieved at that value. The interaction time of 30 minutes was sufficient to achieve the maximum adsorption capacity (). During this time, the poly(HEMA-GMA)-PEI-Cu(II) cryogel binding sites adsorbing Hb were saturated and thus there was no significant increase observed for longer time from that point. The adsorption capacity was increased with the increasing concentration of Hb used in the adsorption experiment, there was no significant changes observed in the adsorbed amount after the point of the Hb concentration values of 1 mg/mL (). This situation can be explained by the saturation of interaction regions of cryogels achieved in terms of target molecules. The decrease observed on the adsorption capacity with increasing temperature and ionic strength is the indicator of the electrostatic interaction expected between poly (HEMA-GMA)-PEI-Cu(II) cryogels and Hb molecules (). The reason for this is the interaction between positively (Na+) charged ions with Hb and negatively (CI-) charged ions with Cu(II) ions. Thus, these interactions preclude the electrostatic interactions expected between adsorbent and target molecules. The electrostatic interaction was weakened with the rise in the temperature causing partial breakage of the bonds and have the adsorption capacity decrease.
Figure 4. The effect of (a) pH. CHemoglobin: 1 mg/mL; interaction time: 30 min.; temperature: 25 °C. (b) Interaction time. CHemoglobin: 1 mg/mL; pH:6.0; temperature: 25 °C. (c) Initial hemoglobin concentration. pH:6.0; interaction time: 30 min.; temperature: 25 °C. (d) Ionic strength. pH:6.0; CHemoglobin: 1 mg/mL; interaction time: 30 min.; temperature: 25 °C. (e) Temperature. pH:6.0; CHemoglobin: 1 mg/mL; interaction time: 30 min., on the hemoglobin adsorption. All experiments were repeated three times with triplicated measurements while applying 95% confidence interval for calculating mean values reported.
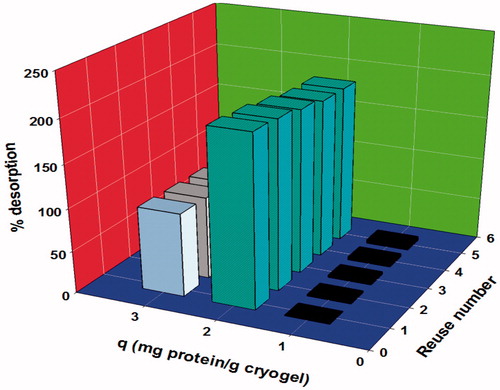
To test the reusability of the poly(HEMA-GMA)-PEI-Cu(II) cryogels, 5 adsorption-desorption cycles were conducted with the same cryogel. At the end of the 5 cycles, there was no significant decrease observed in the adsorption capacity of the cryogel used (from 200.47 to 185.7 mg/g) (). This result confirms the high reusability feature of poly(HEMA-GMA)-PEI-Cu(II) cryogels.
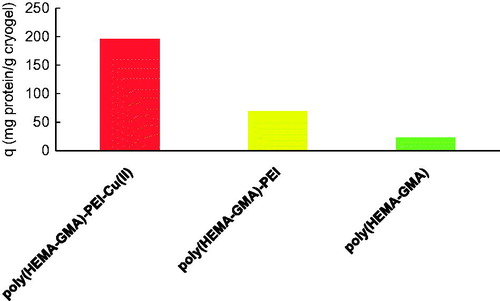
Moreover, the Hb adsorption performances of poly(HEMA-GMA) and poly(HEMA-GMA)-PEI-Cu(II) cryogels were compared under identified optimal conditions (pH: 6.0, the interaction time: 30 min., Hb initial concentration of 1000 mg/L). As seen from the , the Hb adsorption capacities (23.4 mg/g and 69.5 mg/g) of poly(HEMA-GMA) and poly(HEMA-GMA)-PEI cryogels are very low as compared to that (195.6 mg/g) of poly(HEMA-GMA)-PEI-Cu(II) cryogels. Accordingly, the incorporation of PEI and Cu(II) into the structure have the cryogel affinity towards Hb increased significantly.
Hb adsorption performance of cryogels from the real blood hemolysate
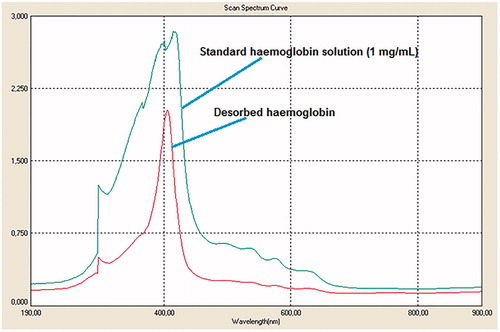
The Hb was adsorbed onto the cryogels in the real human blood hemolysate (Derazshamshir et al. Citation2016, Lu et al. Citation2004). As can be seen from the figure, the peak of the standard Hb solution is almost the same with the solution of Hb desorbed (). This shows the achievement of the desorption process of Hb from the cryogels. The Hb adsorption amount of cryogels was determined as 124.5 mg Hb/g cryogel for the blood sample diluted in 1:3 ratio. The Hb desorption ratio of croygels for real blood sample was found as 48.3%.
The adsorption isotherm models
The adsorption isotherm models were analysed to characterize the nature of the interaction between Hb molecule and poly(HEMA-GMA)-PEI-Cu(II) cryogels. According to the Langmuir adsorption isotherm, the uniform one-layered adsorption phenomena is assumed to happen on the surface (Langmuir Citation1918), whereas the Freundlich adsorption isotherm is not limited to a single layer and there is a heterogeneous interaction (Freundlich Citation1906). The following equations were used to analyse the Langmuir and Freundlich adsorption isotherms.
If linearized,
equation obtained. The y-intercept of the plot, 1/Ceq versus 1/Qeq, is 1/Qmax and the slope is 1/Qmax.b. Wherein, Qeq is the amount (mg/g) of Hb adsorbed, Ceq (mg/L) is the concentration of Hb at equilibrium, b is the Langmuir adsorption constant (L/mg), and Qmax. is the maximum adsorption capacity (mg/g).
wherein, the Kf and n show the Freundlich adsorption isotherm constants. The y-intercept of the plot, lnQeq vs lnCeq, is the Kf and the n value is the slope.
The adsorption and the correlation coefficients of both adsorption isotherms were calculated. According to the experimental results, the correlation coefficient estimated with respect to Langmuir adsorption isotherm model (0.9701) was found higher than that in Freundlich adsorption isotherm model (0.9039). Moreover, the Qmax value calculated according to the Langmuir adsorption isotherm calculations is more consonant with experimental results as compared to that of Freundlich adsorption isotherm model (for Langmuir adsorption isotherm Qmax.= 500.0 mg/g, b = 0.0014 L/mg; for Freundlich adsorption isotherm Kf = 1.44, n = 0.8099, 1/n = 1.24).
Discussion
It was determined that the poly(HEMA-GMA)-PEI-Cu(II) cryogel is a suitable adsorbent for the adsorption of the Hb molecule. It was thought that the interaction takes place between Cu(II) ions in the structure of the cryogels and the charged groups of the Hb molecules. The decrease in the adsorption capacity with increasing temperature and increasing ionic strength confirms the existence of an electrostatic interaction between adsorbent and target molecule. The interaction behavior between the cryogel and Hb fits well to the Langmuir adsorption model. Therefore, according to this model, Hb molecules were adsorbed by poly(HEMA-GMA)-PEI-Cu(II) cryogels in a single layer and homogenously.
Acknowledgements
The author is very grateful to the Assoc. Prof. Dr Dursun Ali Köse, Chemistry Department, Hitit University.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Altıntaş EB, Türkmen D, Karakoç V, Denizli A. 2011. Hemoglobin binding from human blood hemolysate with poly(glycidyl methacrylate) beads. Colloids Surf B Biointerfaces. 85:235–240.
- Anirudhan TS, Rejeena SR. 2013. Selective adsorption of hemoglobin using polymer-grafted-magnetite nanocellulose composite. Carbohydrate Polym. 93:518–527.
- Anirudhan TS, Tharun AR, Rejeena SR. 2011. Investigation on poly(methacrylic acid)-grafted cellulose/bentonite superabsorbent composite: synthesis, characterization, and adsorption characteristics of bovine serum albumin. Indus Eng Chem Res. 50:1866–1874.
- Bhattacharya D, Mukhopadhyay D, Chakrabarti A. 2007. Hemoglobin depletion from red blood cell cytosol reveals new proteins in 2-D gel-based proteomics study. Proteomics Clin Appl. 1:561–564.
- Derazshamshir A, Baydemir G, Andaç M, Say R, Galaev IY, Denizli A. 2010. Molecularly imprinted PHEMA-based cryogel for depletion of hemoglobin from human blood. Macromol Chem Phys. 211:657–668.
- Derazshamshir A, Baydemir G, Yılmaz F, Bereli N, Denizli A. 2016. Preparation of cryogel columns for depletion of hemoglobin from human blood. Artif Cells Nanomed Biotechnol. 44:792–799.
- Dimino ML, Palmer AF. 2007. Purification of bovine hemoglobin via fast performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 856:353–357.
- El-Safty S, Shahat A, Nguyen H. 2011. Nano model memebrane filters for the well controlled separation of biomolecules. Colloids Surfaces A: Physiochem Eng Asp. 377:44–53.
- Freundlich HMF. 1906. Uber die adsorption in losungen. Zeitschrift für Physikalische Chemie. 57:385–471.
- Jin RH, Yuan JJ. 2005. Simple synthesis of hierarchically structured silicas by poly(ethyleneimine) aggregates preorganized by media modulation. Macromol Chem Phys. 206:2160–2170.
- Karakuş C, Uslu M, Yazıcı D, Barik AS. 2015. Evaluation of immobilized metal affinity chromatography kits for thepurification of histidine-tagged recombinant CagA protein. J Chromatography B. 1021:182–187.
- Köse K, Uzun L. 2016. PolyGuanine methacrylate cryogels for ribonucleic acid purification. J Sep Sci. 39:1998–2005.
- Langmuir I. 1918. The adsorption of gas on plane surfaces of glass, mica and platinum. J Am Chem Soc. 40:1361–1370.
- Lezcano NE, Odo N, Kutlar A, Brambilla D, Adams RJ. 2006. Regular transfusion lowers plasma free hemoglobin in children with sickle-cell disease at risk for stroke. Stroke. 37:1424–1426.
- Liu Y, Gong J, Wu W, Fang Y, Wang Q, Gu H. 2016. A novel bio-nanocomposite based on hemoglobin and carboxyl graphene for enhancing the ability of carrying oxygen. Sensors Actuators B. 222:588–597.
- Lu X, Zhao D, Su Z. 2004. Purification of hemoglobin by ion exchange chromatography in flow-through mode with PEG as an escort. Artif Cells Blood Substit Immobil Biotechnol. 32:209–227.
- Ma ZY, Guan YP, Liu HZ. 2005a. Synthesis of monodisperse nonporous crosslinked poly(glycidyl methacrylate) particles with metal affinity ligands for protein adsorption. Polym Int. 54:1502–1507.
- Ma ZY, Guan YP, Liu XQ, Liu HZ. 2005b. Synthesis of magnetic chelator for highcapacity immobilized metal affinity adsorption of protein by cerium initiated graft polymerization. Langmuir. 21:6987–6994.
- Ma ZY, Liu XQ, Guan YP, Liu HZ. 2006. Synthesis of magnetic silica nanospheres with metal ligands and application in affinity separation of proteins. Colloids Surf A. 275:87–91.
- Matsukizono H, Zhu PX, Fukazawa N, Jin RH. 2009. Turbine-like structured silica transcribed simply by pre-structured crystallites of linear poly(ethyleneimine) bounded with metal ions. CrystEngComm. 11:2695–2700.
- Porath J, Carlsson J, Olsson I, Belfrage G. 1975. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 258:598–599.
- Pourreza N, Golmohammadi H. 2015. Hemoglobin detection using curcumin nanoparticles as a colorimetric chemosensor. RSC Adv. 5:1712–1717.
- Ringrose JH, van Solinge WW, Mohammed S, O’Flaherty MC, van Wijk R, Heck AJR, Slijper M. 2008. Highly efficient depletion strategy for the two most abundant erythrocyte soluble proteins improves proteome coverage dramatically. J Proteome Res. 7:3060–3063.
- Sun X, Liu X, Feng J, Li Y, Deng C, Duan G. 2015. Hydrophilic Nb5+-immobilized magnetic core–shell microsphere: a novel immobilized metal ion affinity chromatography material for highly selective enrichment of phosphopeptides. Analytica Chimica Acta. 880:67–76.
- Sun Y, Du H, Lan Y, Wang W, Liang L, Feng C, Yang M. 2016. Preparation of hemoglobin (Hb) imprinted polymer by Hb catalyzed eATRP and its application in biosensor. Biosensors Biosens Bioelectron. 77:894–900.
- Williams LM, Fu ZM, Dulloor P, Yen T, Barron-Casella E, Savage W, et al. 2010. Hemoglobin depletion from plasma: considerations for proteomic discovery in sickle cell disease and other hemolytic processes. Proteomics Clin Appl. 4:926–930.
- Yao DD, Kubosawa H, Souma D, Jin RH. 2016. Shaped crystalline aggregates of comb-like polyethyleneimine for biomimetic synthesis of inorganic silica materials. Polymer. 86:120–128.
- Zhang M, Cheng D, He XW, Chen LX, Zhang YK. 2010. Magnetic silica-coated submicrospheres with immobilized metal ions for the selective removal of bovine hemoglobin from bovine blood. Chem Asian J. 5:1332–1340.
- Zhang M, He XW, Chen LX, Zhang YK. 2011. Preparation and characterization of iminodiacetic acid-functionalized magnetic nanoparticles and its selective removal of bovine hemoglobin. Nanotechnology. 22:065705–065713.
- Zhan T, Wang X, Li X, Song Y, Hou W. 2016. Hemoglobin immobilized in exfoliated Co2Al LDH-graphene nanocomposite film: Direct electrochemistry and electrocatalysis toward trichloroacetic acid. Sensors Actuators B. 228:101–108.