Abstract
This study highlights the facile, reliable, cost effective, and ecofriendly synthesis of silver nanoparticles (AgNPs) using Borago officinalis leaves extract efficiently. The biosynthesis of AgNPs was verified by UV–Vis spectrum which showed the surface plasmon resonance (SPR) band at 422 nm. Transmission electron microscope (TEM) analysis revealed that the particles were spherical, hexagonal, and irregular in shape and had size ranging from 30 to 80 nm. The energy dispersive X-ray spectroscopy (EDX) and elemental mapping have displayed the purity and maximum distribution of silver in the AgNPs. The crystalline nature of AgNPs had been identified using X-ray diffraction (XRD) and selected area diffraction pattern (SAED). The particle size analysis revealed that the Z-average diameter of the AgNPs was 50.86 nm with polydispersity index (PDI) 0.136. Zeta potential analysis displayed the colloidal stability of AgNPs. This work also showed the efficacy of AgNPs against lung cancer cell lines (A549) and cervical cancer cell line (HeLa), in vitro. The AgNPs showed cytotoxicity to the A549 and HeLa cancer cell line at the concentrations 5 and 2 μg/ml. The AgNPs were also explored for the antibacterial activity including biofilm inhibition against pathogenic bacteria. The B. officinalis leaves extract can be used efficiently for green synthesis AgNPs. The biosynthesized AgNPs demonstrated potentials as anticancer and antibacterial agents. This work provides helpful insight into the development of new anticancer and antimicrobial agents.
Introduction
Biosynthesis of nanoparticles as an emerging highlight of the intersection of nanotechnology and biotechnology has received increased attention due to growing need to develop environmentally benign technologies in nanomaterial synthesis (Bhattacharya and Gupta Citation2005). An important and challenging task in nanoparticle synthesis is the development of a simple, rapid, and green method. Various strategies are used for synthesis of nanoparticles. Conventionally, physiochemical techniques underlie the reduction of metal ions, followed by surface modification, the addition of additional capping ligands for stability, and use of organic solvents which raise environmental concerns, because of the toxic compounds used and hazardous by-product (Marimuthu et al. Citation2011). A great deal of effort has been put into the biosynthesis of the metal nanoparticle using microorganisms and plants (Farooqui et al. Citation2010, Mohanpuria et al. Citation2008). Recently, various microorganisms and plant extracts have been reported for the green synthesis of metal nanoparticles in an efficient way (Elavazhagan et al. Citation2011, Singh et al. Citation2016a,Citationb, Citation2015a, Jo et al. Citation2015). The naturally synthesized enzyme, protein, flavonoid, and antioxidant compounds from microorganisms and plant extracts help in reducing and stabilizing the synthesized nanoparticles (Krishnaraj et al. Citation2014, Sriram et al. Citation2012). This green route of synthesis of nanoparticles is the most convenient, facile, ecofriendly way and minimizes the side effects of chemical and physical methods by avoiding the utilization of toxic chemicals and creating hazardous by-products. The green synthesis provides stabilization to the synthesized nanoparticles with no additional capping agent (Raveendran et al. Citation2003). Aspectual, here we report the use of natural and herbal Borago officinalis medicinal leaves for the synthesis of silver nanoparticles (AgNPs).
B. officinalis belongs to the family Boraginaceae and is also known as starflower. The plant is well known for its therapeutic importance to human and reputed as antispasmodic, antihypertensive, antipyretic, aphrodisiac, demulcent, diuretic and is also considered useful to treat asthma, bronchitis, cramps, diarrhea, palpitations, and kidney ailments (Duke et al. Citation2002, Usmanghani et al. Citation1997). A decoction of the plant is used as nerve and cardiac tonic and a home remedy for blood purification (Kybal Citation1980). Reports suggest that Borage oil is of great interest among medical and nutritional research groups due to its high content of γ-linolenic acid and is also known to have antioxidant and reactive oxygen species (ROS) scavenging properties (Bandonien and Murkovic Citation2002, Huang et al. Citation1995). Thus, we explored the plant for application in nanotechnology, for the synthesis of AgNPs in a rapid, facile, and economical way.
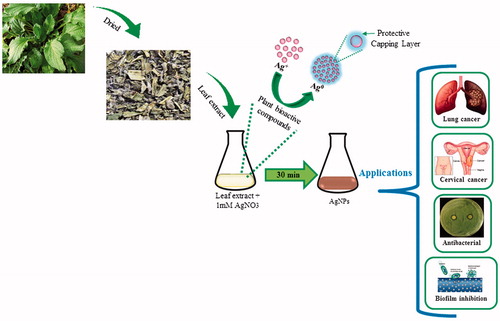
Cancer is an abnormal tissue growth in which the cells exhibit an uncontrolled division, in an autonomous fashion, leading to a progressive increase in the number of the dividing cell (Kanchana and Balakrishna Citation2011). There is increasing demands for anticancer therapy. The discovery and identification of new antitumor drug with low side effects on the immune system have become an essential goal in many studies of immuno-therapies (Xu et al. Citation2009). Despite many efforts, multi drug resistance is still considered as a major drawback in chemotherapy of cancer which has been the subject of exhaustive experiments (Gottesman et al. Citation2002). AgNPs have been proved to have great potential in anticancer activity because they disrupt mitochondrial respiratory chain which leads to the production of ROS and interruption of adenosine triphosphate (ATP) synthesis, thereby causing nucleic acid damage (Husseiny et al. Citation2015). Thus, we are verifying the possible upgrading of cytotoxic action of biosynthesized AgNPs on A549 and HeLa human cancer cell lines relative to normal RAW 264.7 cell line. Further, silver owing to its antimicrobial properties has been applied to treat bacterial infections associated with burns and wounds, for instance, as silver nitrate or silver sulfadiazine. Considering this, this study highlights the antibacterial activity of biosynthesized AgNPs against pathogenic bacteria including Pseudomonas aeruginosa, Escherichia coli, Vibrio parahaemolyticus, and Staphylococcus aureus. In addition, the AgNPs’ potential has tested against biofilm inhibition, as it is a major concern, in different fields such as medical and chemical, where films or membranes are developed to apply in various medical devices like catheter and water filtration membrane (Bjarnsholt et al. Citation2007, Inbakandan et al. Citation2013). shows an overview of this study i.e., from synthesis of AgNPs and its potential application.
Materials
Chemicals
All the media were purchased from Difco, MB cell, Seoul, Korea. All the chemicals were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). B. officinalis (dried leaves) was obtained from Mountain Rose herbs (Eugene, OR). Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), and Pen–Strep (mixture of penicillin and streptomycin) were bought from Gibco BRL (Grand Island, NY).
Cell line and bacteria culture maintenance
Murine macrophage cell lines (RAW 264.7), lung cancer cell lines (A549), and cervical cancer cell line (HeLa) were obtained from the Korean Cell Line Bank (Seoul, South Korea) (KCLB 40071, KCLB 10185, and KCLB 10002). RAW 264.7, A549 and HeLa cell line were cultured in DMEM (supplemented with 10% FBS and 1% Pen-Strep). The cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
The pathogenic bacterial strains P. aeruginosa [KACC 14021], E. coli [CCARM 0237], V. parahaemolyticus [KACC 15069], and S. aureus [KCTC 3881] were obtained from Korean Agricultural Culture Collection (KACC) and Korean Collection for Type Cultures (KCTC). All the bacterial strains were cultured on nutrient agar (NA) media at 28 °C and preserved at −80 °C in glycerol stock vials for further study.
Experimental
Preparation of leaf extract
The dried leaf of B. officinalis was powdered and used to make leaf extract. 10.0 g of leaf powder was extracted with 100 ml deionized water at 100 °C for 30 min. The extract was filtered using a Whatman filter paper and was centrifuged at 10,000 × g for 10 min, to remove any suspended material. The extract was finally filtered through a 0.2 μm PVDF syringe filter (SmartPor, Korea). Further, the filtrate was used to prepare AgNPs.
Biosynthesis of AgNPs
For the synthesis of AgNPs, 10 ml of leaf extract was diluted in 40 ml of sterile deionized water. Aqueous silver nitrate (AgNO3) solutions were added to it, with a final concentration of 1 mM in the reaction mixture. The reaction mixture was kept at 65 °C. Leaves extract and deionized water without AgNO3 solution were used as a control. The dried AgNPs were finally obtained after filtration, centrifugation, and lyophilization. This AgNPs were further used for characterization.
Characterization of AgNPs
The AgNP solution was taken in quartz cuvette for recording the UV–Vis spectrum on the spectrophotometer (UV–Vis) (Ultrospec 2100 Pro, Amersham Biosciences, Piscataway, NJ). The samples were scanned between 300 and 800 nm. Field emission transmission electron microscope (FE-TEM), elemental mapping, selected area diffraction pattern (SAED), and energy dispersive X-ray spectroscopy (EDX) of AgNPs were analyzed with an electron microscope, 2100F (JEOL, Tokyo, Japan) instrument, operated at 200 kV. The sample for TEM analysis was prepared by loading a drop of the nanoparticles colloid on carbon-coated copper grid and could dry at room temperature. The X-ray diffraction (XRD) analyses were performed on X-ray diffractometer, D8 Advance (Bruker, Billerica, MA), Germany, operated at 40 kV, 40 mA, with CuKα radiation, at a scanning rate of 6°/min, step size 0.02, over the 2θ range of 20–80°. Fourier transform infrared spectra of AgNPs and plant extract were recorded on PerkinElmer Spectrum One FTIR spectrometer (PerkinElmer, Waltham, MA) over the range of 4000–450 cm−1 at a resolution of 4 cm−1. For XRD and Fourier transform infrared spectroscopy (FTIR), 3–5 mg of dried samples were used. Dynamic light scattering (DLS) and Zeta potential of AgNPs was measured in triplicate using Zetasizer Nano ZS90 (Malvern Instruments, UK) at 25 °C and at a 12 angle.
Biological activities of AgNPs
Anticancer activity
RAW 264.7, A549, and HeLa cell lines were seeded at a density of 1 × 105 cells well in a 96-well microtiter plate (Corning Costar, Lowell, NY) in DMEM medium containing 10% (v/v) FBS and 1% (v/v) P/S. Cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air for 24 h. When cells reached over 80% confluence, the treatment with AgNPs was performed with various concentrations (1, 2, 5, and 10 μg/ml). After 1 day of treatment, cell viability was measured with an MTT assay. To each cell, 10 μL MTT solution (5 mg/ml) was added and the plates were incubated for 3–4 h; then, the formazan was dissolved in dimethyl sulfoxide (DMSO) and the absorbance of each well was determined by absorbance at 570 nm on an enzyme-linked immunosorbent assay (ELISA) reader (Molecular Devices E09090; San Francisco, CA). Cells without AgNPs were used as a control. All experiments were performed in triplicates and means with standard errors were calculated. Cell viability for a well was calculated by the following equation: CV = (the optical density (OD) value of treated well/the OD value of non-treated control well) × 100%.
Antibacterial activity
Antibacterial effect of AgNPs was evaluated using disc diffusion method against P. aeruginosa, E. coli, V. parahaemolyticus, and S. aureus on Mueller–Hinton agar (MHA) plates. Briefly, an overnight culture of bacterial suspensions was adjusted to 0.5 OD. MHA plates inoculated with above bacterial suspension and spread to form bacterial lawns. Paper discs (8 mm) impregnated with 30 μL of AgNP solution in deionized water (500 ppm). The final concentration of AgNPs was 15 μg/disk. The disks were then placed on inoculated plates. All plates were incubated at 28 °C for 24 h. After incubation, the zones of inhibition around the disc were measured in all the plates (Singh et al. Citation2015a). Experiments were performed in triplicate.
Biofilm inhibition
The biofilm degrading activity of AgNPs was determined by colorimetric method against S. aureus and P. aeruginosa. Briefly, the wells of 96-well microtiter plates (Corning Costar, Lowell) were filled with 100 μl of overnight grown log phase of S. aureus and P. aeruginosa (106 cfu/ml). After culturing for 24 h, different concentrations of AgNPs ranging from 2 to 10 μg/ml were added. The cell culture plates were then incubated for 4 h at 28 °C. After incubation, the media were removed and the wells were washed three times with 200 μL sterile water. Then, the microtiter plate was kept for air drying for 45 min. Then, 200 μL of a 0.1% (v/v) crystal violet solution in water were added to each well and kept for 45 min. The wells were then washed three times with 200 μL sterile water to remove excess stain. The dye incorporated by the adherent cells was solubilized with 200 μL of 95% (v/v) ethanol. The absorbance of each well was measured at 595 nm using ELISA reader (Molecular Devices E09090; San Francisco, CA). The experiments were performed in triplets and results were interpreted in terms of mean ± standard deviation (Bjarnsholt et al. Citation2007).
Results and discussion
Synthesis of AgNPs
Recently, many plant extracts have been reported for the synthesis of AgNPs. The reason for formation of AgNPs was attributed to flavonoids, enzymes, proteins, polyphenols, and some active biomolecules in plant extract for reducing AgNO3 to AgNPs (Krishnaraj et al. Citation2014, Singh et al. Citation2016c). To evaluate the use of B. officinalis in green nanotechnology, we have synthesized AgNPs using B. officinalis leaf extract. The reduction of AgNO3 was visually evident from the color change of the reaction mixture after the incubation period at 65 °C. The reaction mixture turns to brown within 68 s. The brown color of reaction mixture corresponds to surface plasmon resonance (SPR) of AgNPs formed in the reaction mixture. Method for nanoparticles synthesis is rapid and simple. The synthesized nanoparticles were stable.
Characterization of AgNPs
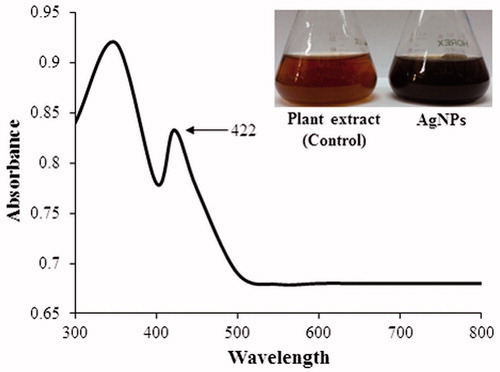
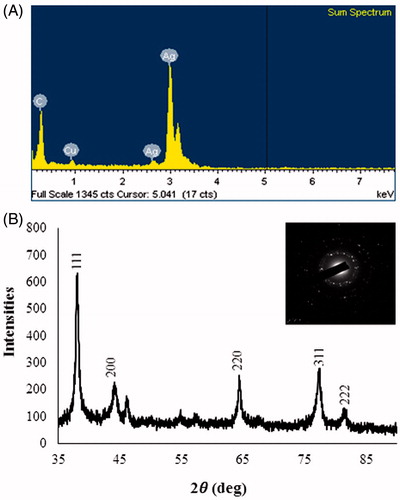
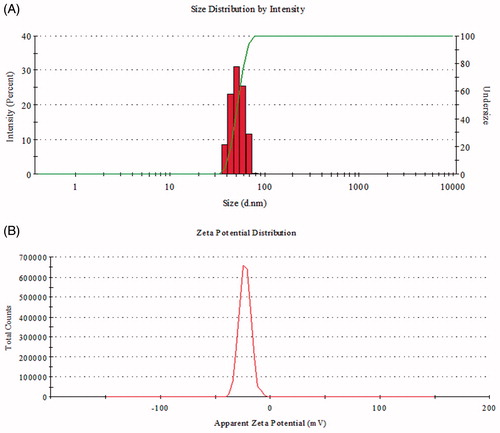
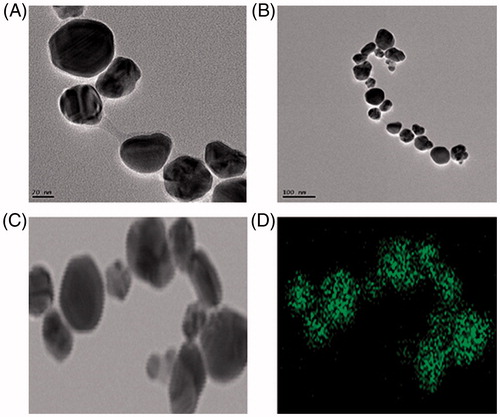
First, UV–Vis spectrophotometer was used to confirm the formation of AgNPs in the aqueous colloidal dispersion. Samples showed a sharp SPR band at 422 nm which is characteristic of AgNPs (). The morphology and size of the AgNPs were analyzed by FE-TEM. The nanoparticles are spherical, hexagonal, and irregular in shape and had size ranging from 30 to 80 nm (). The elemental mapping results show the maximum distribution of silver elements, suggesting that silver was the predominant element in the respective product (). EDX profile peaks obtained confirms Ag+ (). EDX spectrum of nanoparticles showed the highest peak at 3 keV for AgNPs and the results were similar with the previous reports (Singh et al. Citation2015a, Citation2016a). The other metal ions group also appeared in the EDX spectrum which corresponds to the TEM grid used for study (Singh et al. Citation2015b). XRD pattern compared with the standard confirmed spectrum of AgNPs. XRD pattern shows the peaks at 2 θ values of 38.11, 44.27, 64.12, 77.17, and 81.53 corresponding to 111, 200, 220, 311, and 222 facets of the cubic crystalline structure. The intense peaks in the whole spectrum show the AgNPs were to be nanosized. SAED pattern also confirms the face-centered cubic “fcc” crystalline structure of nanoparticles ().
Figure 3. (A and B) FE-TEM images of AgNPs, (B and C) elemental mapping results indicate distribution of elements silver.
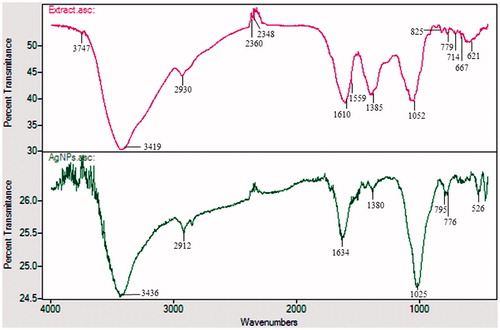
FTIR spectrum of plant extract and biosynthesized AgNPs is shown in . FTIR spectra of extract showed the major absorption bands at 3436, 2912, 1634, 1380, 1025, 795, 776, and 562 cm−1. The bands shown at 3436, 2912, and 1380 cm−1 are due to the stretching vibrations of hydroxyl (O–H) and alkane (C–H). Bending vibration of alkene (C=O) groups was observed at 1634 and 795 cm−1. Amide bands containing carbonyl groups (C=O) were observed at 1025 cm−1. Peak at 776 represents aromatic groups (C=H). The presence of these peaks attributed to the reducing sugars, flavonoids, saccharides, and proteins in the extract (Muniyappan and Nagarajan Citation2014). The reducing sugars among the saccharides are responsible for reduction process. Some proteins and flavonoids acted as protecting agents. Particle size and polydispersity index (PDI) of AgNPs were 50.86 ± 9.5 nm and 0.136, respectively. Zeta potential measurements revealed relative colloidal stability with a negative surface net charge −23.0 mV on the AgNPs (). The stability of nanoparticles was determined by keeping the purified nanoparticles solution at room temperature for different day intervals. There was no observable variation in the UV–Vis spectrum of the nanoparticles solution even after one month, which revealed the stable nature of the nanoparticles.
Anticancer activity of AgNPs
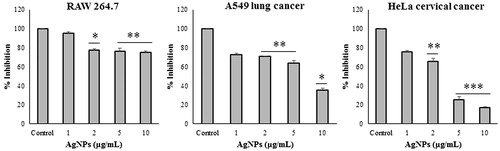
Prior to testing the anticancer effect of AgNPs, they were investigated for their cytotoxic effect on cell growth and viability against RAW 264.7 macrophage cells with an MTT assay. RAW 264.7 cells did not exhibit any significant cytotoxicity, which supports the biocompatibility of the AgNPs in the macrophage cell lines. However, at 2, 5, and 10 μg/ml concentrations of AgNPs, the cells viability percentage was around 75–80%. To check anticancer effect, MTT assay was carried out using A549 and HeLa cancer cell lines. AgNPs showed that it could inhibit proliferation of cancer cells at 1–10 μg/ml concentration. The results of MTT assay is shown in . The results show that there is a gradual decrease in cell viability with an increase in the concentration of AgNPs. However, analysis of our data suggested that AgNPs showed more significant reductions in A549 at 10 μg/ml (35.7%) and HeLa cells at 5 μg/ml (25.1%).
Antibacterial and biofilm inhibition activity of AgNPs
Elemental silver and silver compounds have been used as antimicrobial agents from ancient times. The mechanism of bactericidal effect of silver colloid particles against bacteria is not well known. A possible antibacterial mechanism is due to Ag+ released from AgNPs, which strongly binds to thiol groups found in enzymes and proteins on the cellular surface and can interfere with cell division and lead to bacterial cell death (de Faria et al. Citation2014). Reports also suggest that AgNPs can cause the bactericidal effect by disruption of the cell membrane or cell wall or by damaging DNA, proteins, and other phosphorous- or sulfur-containing cell constituents (Marambio-Jones and Hoek Citation2010, Nel et al. Citation2009). In this study, the antibacterial assay of AgNPs was done on various pathogenic bacteria like P. aeruginosa, E. coli, V. parahaemolyticus, and S. aureus. Zone of inhibition around AgNPs for individual bacterial culture is shown in . The results found that the pathogenic bacteria including P. aeruginosa, E. coli, V. parahaemolyticus, and S. aureus were sensitive to the biosynthesized AgNPs. The results were interpreted in terms of standard deviation of mean diameter of zone of inhibition and are given in .
Figure 8. (A) Zones of inhibition against Pseudomonas aeruginosa [KACC 14021], Escherichia coli [CCARM 0237], Vibrio parahaemolyticus [KACC 15069] and Staphylococcus aureus [KCTC 3881], respectively. (B) Biofilm inhibition by AgNPs against S. aureus [KCTC 3881] and P. aeruginosa [KACC 14021].
![Figure 8. (A) Zones of inhibition against Pseudomonas aeruginosa [KACC 14021], Escherichia coli [CCARM 0237], Vibrio parahaemolyticus [KACC 15069] and Staphylococcus aureus [KCTC 3881], respectively. (B) Biofilm inhibition by AgNPs against S. aureus [KCTC 3881] and P. aeruginosa [KACC 14021].](/cms/asset/a79362d5-896c-4116-8857-2fac5ad17453/ianb_a_1228663_f0008_c.jpg)
Table 1. Antimicrobial activity of the silver nanoparticles synthesized from Borago officinalis leaf extract.
Microorganisms in biofilm-associated infections on indwelling medical devices, such as catheters, are the major concern. Even after antibiotic therapy, it may persist, sometimes results in the removal of the device. It has been established that AgNPs were effective in eradicating biofilm. In this study, a simple biofilm assay was performed to assess the effect of biosynthesized AgNPs on inhibiting biofilm formation. The results suggest that the complete biofilm inhibition was observed for S. aureus and P. aeruginosa at 10 μg/ml concentration of AgNPs () (Singh et al. Citation2016c).
Conclusion
We demonstrate the rapid, facile, convenient, green synthesis of AgNPs in an ecofriendly manner by using B. officinalis leaf extract. The synthesized nanoparticles were of different shapes, 30–80 nm in size, stable, and crystalline. The nanoparticles showed the absorption spectrum at 422 nm and characterized by using different techniques. In vitro MTT assay was carried out to study the effect of AgNPs on cancer cell line. The synthesized AgNPs showed biocompatibility to RAW 245.6 cell line but showed cytotoxicity against A549 and HeLa cancer cells. The AgNPs were capable of showing the antibacterial effect and biofilm inhibition against pathogenic bacteria. Based on these findings, AgNPs may lead to valuable applications in the medical field. This study illustrated an innovative and easy way for the synthesis of the large amount of antimicrobial AgNPs using natural products which can be used in various biomedical applications.
Funding information
This work was conducted under the industrial infrastructure program [No. N0000888] for fundamental technologies which is funded by the Ministry of Trade, Industry and Energy, 10.13039/501100003052 (MOTIE, Korea).
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Bandonien D, Murkovic M. 2002. The detection of radical scavenging compounds in crude extract of borage (Borago officinalis L.) by using an on-line HPLC-DPPH method. J Biochem Biophys Methods. 53:45–49.
- Bhattacharya D, Gupta RK. 2005. Nanotechnology and potential of microorganisms. Crit Rev Biotechnol. 25:199–204.
- Bjarnsholt T, Kirketerp-Moller K, Kristiansen S, Phipps R, Nielsen AK, Jensen PO, et al. 2007. Silver against Pseudomonas aeruginosa biofilms. APMIS. 115:921–928.
- de Faria AF, Martinez DS, Meira SM, de Moraes AC, Brandelli A, Souza Filho AG, Alves OL. 2014. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf B. 113:115–124.
- Duke JA, Bogenschutz-Godwin MJ, DuCelliar J, Duke PK. 2002. Handbook of Medicinal Herbs. 2nd ed. Boca Raton: CRC Press, pp. 373–374.
- Elavazhagan T, Arunachalam KD. 2011. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int J Nanomedicine. 6:1265–1278.
- Farooqui MA, Chauhan PS, Krishnamoorthy P, Shaik J. 2010. Extraction of silver nano-particles from the leaf extracts of Clerodendrum inerme. Dig J Nanomater Biostruct. 5:43–49.
- Gottesman MM, Fojo T, Bates SE. 2002. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2:48–58.
- Huang SY, Lin X, Redden PR, Horrobin DF. 1995. In vitro hydrolysis of natural and synthetic γ-linolenic acid containing triacylglycerol by pancreatic lipase. J Am Oil Chem Soc. 72:625–631.
- Husseiny SM, Salah TA, Anter HA. 2015. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their antibacterial and antitumor activities. J Basic Appl Sci. 4:225–231.
- Inbakandan D, Kumar C, Abraham LS, Kirubagaran R, Venkatesan R, Khan SA. 2013. Silver nanoparticles with anti microfouling effect: a study against marine biofilm forming bacteria. Colloids Surf B. 111:636–643.
- Jo JH, Singh P, Kim YJ, Wang C, Mathiyalagan R, Jin CG, et al. 2015. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif Cells Nanomed Biotechnol. 44:1576–1581.
- Kanchana A, Balakrishna M. 2011. Anti-cancer effect of saponins isolated from solanum trilobatum leaf extract and induction of apoptosis in human larynx cancer cell lines. Int J Pharm Pharm Sci. 3:356–364.
- Krishnaraj C, Muthukumaran P, Ramachandran R, Balakumaran MD, Kalaichelvan PT. 2014. Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Rep. 4:42–49.
- Kybal JA. 1980. Hamlyn Color Guide Herbs and Spices. Vol. 88. London: Hamlyn. 56–57.
- Marambio-Jones C, Hoek EM. 2010. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res. 12:1531–1551.
- Marimuthu V, Palanisamy SK, Sesurajan S, Sellappa S. 2011. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol. 3:143–148.
- Mohanpuria P, Rana NK, Yadav SK. 2008. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 10:507–517.
- Muniyappan N, Nagarajan NS. 2014. Green synthesis of silver nanoparticles with Dalbergia spinosa leaves and their applications in biological and catalytic activities. Process Biochem. 49:1054–1061.
- Nel AE, Mädler L, Velegol D, Xia T, Hoek EM, Somasundaran P, et al. 2009. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 8:543–557.
- Raveendran P, Fu J, Wallen SL. 2003. Completely “green”; synthesis and stabilization of metal nanoparticles. J Am Chem Soc. 125:13940–13941.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2016a. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. 44:1150–1157.
- Singh P, Kim YJ, Singh H, Wang C, Hwang KH, Farh Mel A, et al. 2015a. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomedicine. 10:2567–2577.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2016b. Weissella oryzae DC6-facilitated green synthesis of silver nanoparticles and their antimicrobial potential. Artif Cells Nanomed Biotechnol. 44:1569–1575.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, El-Agamy Farh M, Yang DC. 2016c. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. 44:811–816.
- Singh P, Kim YJ, Singh H, Mathiyalagan R, Wang C, Yang DC. 2015b. Biosynthesis of anisotropic silver nanoparticles by Bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J Nanomater. 2015:234741.
- Sriram MI, Kalishwaralal K, Gurunathan S. 2012. Biosynthesis of silver and gold nanoparticles using Bacillus licheniformis. Methods Mol Biol. 906:33–43.
- Usmanghani K, Saeed A, Alam MT. 1997. Indusyunic Medicine. Karachi: University of Karachi Press. 316–317.
- Xu H, Yao L, Sun H, Wu Y. 2009. Chemical composition and antitumor activity of different polysaccharides from the roots of Actinidia eriantha. Carbohydr Polym. 78:316–322.