Abstract
Many materials such as aluminum hydroxide have been tried as adjuvants to compensate low inherent immunogenicity of subunit vaccines. The aim of this study was to evaluate the specific immune response following the administration of aluminum hydroxide nanoparticles with EsxV antigen. The physiochemical properties of the nanoparticle were characterized in vitro. After subcutaneous immunization, cytokine secretion patterns including IFN-gama,IL-4, and TGF-beta levels were measured by indirect enzyme linked immunosorbent assay (ELISA). Aluminum hydroxide-NPs were demonstrated excellent effects to raise of IFN-γ secretion in compare to EsxV alone. Administration of aluminum hydroxide nanoparticles stimulates strong cellular immune response and could be considered as appropriate adjuvant against TB infection.
Introduction
Tuberculosis (TB) as public health issue lead to infection nearly in one-third of the world’s population and 1.8 million annual deaths (WHO Citation2009). Vaccination remains one of the best strategies to control this devastating global problem. The only approved vaccine is Bacillus calmette–Guerin (BCG) that has a variable efficacy in different populations (da Costa et al. Citation2014). Currently, several vaccines such as viral vector, BCG recombinant strains and subunit vaccines in combination with drastic adjuvants have been tried to induce efficient protection against TB (Kaufmann Citation2012).
Subunit vaccines that consist of protective proteins have shown suitable safety and immunity. Several subunit vaccines such as H1 + IC31, ID93 + GLA-SE, and M72 + AS01E are developed against tuberculosis. However, using the efficient adjuvant is the main strategy to create protective and strong immune response.
T helper (Th-1) assay demonstrated that M.tuberculosis has three immunodominant antigens with low molecular weights, which are deleted in all strain of BCG vaccine, i.e., Rv3619c, Rv3874, andRv3875. These proteins emerged as a suitable vaccine candidate to promote specific Th-1 immune response against M.tuberculosis. To support this, several studies indicated that Rv3619c (esxV) is a potent T-cell inducer antigen and if it used with efficient adjuvants, it may create preventive response against tuberculosis (Knudsen et al. Citation2014, Mahmood et al. Citation2011).
Unfortunately, many of new generation vaccines especially subunit vaccines have a low inherent immunogenicity (Brandt et al. Citation2000). To compensate low inherent immunogenicity in modern generation of vaccines, such as subunit vaccine, many of materials tried as adjuvant. Adjuvants are vaccine additive compounds without any specific antigenic properties for stimulation of specific immune response against vaccine. Activation of dendritic cells trough pattern recognition receptors and Toll-like receptors is a main mechanism that is applied by adjuvants such as CpG (Brito and O'Hagan, Citation2014, Pulendran and Ahmed Citation2006). In contrast to many other adjuvants, several mechanisms have been suggested to describe aluminum hydroxide ability in inducing immune response against different antigens.
First, the aggregation of antigen with aluminum hydroxide adjuvant improved the process of antigen uptake by APC cells in the site of injection (Glaziou et al. Citation2015). This phenomenon is called depot effect, which influenced by several properties of aluminum adjuvants such as electric charge, surface area, etc. Recent studies reported that the aluminum hydroxide activate nucleotide binding oligomerization domain (NOD) like receptor protein 3 (NALP3) to secrete pro-inflammatory adjuvant by macrophages (Kool et al. Citation2008, Li et al. Citation2007).
In addition to traditional components such as aluminum hydroxide, new class of adjuvants including polymeric nanoparticle, liposomes, and engineered nanomaterials are used to regulate a cognitive immune response (Amini et al. Citation2016, Sun et al. Citation2013). Because of pathogen-mimicking properties including size and pathogen-associated molecular patterns (PAMP), nano-vaccines are promising strategy to demonstrate various advantages for tuning both cellular and humoral immune response (Yang et al. Citation2016). Because of smaller particle size and higher surface area, aluminum hydroxide adjuvants in nano-scale forms show higher adsorption ability (Glaziou et al. Citation2015). In addition, nano-vaccines are homogenized, which is an important property to induce immune response. So that, aluminum hydroxide in Nano-scale form can be considered as an advanced technique to promote adjuvant influences. Previous studies have been demonstrated that nano-scale form of aluminum adjuvants possess stronger ability to stimulate more cellular and humoral immune response in compare to traditional alum (Glaziou et al. Citation2015, Tang et al. Citation2008). In this study, we therefore evaluated the ability of aluminum hydroxide in nanoparticle scale as an acceptable adjuvant to increase cellular immune response against the EsxV antigen.
Material and methods
Aluminum hydroxide nanoparticles preparation
Aluminum hydroxide nanoparticles were synthesized according to the previously described method (Li et al. Citation2014). Briefly, an equal volume of 3.6 mg/ml AlCl3.6H2O was added to 0.04 M NaOH solution. After adjusting pH to 7.0, solution was stirred at room temperature for 30 min. In the next step, solution was sonicated for 10 min. Finally, NaCl was removed by Amicon® Ultra-4 Centrifugal Filter Devices (Milipore, Germany).
Nanoparticles characterization
To determine zeta potential and size of the Aluminum hydroxide nanoparticles dynamic light scattering (DLS) (Malvern 3000, UK) was used. Morphology of the nanoparticle was characterized by scanning electron microscope (SEM). Moreover, the stability of nanoparticles suspension was studied in 4 °C for 15 days. Nanoparticles size and zeta potential were measured on the first day by DLS and were repeated for 15 days.
Cell culture
Cell line (Hela) was purchased from the national cell bank of Iran and cultured in cell culture flasks. The cells centrifuged for 5 min at 1200 g and resuspended in fresh medium. The cells were counted using Trypan Blue exclusion (99% viability) and cells concentration were adjusted to 105 cells/ml and 100 μl aliquot of different concentration of nanoparticle suspension was added to each well of 96-well cell culture plates (Corning) with RPMI-1640 medium (Cambrex Bioscience) supplemented with L-glutamine (2 mM, Gibco, MA), penicillin–streptomycin (100 IU/ml penicillin, 100 mg/ml streptomycin (Gibco, MA)), and heat inactivated fetal bovine serum (Biowest, Nuaillé, FRA) 10% (v/v). The cells were incubated at 37 °C in the humidified incubator with 5% CO2 atmosphere (Memmert, Germany).
MTT assay
MTT solution (USB Corporation, Cleveland) was added to each well and the plates were incubated for 4 h at 37 °C. Then, MTT solution was removed and DMSO was added to dissolve formazan crystals. The absorbance at 540 nm was read on a 680Microplate Reader (Bio-Rad Laboratories, Hercules, CA). The percentage of viability was calculated by the below equation:
Viability = AT/AC ×100
AT and AC are the absorbance of treated and control cells, respectively.
Protein adsorption on the nanoparticles preparation and characterization
Protein at a weight ratio of 1:1 and1:3 was added to aluminum hydroxide nanoparticles and mixed gently by stirring for 10 min, 30 min and overnight at 4 °C. The amount of EsxV protein adsorbed on aluminum hydroxide nanoparticles was determined by SDS-PAGE and Micro Bicinchoninic Acid (BCA) by the below equation:
After addition of manitol as a cryoprotectant, EsxV-nanoparticles (EsxV-NP) were lyophilized and stored at −20 °C.
SDS-PAGE
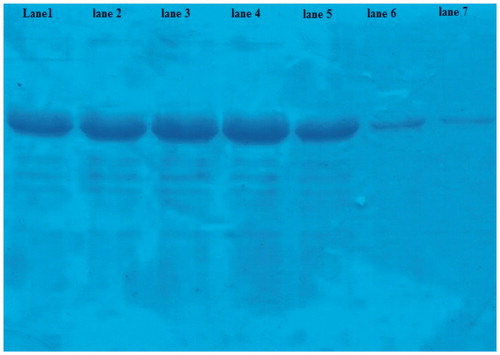
12% Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was used for protein separation. The lyophilized EsxV-NP resuspended in de-ionized water and mixed with sample buffer. Samples with proteins standard (Parstoos) were run at 130V for 1 h.
Experimental groups and immunization
Female BALB/c (six- to eight-weeks old) mice purchased from the local animal facility (Razi Vaccine and Serum Research Institute, Mashhad-Iran). The mice were maintained under controlled environment and separated into four groups (eight animal per group). They were immunized three times in 2-week intervals with 20 μg protein/200 μl formulation/per mouse. Groups were immunized with PBS, EsxV protein, EsxV-NP and BCG by subcutaneous injection.
Cytokine assay
Two weeks after the last immunization, all mice were killed and their spleens were dissected aseptically. The spleens were homogenized. Splenocytes suspension was prepared and erythrocytes removed by lysis buffer (ammonium chloride). Then, isolated spleen cells were cultured in duplicate (2 × 105 cells/well) in RPMI-1640 medium supplemented with penicillin/streptomycin solution (Penstrep, Biosera, UK) and 10% fetal calf serum (GIBCO, UK) in the presence of 5 μg/ml of EsxV protein. The Splenocytes incubated for 72 h at 37 °C in an atmosphere of 5% CO2. Positive controls were stimulated with phytohemagglutinin-A (PHA) (2 μg/ml) and negative controls were left un-stimulated. Finally, Cell culture supernatants were harvested for the measurement of IFN-γ, TGF-beta, IL-4 by ELISA kits (Ebioscience, AUT) according to the manufacturer’s instructions.
Statistical analysis
Data were reported as mean ± standard error of the mean (SEM). Differences between groups were analyzed using One-way ANOVA and Tukey-Kramer post-test by SPSS 13.0 (IBM, Armonk, NY) software. p Values p < .05 was considered statistically significant.
Results
Nanoparticles characterization
According to the obtained results via DLS, the aluminum hydroxide nanoparticles possess suitable size and zeta potential. As the size of aluminum hydroxide nanoparticles were 141.1 ± 8.12 nm and after protein adsorption it changed to 195.4 ± 11.3 nm. Moreover, zeta potential of both the blank and EsxV nanoparticles were positive. However, protein adsorption on the nanoparticles leads to decrease zeta potential from 43 ± 7.6 mV to 17.8 ± 5.2 mV. Stability evaluation of nanoparticles demonstrated that nanoparticles were stable at 4 °C. The size of nanoparticles increased from 198.7 ± 8.25 to 221.73 ± 6.53 after 15 days. Morphology of the EsxV-NP nanoparticles was studied by SEM and confirmed spherical and smooth shapes of nanoparticles ().
Cytotoxicity assay
The prepared aluminum hydroxide nanoparticles were demonstrated by low cytotoxicity. The cytotoxicity of nanoparticles was dose-dependent and cell viability was decreased with increase in aluminum hydroxide nanoparticle dosage. However, MTT values were not significantly different among different dosage. The results of this study indicated that aluminum hydroxide nanoparticles have low toxicity (data not shown).
Protein adsorption on the nanoparticles
The nanoparticles ratio and time of stirring had effect on the protein adsorption. By using an equal ratio of nanoparticle to protein, proteins adsorption after 10, 30 minutes, and overnight were 10.3, 18.4, and 35.4%, respectively. But with increase in the amount of nanoparticles to 3-fold, after 10, 30 minutes, and overnight adsorption depending manner to time was increased to 42, 78, and 93%, respectively ().
Immune responses
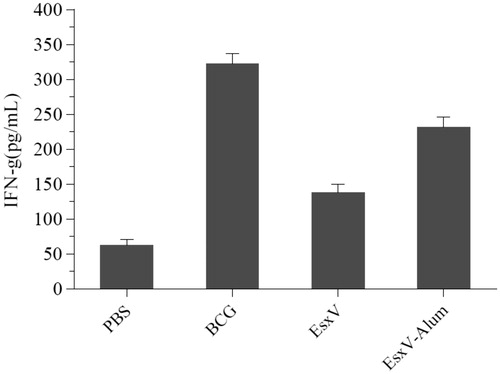
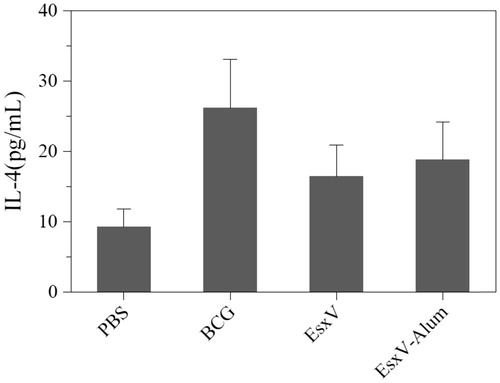
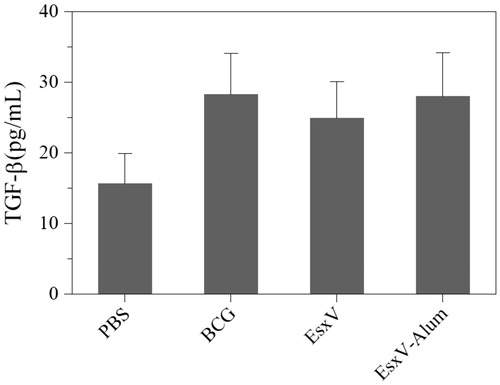
To evaluate the immune response against M.tuberculosis antigen, cytokine secretion pattern including IFN-γ, TGF-beta, and IL-4 was used. The IFN-γ secretion showed significant increase in BCG, EsxV, and EexV-NP groups (p < .001) (). Although, BCG group had a higher ability to raise of IFN-γ secretion, aluminum hydroxide-NPs also were demonstrated promising effects (p < .001). IL-4 in all immunized group was higher than PBS group, while, BCG group only showed significant higher level of secretion (p < .05). There was no significant difference between BCG and EsxV-NP groups (). But, the secretion of TGF-beta, as a marker of T-reg, in all groups was higher than PBS group (p < .05). No significant differences were observed between the BCG group and EsxV-NP group ().
Discussion
In spite of numerous innovation to design of efficient vaccine, still tuberculosis remains a serious public health problem in recent years. Since, the Th1-type cellular immunity play significant role by creating protection, new vaccine candidates have focused on the induction CD4 + and CD8 + immune response. The traditional aluminum hydroxide is safe and applied with many vaccines, but it promotes immune response toward Th2-type. Therefore, to change of this property, we examined aluminum hydroxide in nanoparticles form to create suitableTH-1 type immune response against M. tuberculosis antigen.
Although, pervious study indicated that particles smaller than 1 μm easily adsorbed by dendritic cells, Kanchan et al. showed nanoparticles (200–600 nm) were more efficiently taken up by macrophages (Kanchan and Panda Citation2007, Nagamoto et al. Citation2004). Other studies indicated that nanoparticles-containing antigen were adsorbed by phagocytosis or endocytosis, while macropynocytosis is a predominant mechanism that used for adsorption of eluted antigen with adjuvants. Hence, the potency of aluminum hydroxide nanoparticle to facilitate the antigen internalization by APC is related to their potent adjuvant properties (Li et al. Citation2014). Since, the EsxV nanoparticles size obtained in this study were 195 nm, which easily adsorbed macrophage by phagocytosis and endocytosis way. Moreover, because of smaller size, larger surface and higher surface reactivity of NP, aluminum hydroxide nanoparticles had more capacity for antigen adsorption when compared to same amount of traditional aluminum hydroxide adjuvant (He et al. Citation2015).
Studies that were carried out by Li et al. and Tang et al. emphasized on aluminum hydroxide NPs ability for induction strong cellular and humoral immune response. As expected, EsxV antigen adsorbed on aluminum hydroxide NPs, secrets significantly higher levels of IFN-γ, as compared with antigen alone. These outcome may be reflects aluminum hydroxide NP ability to create strong immune response against tuberculosis infection. Many studies indicated the principal important of humoral immune response in prevention of TB infection (Zuñiga et al. Citation2012). In our study, EsxV NP and BCG vaccinated groups exhibited high levels of IL-4 as a marker of Th2 immune response. Therefore, aluminum hydroxide NP had a significant effect on the immunogenicity of EsxV to induce humoral response. The same results were observed by He et al. they demonstrated the Anti-HBsAg titers in aluminum hydroxide NP group was higher than other groups (He et al. Citation2005).
Based on the accumulated research results, aluminum hydroxides NP possess strong adjuvant properties to induce cellular and humoral immune response.
Conclusions
Aluminum hydroxide NP demonstrated suitable adjuvant ability and it can be applied for designing of new and efficient vaccines against TB. Moreover, complementary studies are required to evaluate the other immunological potentials of aluminum hydroxide NPs.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Amini Y, Tebianian M, Mosavari N, Fasihi Ramandi M, Ebrahimi SM, Najminejad H, et al. 2016. Development of an effective delivery system for intranasal immunization against Mycobacterium tuberculosis ESAT-6 antigen. Artif Cells Blood Substit Immobil Biotechnol. 1–6.
- Brandt L, Elhay M, Rosenkrands I, Lindblad EB, Andersen P. 2000. ESAT-6 Subunit Vaccination against Mycobacterium tuberculosis. Infect Immun. 68:791–795.
- Brito LA, O'Hagan DT. 2014. Designing and building the next generation of improved vaccine adjuvants. J Control Release. 190:563–579.
- da Costa AC, de Oliveira Costa-Junior A, de Oliveira FM, Nogueira SV, Rosa JD, Resende DP, et al. 2014. A new recombinant BCG vaccine induces specific Th17 and Th1 effector cells with higher protective efficacy against tuberculosis. PLoS One. 9:e112848.
- Glaziou P, Sismanidis C, Floyd K, Raviglione M. 2015. Global epidemiology of tuberculosis. Cold Spring Harb Perspect Med. 5:a017798.
- He P, Lu F, Chen Y, Zuo G, Li Y, He F. 2005. Synthesis of nanoparticulate alum adjuvant and its application to the HBsAg and rabies virus. J Immunol. 22:90–93.
- He P, Zou Y, Hu Z. 2015. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum Vaccin Immunother. 11:477–488.
- Kanchan V, Panda AK. 2007. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 28:5344–5357.
- Kaufmann SH. 2012. Tuberculosis vaccine development: strength lies in tenacity. Trends Immunol. 33:373–379.
- Knudsen NPH, Nørskov-Lauritsen S, Dolganov GM, Schoolnik GK, Lindenstrøm T, Andersen P, et al. 2014. Tuberculosis vaccine with high predicted population coverage and compatibility with modern diagnostics. Proc Natl Acad Sci USA. 111:1096–1101.
- Kool M, Pétrilli V, De Smedt T, Rolaz A, Hammad H, van Nimwegen M, et al. 2008. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 181:3755–3759.
- Li H, Nookala S, Re F. 2007. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 178:5271–5276.
- Li X, Aldayel AM, Cui Z. 2014. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 173:148–157.
- Mahmood A, Srivastava S, Tripathi S, Ansari MA, Owais M, Arora A. 2011. Molecular characterization of secretory proteins Rv3619c and Rv3620c from Mycobacterium tuberculosis H37Rv. FEBS J. 278:341–353.
- Nagamoto T, Hattori Y, Takayama K, Maitani Y. 2004. Novel chitosan particles and chitosan-coated emulsions inducing immune response via intranasal vaccine delivery. Pharm Res. 21:671–674.
- Pulendran B, Ahmed R. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 124:849–863.
- Sun B, Ji Z, Liao Y-P, Wang M, Wang X, Dong J, et al. 2013. Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano. 7:10834–10849.
- Tang C, Huang X, Yang F, Li M, Fan G, Yue H. 2008. Adjuvant effect of aluminum hydroxide nanoparticles on Newcastle diseases antigen in chichens. Chinese Veterinary Sci. 38:1060–1064.
- WHO. 2009. Global Tuberculosis Control 2009. Geneva: World Health Organization.
- Yang L, Li W, Kirberger M, Liao W, Ren J. 2016. Design of nanomaterial based systems for novel vaccine development. Biomater Sci. 4:785–802.
- Zuñiga J, Torres-García D, Santos-Mendoza T, Rodriguez-Reyna TS, Granados J, Yunis EJ. 2012. Cellular and humoral mechanisms involved in the control of tuberculosis. Clin Develop Immunol. 2012.