?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, we synthesized poly(N-vinylcaprolactam)-g-collagen (PNVCL-g-Col) by grafting collagen with carboxyl group-terminated thermosensitive PNVCL-COOH. The resulting biopolymer was evaluated for its structural, thermal, and rheological properties. Aqueous solutions of PNVCL-g-Col exhibited a temperature-dependent phase transition around human physiological temperature (at ∼38.5 °C), and temperature-dependent tunability. PNVCL-g-Col exhibited temperature-dependent release of the model drugs, lidocaine hydrochloride and bovine serum albumin. Thus, PNVCL-g-Col biopolymer may have wide potential use in various biomedical applications, including controlled release and tissue engineering.
Introduction
Interest in hydrogels is increasingly focusing on stimuli-sensitive intelligent polymeric systems (Seker et al. Citation2014, Stuart et al. Citation2010). These systems preserve bioactive agents from hostile effects, such as enzymatic degradation by proteinases or oxidation. Moreover, thermosensitive biomaterials are capable of phase transition, which is useful in mediating delivery of various therapeutic agents. Thermosensitive hydrogels are of particular interest as they consistently change form via simple alterations of the ambient temperature (Guan et al. Citation2015, Hoarea and Kohaneb Citation2008). Thermosensitive hydrogels are characterized by sudden reversible phase separation with transition between the extended and coiled forms of the polymer, in response to a certain temperature change, which functions as an on–off switch (Gill and Hudson Citation2004, Ward and Georgiou Citation2011). They are particularly useful as controlled-release/drug delivery systems (Guan et al. Citation2015, Mura et al. Citation2013, Zhou et al. Citation2016).
Poly(N-isopropylacrylamide) (PNIPAAm) is a prominent thermosensitive polymer that displays a sharp phase-transition in water (also represented by low critical solution temperature, LCST) at ∼32 °C (Schild Citation1992). PNIPAAm and its copolymers have various applications in drug delivery, microfluidics, nuclear medicine, cell culture technologies, and tissue engineering (Krause et al. Citation2016, Sarkar and Kumar Citation2016, Sedláček et al. Citation2016). In particular, this thermosensitive polymer is useful for cell sheet engineering in attaining a functional cell graft in monolayer form (Sakuma et al. Citation2016, Shimizu et al. Citation2003).
There are contradictory results on the cytotoxicity of NIPAAm, and its polymer, PNIPAAm. Findings state that cellular sensitivity may be dose-dependent and varies according to cell-type (Cooperstein and Canavan Citation2013, Joseph et al. Citation2010, Prabaharan et al. Citation2008). Copolymerization may have a positive influence on the compatibility of PNIPAAm for some biomedical applications.
Poly(N-vinylcaprolactam) (PNVCL) is a nontoxic synthetic polymer (Vihola et al. Citation2005). The hydrolysis of PNVCL does not generate small amide compounds, as the amide groups bind to the hydrophobic backbone chain (Lau and Wu Citation1999, Vihola et al. Citation2005). PNVCL exhibits a continuous phase-transition behavior with a LCST ranging between 30 and 50 °C (Jiří Citation2009, Laukkanen et al. Citation2004), which strongly correlates with the molecular weight and the end groups of the polymer. Among others, biomedical applications of PNVCL include drug delivery, tissue engineering, and wound healing (Emin et al. Citation2008, Kavitha et al. Citation2016, Lee et al. Citation2013, Liu et al. Citation2014, Strukova et al. Citation2001).
Strategies for developing biomaterials based on natural and synthetic polymers (hybrid) are central for the advancement of the biomedical field (Demirdögen et al. Citation2010, Lau and Kiick Citation2015, Linn et al. Citation2003). PNVCL is a valuable tool, since it offers thermo-sensitive properties as well as biocompatibility, and ability to form complexes with different therapeutic agents (Galaev and Mattiasson Citation1999, Laukkanen et al. Citation2004). Thus its natural polymer-conjugates can be useful for cellular applications. Within this context, Prabaharan et al. (Citation2008) have proposed chitosan-PNVCL as a controlled-delivery drug carrier, and folic acid coupled PNVCL-b-PEG as an anti-cancer drug carrier (Prabaharan et al. Citation2009).
Collagen is among the most commonly used natural biopolymers, being the main structural protein of animal tissues (Bauer et al. Citation2014, Sionkowska Citation2012). Collagen contains carbonyl moieties, amide bonds, and hydroxyl side groups. Furthermore, free amino groups of hydroxylysine and lysine residues of collagen facilitate a bridge with target molecules. In combination with N-hydroxysuccinimide (NHS), zero-length cross-linker carbodiimide [N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC)] is a commonly preferred system for cross-linking. Studies prove that the EDC/NHS-crosslinked collagen samples display non-cytotoxic properties under in vitro (van Luyn et al. Citation1992) and in vivo conditions (van Luyn et al. Citation1995).
In the present study, we have developed a novel thermosensitive PNVCL-g-collagen copolymer that exhibits a temperature-dependent phase change near human physiological temperature. We have performed structural, thermal, and rheological analyses, and also explored water uptake capacity, model drug release and in vitro cytotoxicity properties of the novel copolymer.
Materials and methods
Chemicals
N-Vinylcaprolactam (NVCL), EDC, NHS, and 3-mercaptopropionic acid (MPA) were purchased from Sigma-Aldrich (St. Louis, MO). 2,2′-Azoisobutyronitrile (AIBN) was supplied from Acros Organics (Thermo Fisher Scientific, Geel, Belgium). All other chemicals and solvents were supplied from either Sigma or Merck (Darmstadt, Germany). We used ultrapure water (20 MΩ cm at 25 °C) in all experiments.
Synthesis of PNVCL-COOH and PNVCL-g-collagen
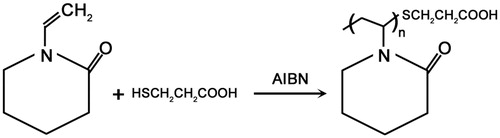
We used free radical polymerization method for the synthesis of PNVCL-COOH (Prabaharan et al. Citation2008). We solubilized NVCL (37.35 mmol), MPA (3.278 mmol), AIBN (0.304 mmol) in 50 mL of pure ethanol. The solution was bubbled through dried nitrogen for 20 min. Polymerization was performed under nitrogen atmosphere at 75 °C, and continued for 8 h. Samples were precipitated in diethyl ether. Upon drying in vacuum, the solid was re-suspended in ultrapure water. Later, the solution was dialyzed for 3 days against ultrapure water in cellulose membrane tubing (MW cutoff 1000 Da), followed by lyophilization.
In order to form PNVCL-g-Col, the synthesized PNVCL-COOH was conjugated with collagen using cross-linking agents (EDC/NHS) to form amide bonds at room temperature. Collagen (isolated from rat-tail tendon) solution (1% w/v) was prepared in 0.1% acetic acid. Ten milliliter collagen solution (1%) was mixed with aqueous PNVCL-COOH solution. Later, 0.1 mL solution of NHS (0.023 g) and EDC (0.0384 g) in ultrapure water was introduced (PNVCL-COOH/Collagen, 5:1). The reaction was performed under constant stirring for 4 h at room temperature.
We performed rheology analyses using the gel form of PNVCL-g-Col. Other experiments were realized by using lyophilized PNVCL-g-Col. After freezing the copolymer at –80 °C for 72 h, lyophilization was performed at −56 °C (Christ Alpha 1-4 LD, Osterode am Harz, Germany) for 24 h. Lyophilized PNVCL-g-Col was mechanically stable to UV sterilization (at 254 nm for 30 min) and could be UV-sterilized for the cytotoxicity study.
FTIR analysis
Structural assessment of the biopolymer product was performed by using attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR Spectrum 100, Perkin Elmer, Shelton, CT). Prior to characterization, the samples were thoroughly dried and powdered finely. Spectral measurements were performed in the region of 400–4000 cm−1 with an accumulation of four scans.
NMR analysis
We employed a Varian Mercury 400 MHz (Agilent, Palo Alto, CA) for NMR experiments, equipped with a 5-mm broadband-observed probe head, at proton resonance frequency of 100.6243 and 400.1779 MHz for carbon. For NMR spectrum optimization, we used Agilent VnmrJ version 3.2 revision (Santa Clara, CA).
GPC analysis
Gel permeation chromatography (GPC) was performed on a Viscotek 270 model dual detector (Malvern Instruments, Malvern, UK), with A3000 Aq and A4000 Aq type columns. Tetrahydrofuran (THF) was the preferred solvent, and was introduced with a flow rate of 1 mL/min at 35.0 °C. The number average (Mn) and weight average (Mw) molecular weights of PNVCL-COOH were measured by evaluating the GPC data using OmniSEC 4.7 software (Malvern Instruments, Malvern, UK).
Phase transition temperature
Phase transition temperature (LCST) of PNVCL-COOH, Collagen, and PNVCL-g-Col was determined by using a Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, CA) at 480 nm. The solubilized samples (5%) were placed into the wells of a polystyrene 96-well culture plate (Corning Inc., Tewksbury, MA) under temperature change rate of 1 °C/min between 27 and 42 °C. Decrease in optical transmittance was followed in order to determine transition from liquid to a 3D gel (LCST).
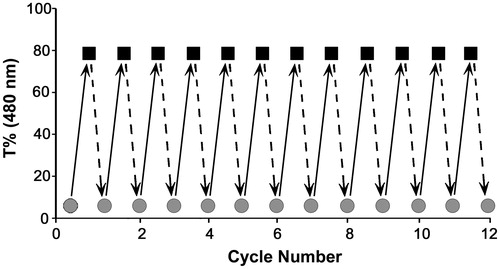
Reproducibility of phase transition was evaluated by exposing the thermo-sensitive PNVCL-g-Col to repetitive cycles of heating and cooling between 25 °C and 40 °C, up to 12 cycles (with 10 min intervals). Temperature measurements were performed when the complete sol-to-gel, or gel-to-sol transition was observed (from transparent fluid to white opaque gel, or vice versa).
Differential scanning calorimetry (DSC) analysis
Thermal properties of the samples were determined by Q2000 calorimeter (TA Instruments, New Castle, DE). Lyophilized PNVCL-COOH, Collagen, and PNVCL-g-Col were placed in aluminum holders and heated between 25 and 250 °C, under nitrogen atmosphere, at 10 °C/min.
Thermogravimetric analysis (TGA)
Lyophilized PNVCL-COOH, Collagen, and PNVCL-g-Col samples were cut into pieces weighing ∼4–5 mg. Weight change of the samples during temperature change was measured with a Q500 model thermogravimetric analyzer (TA Instruments, New Castle, DE). The experiments were performed inside alumina crucibles. Samples were heated at 20 °C/min increments between 20 and 800 °C, at nitrogen gas flow-rate of 100 mL/min.
Rheological analysis
Rheological characterization was performed with a Pysica MCR 301 model strain-controlled rheometer (Graz, Austria). To assess gelation characteristics and reversibility, rheological properties were measured during phase transition as a function of stress, time, temperature, and frequency sweeps. We formed a 1 °C/min increase temperature ramp from 25 to 50 °C by using a Peltier temperature control-module. This temperature profile was used to graph the change in stored elastic modulus (G′) and the viscous loss modulus (G″) in relation with temperature. In order to evaluate the in situ gelation behavior and evolution of G′ and G″, we performed a constant shear frequency (10 rad/s) time-sweep at the linear viscoelastic region (LVE). Strain sweep tests were carried out at 25 °C in the LVE, 0.01–100 rad/s for each sample. Oscillatory shear frequency sweeps were acquired at 25 °C and 38.5 °C in order to characterize the transition from viscous liquid to elastic solid.
Water uptake analysis
Weights of the dry lyophilized samples (Wdry) were measured and the samples were placed in ultrapure water, to document the swelling characteristics of PNVCL-g-Col. We weighed the swollen samples (Wwet) at predetermined time points. The swelling ratio of the samples was calculated according to the equation below [EquationEq. (1)(1)
(1) ]:
(1)
(1)
in which, the weights of the wet and dried samples are represented as Wwet and, Wdry, respectively. Temperature dependent swelling behavior was studied at 4 °C and 40 °C.
In vitro release of lidocaine and BSA
To assess the in vitro release properties of the copolymer, lyophilized PNVCL-g-Col samples (in sponge form) were loaded either with lidocaine hydrochloride [2-(diethylamino)-N-(2,6-dimethylphenyl) acetamide HCl], or with BSA. Analyses were performed under static conditions. This experiment was done to document distinct states of the proposed material, rather than the properties during the transition. To elaborate, 4 °C was selected to guarantee maximal hydration and 40 °C was selected to observe the PNVCL-g-Col performance in maximum dehydration conditions. Moreover, sampling at 37 °C is problematic, as the sample temperature drops quickly, causing inadvertent phase change that interferes with the release dynamics. 40 °C was preferred to compensate for the temperature change without causing phase change.
The local anesthetic lidocaine hydrochloride (MW: 270.79822 g/mol) was selected as low-molecular-weight model drug. Five hundred microliters of the lidocaine solution (2.5 mg/mL in ultrapure water) was dropped into dry cylindrical PNVCL-g-Col sponges and incubated overnight at 4 °C. Then, each PNVCL-g-Col sponge (weighing 5 mg; ø: 15 mm; h: 4 mm) was placed inside 15 mL centrifuge tubes containing 3 mL ultrapure water, and incubated at either 4 °C or 40 °C, for up to 72 h. We collected media samples at pre-determined time-points (at 1, 3, 6, 9, 12, 24, 48, and 72 h) and replaced with fresh medium. The samples were stored at −40 °C, and the amount of released lidocaine was determined by using the lidocaine standard curve. We measured the absorbance at 262 nm by using a UV–Vis spectrophotometer. Bovine serum albumin, with a MW of ∼66.5 kDa was preferred as high MW model protein. We implemented the same loading and release conditions for BSA. The amount of BSA in the solution was measured by using Lowry assay at 750 nm, and BSA standard curve.
In vitro cytotoxicity analysis
Indirect extract testing (International Organization for Standardization; ISO 10993-5) was employed to evaluate potential cytotoxicity of the polymer on human dermal fibroblasts through MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays. Cells were seeded at 3.0 × 104 cells/cm2 inside 24 well cell culture plates, and cultured in DMEM F-12 medium (Lonza, Basel, Switzerland) supplemented with 10% fetal bovine serum, in 5% CO2, at 37 °C for ∼6–7 days (SM), to reach ∼80% confluence.
At the same time, lyophilized PNVCL-g-Col sponges (UV-sterilized) were incubated for 24 h in fresh culture medium (polymer extract medium; EM). The following groups were cultured for 48 h: (1) SM serving as control, (2) extract culture with EM, and (3) the positive control (SM containing 400 μM phenol). MTT (30 μL) and DMEM F-12 (270 μL) were added to each well and incubated for 4 h. The formed formazan crystals were solubilized by isopropanol and absorbance measurements performed at 570 nm. Cell viability percentage of group 1 was taken as 100%.
Statistical analysis
All quantitative experiments were performed in triplicates. Results were expressed as mean ± standard deviation, and evaluated with one-way analysis of variance. Statistical significance value was set at P ≤ 0.05.
Results and discussion
Synthesis and structural characterization of PNVCL-COOH and PNVCL-g-Col
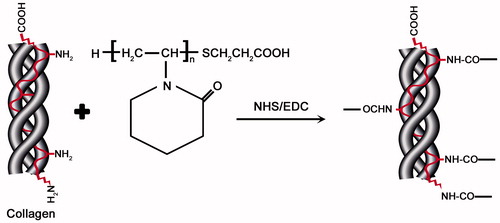
Polymerization of NVCL yielded carboxylic acid-functionalized PNVCL-COOH, in the presence of the chain transfer reagent MPA. NHS and EDC were used to activate the carboxylic acid groups located at chain termini, which subsequently reacts with the amine groups of collagen. shows the reaction of the introduction of carboxyl groups using MPA as chain transfer agent for the synthesis of PNVCL-COOH.
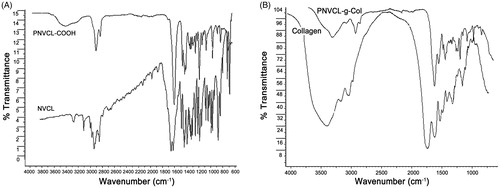
ATR-FTIR analysis showed that, the C=C stretching bands, which represent the NVCL monomer spectrum at 1658 cm−1 and 3108 cm−1 (CH= and CH2=) had disappeared in the PNVCL-COOH spectrum (). PNVCL-COOH exhibited characteristic peaks at ∼3425 cm−1 (=OH stretching vibration), ∼1620 cm−1 (C=O stretching vibration) and at 1480–1443 cm−1 (C=N stretching vibration), while molecular signature for NVCL had disappeared.
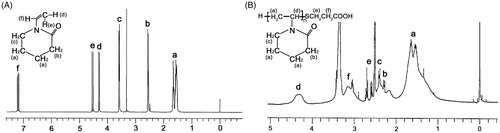
depicts 1H NMR spectra that represents NVCL monomer () and the PNVCL-COOH polymer (). shows exhibited peaks of PNVCL-COOH at δ = 4.3 ppm (1H, =NCH), δ = 3.1 ppm (2H, =NCH2), δ = 1.4–1.8 ppm (8H, =CH2=), δ = 2.4 ppm (2H, =COCH2=), and chain-transfer agent (S-CH2CH2COOH) peaks at δ = 2.6–2.7 ppm. Moreover, the peak of vinyl proton of the monomer at δ = 7 ppm () was not detected in the polymer, indicating the opening of the vinyl double bond and polymerization.
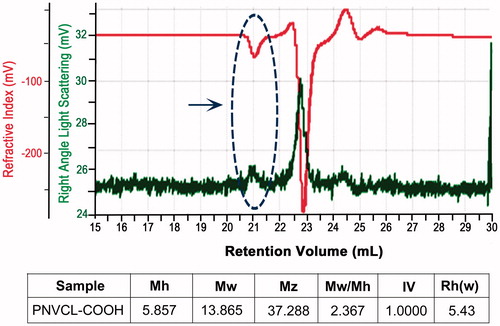
GPC results show number average (Mn) and weight average (Mw) molecular weights of PNVCL-COOH in THF. The Mn of PNVCL-COOH of two different samples was 5857 and 13,865. Weight average molecular weights of PNVCL-COOH of the two different samples were 13,854 and 19,190 (; indicated by an arrow).
Thermosensitive PNVCL-g-Col was successfully synthesized by forming amide bonds between the –COOH and –NH2 groups using the NHS/EDC system (). PNVCL-g-Col solution was dialyzed using 12–14 kDa cut-off dialysis membrane in ultrapure water for 3 days. The IR spectrum of conjugated biopolymer indicated successful grafting, which was confirmed by the characteristic peaks of PNVCL-COOH (1480–1443 cm−1) and the secondary amide peaks of collagen around 1552 cm−1 ().
Characterization of thermal properties
Phase transition temperature
shows the temperature-dependent phase transition findings for PNVCL, Collagen, and PNVCL-g-Col. The phase transition temperature of pure collagen was absent in the temperature range of 20–45 °C. However, after conjugation with the thermosensitive PNVCL-COOH, the PNVCL-g-Col copolymer exhibited a temperature-dependent transmittance change. PNVCL-COOH and PNVCL-g-Col copolymer samples, both in solution and lyophilized sponge forms underwent rapid gelation at their respective LCST, with a rapid thermosensitive phase transition from clear transparent liquid to opaque white gel (inset of ), as indicated by a sudden decrease in transmittance. The LCST value of PNVCL-g-Col copolymer was (∼38.5 °C) higher than that of PNVCL, which can be attributed to the increase in the hydrophilic character of the copolymer. Thus, the phase transition temperature of PNVCL-g-Col is close to physiological human body temperature, which may be useful in a variety of biomedical applications.
Figure 6. Temperature dependent phase change of PNVCL, collagen, and PNVCL-g-Col. Inset photo showing PNVCL-g-Col hydrogel before (transparent fluid at 4 °C) and after gelation (white opaque gel at 40 °C).
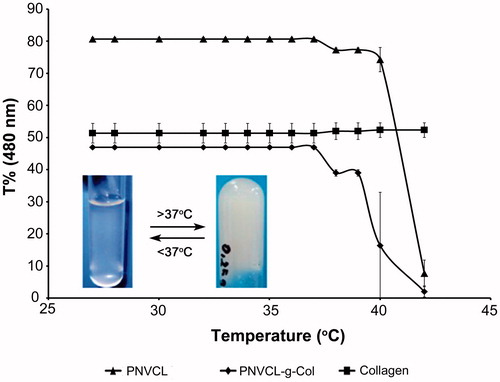
Increase in LCST is attributed to the inclusion and strong interaction of hydrophilic and charged groups (Chilkoti et al. Citation2002, Wu et al. Citation2005). For example, while PNIPAAm exhibits a LCST of 33.5 °C, which is 2–3 °C higher for PNIPAAm-COOH (Lee and Kim Citation2010). Additionally, Curcio et al. (Citation2010) have shown that the LCST of thermosensitive microspheres could be elevated (from 35.4 to 36.9 °C) by including hydrolyzed methacrylated gelatin moieties, bearing hydrophilic carboxylic acid groups. The LCST of thermosensitive PNVCL could be increased by folate-conjugation and poly(ethylene glycol) copolymerization (Prabaharan et al. Citation2009).
Reproducibility of phase transition is significant for potential applications of a thermosensitive hydrogel. Findings of the iterative sol–gel transition experiment are shown in . From the figure, reversibility of phase transition below (25 °C) and above (40 °C) the LCST of PNVCL-g-Col was evident for at least 12 cycles. Complete transition, both from sol-to-gel and from gel-to-sol cycle takes approximately 1.5–2 min.
DSC and TGA analyses
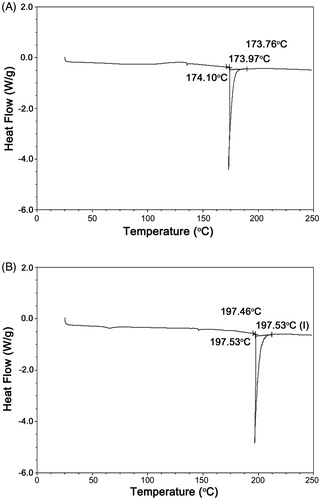
DSC findings of the PNVCL-COOH and PNVCL-g-Col copolymer are given in . The PNVCL-COOH peak was observed at 174 °C, which was a result of the removal of free water (). On the other hand, the PNVCL-g-Col copolymer phase transition was observed at a higher temperature of 197 °C (). We attribute this to the incorporation of collagen with highly hydrated domains, which formed bonds with the copolymer. The removal of the bound water was more difficult and there was a shift toward higher temperature at the phase transition temperature.
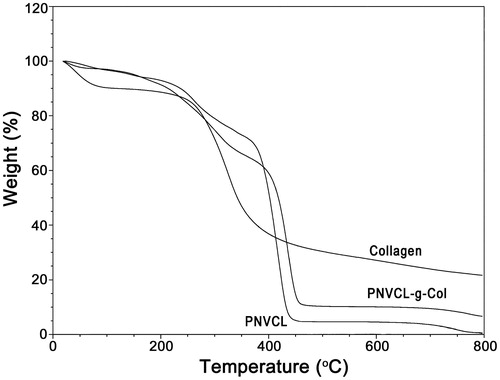
TGA analysis confirmed the DSC results. Thermodynamic evaluations emphasized that PNVCL-g-Col copolymer was more stable than pure collagen. This is a result of the increased stability with crosslinking following copolymerization of collagen with PNVCL.
The TGA thermogram of collagen shows two transition bands (). By the loss of the bound water, the primary band appeared at the 90–110 °C temperature range. The second band at 300–380 °C temperature range was probably due to loss of weight (∼30%). The loss of weight of PNVCL-COOH was observed at 180 °C with the removal of the free and bound water. The band between 190 and 380 °C may be due to maximum thermal degradation/carbonization of the synthetic polymer. Owing to the high carbon content of PNVCL-COOH molecules, the gel was more readily carbonized and observed weight-loss was higher compared to the carbonization of pure collagen. Following conjugation of collagen with PNVCL-COOH, the related loss of weight of the band shifted toward a higher temperature range of 390–420 °C (resulting in 90% weight loss).
Rheological analysis
Rheological analysis is instrumental in determining the relationship between structural and mechanical properties of the materials. We performed ramp experiments to further assess the effect of temperature on rheological properties. Two dynamic mechanical properties influence the mechanism of the gelation process in hydrogels. The elastic modulus G′ stands for the reversibly stored energy of the system. While, the viscous modulus G″, represents the irreversible energy loss. Temperature-sensitive gelation process is a gradual transition from a viscoelastic liquid to a viscoelastic solid. As the temperature increased, the solutions of PNVCL-g-Col turned into a gel-like state, which implies that both moduli had increased (). G′ was almost equal to G″ and slightly greater than G″ in all experiments. The increase rate in G′ was higher than that of G″ due to the increase in elastic properties, since collagen has a flexible polymer chain. Below the LCST, via hydrogen bonding, favorable interactions caused dissolution of the polymer in aqueous environment (). A likely explanation is that, above the LCST, PNVCL-g-Col molecules aggregated in solution due to the dehydration of hydrophobic PNVCL blocks of PNVCL-g-Col. At critical point, gel structure became “tighter” as the molecular hydrophobic interactions increased. We address the temperature-induced association behavior to the hydrophobic groups present in the structure of PNVCL chains. As the temperature increased to 36 °C, the G′ and G″ values overlapped, indicating that PNVCL-g-Col underwent a sol–gel transition, transforming into an elastic network.
Figure 10. Rheological assessments during phase transition, as a function of stress, time, temperature, and strain sweep. G′ is the stored elastic modulus and G″ is the viscous loss modulus.
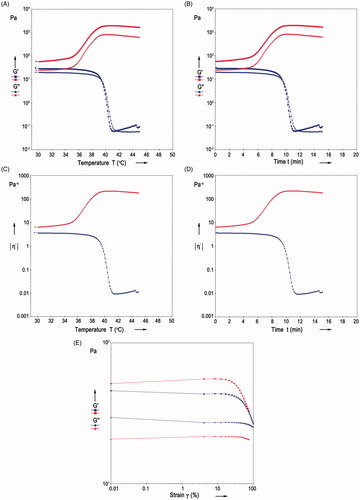
With elevation in temperature, the structure reached a plateau at ∼38 °C, which is indicative of a stable elastic network. However, the G′ and G″ values of the solutions of pure collagen decreased drastically with the increase of temperature to nearly 38.5 °C. This is probably due to the decomposing of collagen, leading to the loss of elastic and viscous properties.
We performed rheological assessments during phase transition as a function of time, in order to characterize gelation and its reversibility (). Alterations in storage modulus in respect to time evolution, complies with our proposed mechanism for gelation. The time sweep profiles of the viscous (G″) and elastic (G′) moduli of collagen and PNVCL-g-Col exhibited similar results with temperature sweep profiles that G′ was equal to G″ or slightly greater than G″ in all experiments. The G′ and G″ values for PNVCL-g-Col solutions displayed a steep increase between 6 and 9 min when the solutions began to turn into gel-like state accompanied by transformation into an elastic network (). However, the G′ and G″ of the collagen solutions decreased sharply within nearly 8 min due to decomposition of elastic and viscous networks.
We investigated complex viscosity in relation to temperature (), and time (). The complex viscosity of PNVCL-g-Col hydrogel significantly increased with temperature and reached a plateau above phase transition (at ∼38.5 °C).
Strain sweep is useful in determining the LVE with respect to strain. Overall, the G″ was smaller than G′. After running the strain sweep, a stable gel was formed, indicating transition from liquid to elastic form. At low and intermediate values of strain, stress response against steady shearing for elastic materials, is independent from the deformation rate. Our results showed that (), collagen and PNVCL-g-Col displayed a linear strain region up to 12% strain.
Water uptake
We measured the change in water uptake according to immersion time and is shown in . The water uptake of the conjugated polymer is influenced by temperature. Water uptake of PNVCL-g-Col copolymer was higher at +4 °C (at which hydrophilicity is maximum). Water molecules penetrated into the hydrogel structure more easily because of increased hydrophilicity, i.e., the increase in hydrogen bonds between water molecules and hydrophilic chain segments. Above the LCST, the bound water was released from the PNVCL-g-Col copolymer. In turn, hydrophobic interactions between the PNVCL chains in the PNVCL-g-Col increased. As a result, the copolymer collapsed/shrunk and the swelling ratio of PNVCL-g-Col decreased by approximately 50% within 6 h.
Figure 11. Water uptake of PNVCL-g-Col depending on temperature at (A) 4 °C (below the LCST) and (B) 40 °C (above the LCST).
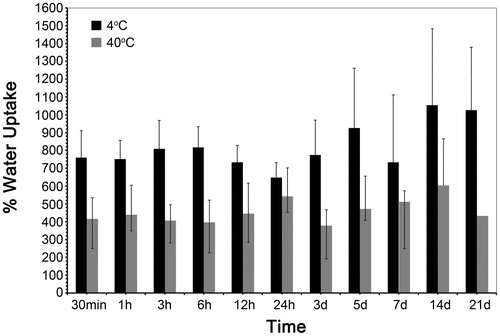
PNVCL-g-Col exhibited temperature-dependent water uptake: Below LCST, absorbed larger amounts of water related to its hydrophilic character. Above the LCST, transformed into the hydrophobic form leading to the aggregation of hydrophobic chains and showed decrease in water uptake. Temperature-sensitive swelling performance of any gel material is dependent on the equilibrium between hydrophilicity and hydrophobicity (Makhaeva et al. Citation1996). Doleski et al. (Citation2010) have shown that the swelling degree of collagen-PNIPAAm increases with the increase in relative collagen content. Besides, water molecules that cluster inside porous structures exist as free water, which adds to the swelling capacity of hydrogels.
In vitro release of lidocaine and BSA
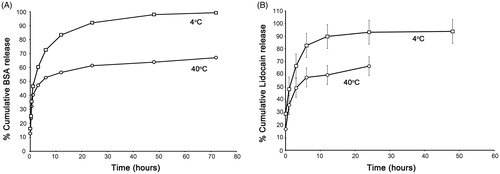
We investigated the release of model drugs; BSA () and lidocaine hydrochloride () from PNVCL-g-Col sponge as a function of time. Release patterns are similar as we observed a rapid release in the first couple of hours, then reaching asymptote at around 72 h and 48 h, respectively for BSA and lidocaine. We observed that the release from PNVCL-g-Col was tunable by temperature changes. In vitro drug release properties were compatible and in correlation with the water uptake characteristics of the PNVCL-g-Col. Cumulative release at 4 °C was higher than at 40 °C at all time-points (). This can be explained by the swollen state of PNVCL-g-Col below the LCST (4 °C), allowing drug release. Due to the collapse of the hydrogel network, percent cumulative release above the LCST (40 °C) was ∼40% lesser for BSA, and 30% lesser for lidocaine.
In vitro cytotoxicity
Cytotoxicity analysis is presented in . Indirect extraction experiments demonstrated that the synthesized copolymer was not toxic to human dermal fibroblasts, with a cell viability of 89.6%±4.9%, after 48 h of incubation with the PNVCL-g-Col extract (which is within the in vitro cytotoxicity limits of the ISO extract test). On the other hand, the viability of the cells incubated with the positive control (standard medium containing 400 μM phenol) was almost completely lost (4.1%±2.3% viability). This finding supports the potential future use of the thermosensitive copolymer in in vivo applications.
Table 1. In vitro cytotoxicity evaluation based on indirect extract testing (ISO 10993-5).
Conclusions
In this study, we have synthesized a new thermosensitive copolymer and have extensively evaluated its structural, thermal, and rheological properties. The temperature dependent phase change of PNVCL-g-collagen is slightly above the human physiological temperature, and is tunable through temperature-dependent switch on–off mechanism. This non-toxic thermosensitive biopolymer holds the potential for use in diverse biomedical applications, including controlled release and tissue engineering.
Disclosure statement
The authors declare no competing financial interests in relation to this article. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Bauer AJP, Liu J, Windsor LJ, Song F, Li B. 2014. Current development of collagen-based biomaterials for tissue repair and regeneration. Soft Mater. 12:359–370.
- Chilkoti A, Dreher MR, Meyer DE, Raucher D. 2002. Targeted drug delivery by thermally responsive polymers. Adv Drug Deliv Rev. 54:613–630.
- Cooperstein MA, Canavan HE. 2013. Assessment of cytotoxicity of (N-isopropyl acrylamide) and poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases. 8:19.
- Curcio M, Puoci F, Spizzirri UG, Iemma F, Cirillo G, Parisi OI, et al. 2010. Negative thermo-responsive microspheres based on hydrolyzed gelatin as drug delivery device. AAPS PharmSciTech. 11:652–662.
- Demirdögen B, Elçin AE, Elçin YM. 2010. Neovascularization by bFGF releasing hyaluronic acid-gelatin microspheres: in vitro and in vivo studies. Growth Factors. 28:426–436.
- Doleski S, Yao L, Pandit A, Elvira C. 2010. NGF release from thermo-responsive collagen-polyNIPAam polymer networks supports neuronal cell growth and differentiation. J Biomed Mater Res A. 94:457–465.
- Emin N, Koc A, Durkut S, Elcin AE, Elcin YM. 2008. Engineering of rat articular cartilage on porous sponges: effects of TGF-beta 1 and microgravity bioreactor culture. Artif Cells Blood Substit Immobil Biotechnol. 36:123–137.
- Galaev IY, Mattiasson B. 1999. 'Smart' polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 17:335–340.
- Gill ES, Hudson SM. 2004. Stimuli-responsive polymers and their bioconjugates. Prog Polym Sci. 29:1173–1222.
- Guan Y, Li Z, He F, Huang Y, Song W, Hu J. 2015. “On-off” thermoresponsive coating agent containing salicylic acid applied to maize seeds for chilling tolerance. PLoS One. 10:e0120695.
- Hoarea TR, Kohaneb DS. 2008. Hydrogels in drug delivery: progress and challenges. Polymer. 49:1993–2007.
- Jiří S. 2009. NMR investigations of phase transition in aqueous polymer solutions and gels. Curr Opin Colloid Interface. 14:184–191.
- Joseph N, Prasad T, Raj V, Kumary TV. 2010. A cytocompatible poly(N-isopropylacrylamide-co-glycidylmethacrylate) coated surface as new substrate for corneal tissue engineering. J Bioact Compat Polym. 25:58–74.
- Kavitha T, Kim JO, Jang S, Kim DP, Kang IK, Park SY. 2016. Multifaceted thermoresponsive poly(N-vinylcaprolactam) coupled with carbon dots for biomedical applications. Mater Sci Eng C Mater Biol Appl. 61:492–498.
- Krause AT, Zschoche S, Rohn M, Hempel C, Richter A, Appelhans D, Voit B. 2016. Swelling behavior of bisensitive interpenetrating polymer networks for microfluidic applications. Soft Matter. 12:5529–5536.
- Lau ACW, Wu C. 1999. Thermally sensitive and biocompatible poly(N-vinylcaprolactam): synthesis and characterization of high molar mass linear chains. Macromolecules. 32:581–584.
- Lau HK, Kiick LK. 2015. Opportunities for multicomponent hybrid hydrogels in biomedical applications. Biomacromolecules. 16:28–42.
- Laukkanen A, Valtola L, Winnik FM, Tenhu H. 2004. Formation of colloidally stable phase separated poly(N-vinylcaprolactam) in water: a study by dynamic light scattering, microcalorimetry, and pressure perturbation calorimetry. Macromolecules. 37:2268–2274.
- Lee B, Jiao A, Yu S, You JB, Kim DH, Im SG. 2013. Initiated chemical vapor deposition of thermoresponsive poly(N-vinylcaprolactam) thin films for cell sheet engineering. Acta Biomater. 9:7691–7698.
- Lee EJ, Kim YH. 2010. Synthesis and thermo-responsive properties of chitosan-g-poly (N-isopropylacrylamide) and HTCC-g-poly(N-isopropylacrylamide) copolymers. Fiber Polym. 11:164–169.
- Linn T, Erb D, Schneider D, Kidszun A, Elcin AE, Bretzel RG, Elcin YM. 2003. Polymers for induction of revascularization in the rat fascial flap: application of vascular endothelial growth factor and pancreatic islet cells. Cell Transplant. 12:769–778.
- Liu J, Debuigne A, Detrembleur C, Jerome C. 2014. Poly(N-vinylcaprolactam): a thermoresponsive macromolecule with promising future in biomedical field. Adv Healthcare Mater. 3:1941–1968.
- Makhaeva EE, Thanh LTM, Starodoubtsev SG, Khokhlov AR. 1996. Thermoshrinking behavior of poly(vinylcaprolactam) gels in aqueous solution. Macromol Chem Phys. 197:1973–1982.
- Mura S, Nicolas J, Couvreur P. 2013. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 12:991–1003.
- Prabaharan M, Grailer JJ, Steeber DA, Gong S. 2008. Stimuli-responsive chitosan-graft-poly(N-vinylcaprolactam) as a promising material for controlled hydrophobic drug delivery. Macromol Biosci. 8:843–851.
- Prabaharan M, Jamison JG, Steeber DA, Gong S. 2009. Thermosensitive micelles based on folate-conjugated poly(N-vinylcaprolactam)-block-poly(ethylene glycol) for tumor-targeted drug delivery. Macromol Biosci. 9:744–753.
- Sakuma M, Kumashiro Y, Nakayama M, Tanaka N, Haraguchi Y, Umemura K, et al. 2016. Preparation of thermoresponsive nanostructured surfaces for tissue engineering. J Vis Exp. 109:e53465.
- Sarkar J, Kumar A. 2016. Thermo-responsive polymer aided spheroid culture in cryogel based platform for high throughput drug screening. Analyst. 141:2553–2567.
- Schild HG. 1992. Poly(N-isopropylacrylamide): experiment, theory and application. Prog Polym Sci. 17:163–249.
- Sedláček O, Černoch P, Kučka J, Konefal R, Stepanek P, Vetrik M, et al. 2016. Thermoresponsive polymers for nuclear medicine: which polymer is the best? Langmuir. 32:6115–6122.
- Seker S, Arslan YE, Durkut S, Elcin AE, Elcin YM. 2014. Nanotechnology for tissue engineering and regenerative medicine. In: Iniewski K, Selimovic S, Eds. Nanopatterning and Nanoscale Devices for Biological Applications. Boca Raton: CRC Press, pp. 339–367.
- Shimizu T, Yamato M, Kikuchi A, Okano T. 2003. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 24:2309–2316.
- Sionkowska A. 2012. Collagen based materials for biomedical applications: preparation and properties. Mater Sci Forum. 706–709:595–599.
- Strukova S, Dugina T, Chistov I, Lange M, Markvicheva EA, Kuptsova S, et al. 2001. Immobilized thrombin receptor agonist peptide accelerates wound healing in mice. Clin Appl Thromb Hemost. 7:325–329.
- Stuart MAC, Huck WTS, Genzer J, Müller M, Ober C, Stamm M, et al. 2010. Emerging applications of stimuli-responsive polymer materials. Nat Mater. 9:101–113.
- Van Luyn MJA, Van Wachem PB, Damink LO, Dijkstra PJ, Feijen J, Nieuwenhuis P. 1992. Relations between in vitro cytotoxicity and crosslinked dermal sheep collagens. J Biomed Mater Res. 26:1091–1110.
- Van Luyn MJA, Van Wachem PB, Dijkstra PJ, Olde Damink LHH, Feijen J. 1995. Calcification of subcutaneously implanted collagens in relation to cytotoxicity, cellular interactions and crosslinking. J Mater Sci Mater Med. 6:288–296.
- Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. 2005. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials. 26:3055–3064.
- Ward MA, Georgiou TK. 2011. Thermoresponsive polymers for biomedical applications. Polymers. 3:1215–1242.
- Wu JY, Liu SQ, Heng PWS, Yang YY. 2005. Evaluating proteins release from, and their interactions with, thermosensitive poly (N-isopropylacrylamide) hydrogels. J Control Release. 102:361–372.
- Zhou X, Zhao Y, Chen S, Han S, Xu X, Guo J, et al. 2016. Self-assembly of pH-responsive microspheres for intestinal delivery of diverse lipophilic therapeutics. Biomacromolecules. 17:2540–2554.